Abstract
Autophagy is not only a survival response to growth-factor or nutrient deprivation but also an important mechanism for tumor-cell suicide, including melanoma. Polygonatum odoratum lectin (POL) displays apoptosis- and autophagy-inducing effects in many human tumors. POL also inhibits the growth of melanoma cells, but its role and molecular mechanism in malignant melanoma remain unclear. In this study, we found that POL suppressed proliferation and induced autophagy in melanoma cells. miR1290 was upregulated and inhibited autophagy in melanoma. BECN1 is the direct functional effector of miR1290. Furthermore, we found that POL promoted BECN1 expression though inhibition of miR1290, thus inducing melanoma-cell autophagy. This finding elucidates a new role and mechanism for POL in melanoma, and provides a potential antineoplastic agent for melanoma treatment.
Introduction
Malignant melanoma, the most aggressive skin tumor, is the primary cause of death of skin tumors.Citation1–Citation3 The global incidence of malignant melanoma has been increasing in recent years.Citation4,Citation5 Melanoma is developing rapidly with early metastasis, and there is no effective treatment for patients with advanced malignant melanoma.Citation1 Therefore, it is important to develop new treatments for melanoma.
Cancer is associated with programmed cell death (PCD), which plays important roles in the development and homeostasis of multicellular organisms.Citation6 Apoptosis and autophagy are two main types of PCD. Apoptosis is an intrinsic mechanism for cell suicide that is regulated by numerous molecular signaling pathways.Citation7 Autophagy is independent of phagocytes and a conserved eukaryotic cellular degradative process that clears damaged or superfluous macrocomplexes and organelles.Citation8
Plant lectins are a class of non-immune-origin carbohydrate-binding proteins, and they can agglutinate cells or precipitate polysaccharides and glycoconjugates.Citation9,Citation10 Several major plant-lectin families have been reported to possess antitumor activities via inducing apoptosis and autophagy in many human malignant tumor cells, including melanoma.Citation11,Citation12 Polygonatum odoratum lectin (POL), isolated from traditional Chinese medicine herb P. odoratum (Mill.). Druce, is a mannose binding-specific Galanthus nivalis agglutinin-related family lectin.Citation13 POL displays remarkable apoptosis- and autophagy-inducing effects in human lung adenocarcinoma cells and murine fibrosarcoma cells.Citation14,Citation15 POL also inhibits the proliferation of melanoma cells.Citation13 However, the effect and molecular mechanism of POL in melanoma remains unclear.
In this study, we demonstrated that POL suppressed the proliferation of melanoma cells. We also found that POL induced autophagy in melanoma cells through upregulated expression of BECN1. BECN1 is involved in autophagy at every major step in the autophagic pathway.Citation16 We found that miR1290 was upregulated and inhibited autophagy by targeting BECN1 in melanoma. Moreover, we found that POL regulated BECN1 by inhibiting the expression of miR1290. These results suggest that POL may serve as a potential antineoplastic agent in future melanoma treatment.
Materials and methods
Human tissue samples
Thirty primary malignant melanoma-tissue and adjacent normal tissue samples were collected from melanoma patients undergoing surgery at the Affiliated People’s Hospital of Jiangsu University. Two professional pathologists independently diagnosed the clinical and pathological features of the melanoma patients. Every sample was preserved in liquid nitrogen immediately after collection. All patients provided informed consent for the use of their tissue in this research. The study was approved by the Human Research Ethics Committee of the Affiliated People’s Hospital of Jiangsu University.
Cell lines and cell culture
The human malignant melanoma cell line A375 was obtained from the Chinese Academy of Sciences cell bank (Shanghai, China). A375 cells were cultured in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) with 10% FBS (Thermo Fisher Scientific). Human epidermal melanocytes (HEMa-LP) were purchased from Thermo Fisher Scientific, and the cells were maintained in medium 254 and human melanocyte growth supplement (Thermo Fisher Scientific). The cell line was maintained in an atmosphere of 37°C containing 5% carbon dioxide.
Oligonucleotides, plasmids, and reagents
POL was purified as described previously.Citation13 Oligonucleotides were purchased from GenePharma (Shanghai, China). Sequences were: Hsa-miR1290 mimic, 5′-UGGAUUUUUGGAUCAGGGA-3′; BECN1 siRNA (siBECN1), 5′-CCCAGCCAGGAUGAUGUCUACAGAA-3′, and 5′-GCUAACUCAGGAGAGGAGCCAUUUA-3′; negative control, 5′-UUCUCCGAACGUGUCACGUTT-3′; Has-miR1290 inhibitor, 5′-UCCCUGAUCCAAAAAUCCA-3′; inhibitor negative control, 5′-CAGUACUUUUGUGUAGUACAA-3′.
Extraction of RNA and quantitative RT-PCR
We used Trizol (Thermo Fisher Scientific) to extract total RNA from cells and tissue. Fermentas reverse-transcription (RT) reagents and the Applied Biosystems TaqMan microRNA RT kit was used to conduct RT polymerase chain reaction (PCR). The amplification reaction was performed with an ABI StepOne Plus system according to predetermined conditions. The specific primer for miR1290 was chemically synthesized in RiboBio (Guangzhou, China). The following primers were used: BECN1 (forward) 5′-AACCAGATGCGTTATGCCCA-3′, (reverse) 5′-CTGTCCACTGTGCCAGATGT-3′. Relevant data were analyzed using 2−ΔΔCt.
CCK-8 assay
CCK-8 (Beyotime, Haimen, China) assay was used to assess melanoma-cell viability. A375 cells (5×103) were inoculated on 96-well plates. Cells were then treated with different concentrations of POL. Fresh culture media (100 μL) containing 10% CCK-8 was added to A375 cells at different times. A microplate reader (Multiskan FC; Thermo Fisher Scientific) was used to measure absorbance at 450 nm wavelength.
Transmission electron microscopy
A375 cells were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer for 1 hour. After being washed three times using 0.1 M PBS, cells were fixed with 1% osmium tetroxide in 0.1 M PBS. Samples were dehydrated with increasing ethanol concentrations and embedded in epoxy resin. Transmission electron microscopy was used to observe the ultrastructure of A375 cells.
Luciferase reporter assay
A BECN1 promoter luciferase reporter plasmid (contains 949 bp human BECN1 promoter) was obtained from SwitchGear Genomics (Menlo Park, CA, USA). The BECN1 3′-UTR fragment containing the miR1290-binding sequences was inserted into pMiR-Report vectors. Mutated plasmid was used as a control. A375 cells were transfected with the related reporter plasmids and Hsa-miR1290 mimic. After transfection for 48 hours, a dual luciferase reporter-assay system (Promega, Fitchburg, WI, USA) was used to detect luciferase activity.
Western blot
We used radioimmunoprecipitation-assay buffer (KenGen, Nairobi, Kenya) to extract total protein from cells, and a bicinchoninic acid protein-assay kit (Beyotime) was used to determine protein concentrations. Equal amounts of protein were separated by sodium dodecyl sulfate polyacrylamide-gel electrophoresis and transferred to polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA), and membranes were closed with milk and incubated overnight with diluted antibodies against BECN1 (1:1,000; Abcam, Cambridge, UK), LC3B (1:3,000; Abcam), and p62 (1:1,000; Abcam). Membranes were incubated with HRP-conjugated secondary antibody (1:2,500; Santa Cruz Biotechnology, Dallas, TX, USA), with GAPDH used as control (1:2,500; Abcam).
Statistical analysis
Data are presented as means ± standard error, and SPSS 13.0 was used to analyze the data. Data were analyzed with independent t-tests (two-sided) and one-way analysis of variance. P<0.05 was considered statistically significant. Pearson correlation analysis was performed using MatLab. All experiments were repeated in triplicate.
Results
POL suppressed proliferation and induced autophagy in melanoma cells
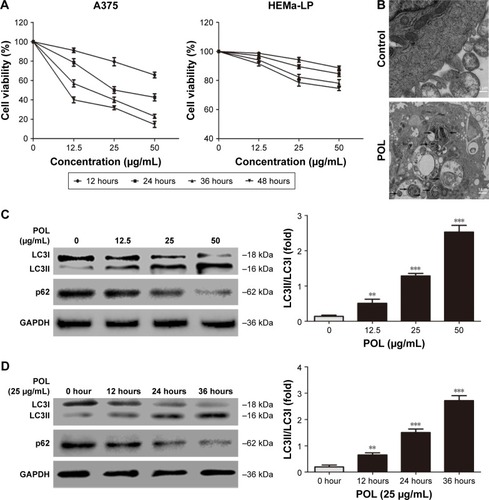
We initially explore the role of POL in melanoma cells. CCK-8 assays revealed that POL significantly inhibited the growth of human melanoma A375 cells in a dose- and time-dependent manner, and the inhibitory rate reached nearly 50% after treatment with 25 μg/mL POL for 24 hours (). However, only a small percentage of CD was found in the human epidermal melanocytes (HEMa-LP) after treatment with POL (). We next used transmission electron microscopy to observe the effect of POL on autophagy. Formation of autophagosome-related structures was readily observed in the POL-treatment group (). LC3I transforming into LC3II is characteristic of autophagy; p62, also known as Sqstm1, bound directly to LC3, and p62 degradation is a result of autophagy. As shown in , POL induced the accumulation of LC3II and the degradation of p62 in A375 cells in a dose- and time-dependent manner. These results demonstrated that POL was effective in inducing autophagy in melanoma cells.
Figure 1 POL suppressed proliferation and induced autophagy in melanoma cells.
Abbreviation: POL, Polygonatum odoratum lectin.
POL increased levels of BECN1 mRNA and protein in melanoma cells
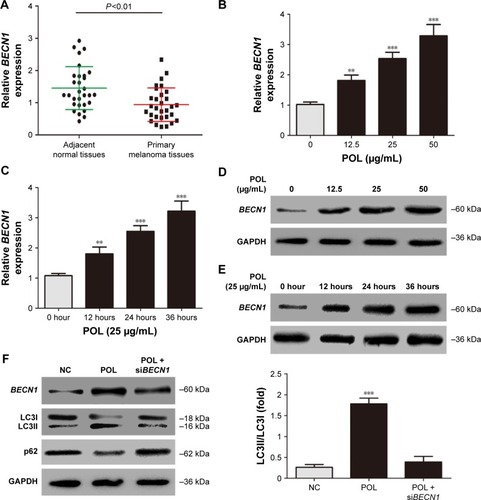
BECN1 is a key factor in autophagosome formation and involved in autophagy at every major step.Citation16 BECN1 was markedly decreased in primary melanoma tissues compared to adjacent normal tissues (). A375 cells were treated with various concentrations of POL for 24 hours and 25 μg/mL POL for different times. BECN1 mRNA and protein expression increased progressively in response to 0–50 μg/mL POL (). Additionally, 25 μg/mL POL led to an increase in expression of BECN1 mRNA and protein in a time-dependent manner in A375 cells (). Importantly, siBECN1 rescued the effect of POL on autophagy (). These results suggested that POL induced autophagy in melanoma cells through increasing BECN1 expression.
Figure 2 POL increased levels of BECN1 mRNA and protein in melanoma cells.
Abbreviation: POL, Polygonatum odoratum lectin.
POL may regulate BECN1 expression though miR1290
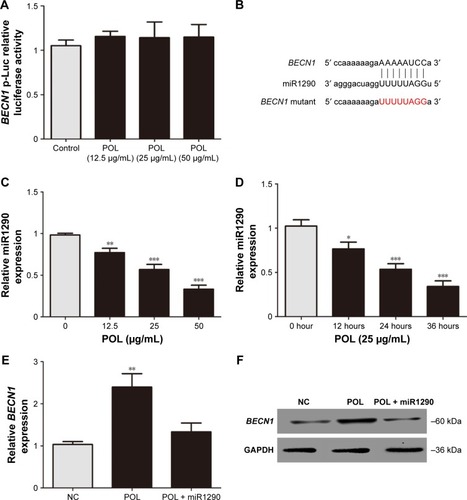
We further explored the mechanism by which POL increased BECN1 levels. We first generated the BECN1 promoter luciferase reporter construct and transfected plasmid into A375 cells. After 24 hours’ transfection, the cells were treated with different concentrations of POL. No significant change was detected in luciferase activity between the negative-control group and the POL-treatment group (). This result suggested that POL may have regulated BECN1 expression at the posttranscriptional level. miRNAs have been shown to influence gene expression by cleaving or inhibiting the translation of target mRNA.Citation17,Citation18 We next explored whether POL regulated BECN1 expression via targeting specific miRNAs. miR1290 was predicted to bind to the BECN1 3′-UTR with TargetScan (). Real-time PCR showed that miR1290 levels were reduced in POL-treated cells (). Moreover, miR1290 mimics abolished the effect of POL on the expression of Becn1 (). These findings indicated that POL may have regulated BECN1 though miR1290.
Figure 3 POL may regulate BECN1 expression though miR1290.
Abbreviations: POL, Polygonatum odoratum lectin; PCR, polymerase chain reaction; NC, negative control.
miR1290 inhibited autophagy in melanoma cells by targeting BECN1
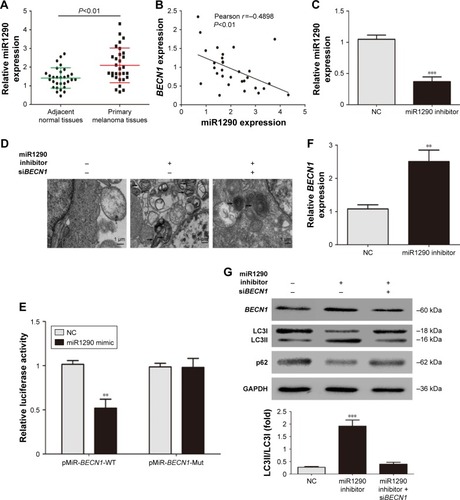
We further investigated the role and mechanism of miR1290 in malignant melanoma. miR1290 levels were markedly increased in primary melanoma tissues compared to adjacent normal tissues (). Analysis indicated negative Pearson correlations between miR1290 and BECN1 (). The miR1290 inhibitor was transfected into A375 cells (). Transmission electron microscopy showed that miR1290 inhibitor was effective in inducing autophagy (). Western blotting showed that miR1290 inhibitor led to an increase in LC3II levels and p62 clearance (). As mentioned before, miR1290 was predicted to bind to BECN1 3′-UTR. We constructed the luciferase reporter plasmids containing the wild-type and mutant-binding sites of the 3′-UTR of BECN1 (). Luciferase activity in cells with wild-type plasmid was decreased in the miR1290-mimic transfection group, but no significant change was detected in the mutant plasmid group (). Meanwhile, miR1290 downregulation caused an increase in endogenous BECN1 levels (). The effect of miR1290 inhibitor on A375 melanoma-cell autophagy was rescued by siBECN1 (). These results showed that miR1290 inhibited autophagy by targeting BECN1 in melanoma cells.
Figure 4 miR1290 inhibited autophagy in melanoma cells by targeting BECN1.
Abbreviations: PCR, polymerase chain reaction; NC, negative control; WT, wild type; Mut, mutant.
POL induced autophagy though regulating miR1290 in melanoma cells
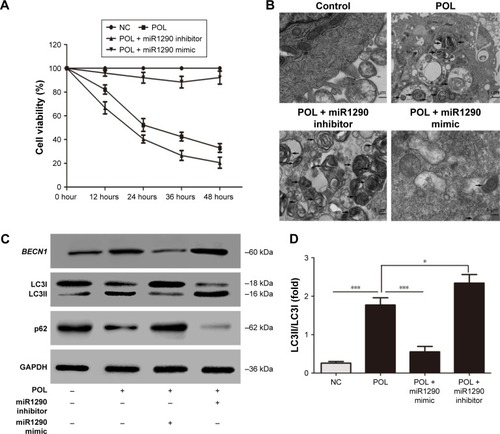
We further investigated whether melanoma-cell autophagy induced by POL could be affected by miR1290. After treatment with 25 μg/mL POL, A375 cells were transfected with miR1290 mimics or inhibitor. Overexpression of miR1290 repressed the melanoma CD induced by POL, while knockdown of miR1290 led to the reverse effect (). As shown in , overexpression of miR1290 reversed the A375-cell autophagy induced by POL, while knockdown of miR1290 amplified the POL effect. Meanwhile, the effect of POL on Becn1, LC3II, and p62 expression was reversed by the miR1290 mimic and amplified by the miR1290 inhibitor (). These results suggested that POL induced autophagy in melanoma via regulating miR1290.
Figure 5 POL induces autophagy though regulating miR1290 in melanoma cells.
Abbreviations: POL, Polygonatum odoratum lectin; NC, negative control.
Discussion
Autophagy, type II PCD, is distinguished from apoptosis by the presence of autophagosomes and autolysosomes and an intact nucleus.Citation19 Autophagy is a eukaryotic cell-degradation process that clears damaged or superfluous macrocomplexes and helps in maintaining cellular metabolism and homeostasis.Citation7,Citation8 Autophagy also plays an important role in tumor-cell suicide.Citation20 In recent years, research has effectively inhibited melanoma-cell growth via inducing autophagic CD.Citation21,Citation22 As such, studies on melanoma autophagy may provide novel therapeutic strategies.
POL is a mannose-binding G. nivalis agglutinin-related family lectin.Citation13 POL can induce apoptosis in murine fibrosarcoma cells through death-receptor and mitochondrial pathways.Citation15 It also can induce autophagy in human lung adenocarcinoma cells by regulation of special miRNAs.Citation14,Citation23 POL also inhibits melanoma-cell growth,Citation13 but its specific role and molecular biological mechanism in malignant melanoma remain to be confirmed. Here, we found that POL significantly inhibited growth and induced autophagy in melanoma A375 cells. We further explore the mechanisms thereof.
Accumulating evidence indicates that BECN1 is involved in autophagy at every major step in the autophagic pathway.Citation16 We demonstrated that POL induced autophagy by increasing BECN1 expression in melanoma cells. BECN1 promoter luciferase reporter assays showed that POL may regulate BECN1 expression at the posttranscriptional level. miRNA, an sncRNA with a length of about 22 nucleotides, plays an important role in regulating gene expression at the post-transcriptional level by biding to the 3′-UTR of its target mRNAs.Citation24 In recent years, the role of miRNA in tumorigenesis has been studied extensively.Citation17,Citation25 miR1290 functions as an oncoMiR in diverse human tumors.Citation26,Citation27 miR1290 promotes gastric tumor-cell proliferation and metastasis by targeting FoxA1.Citation28 miR1290 also acts as a tumor oncogene in the progression of esophageal squamous-cell carcinoma by targeting NFIX.Citation26 However, the role of miR1290 in melanoma has rarely been studied. We found that miR1290 levels were significantly increased in primary melanoma tissues and induced autophagy in melanoma cells. BECN1 is the direct functional effector of miR1290. Moreover, we revealed that POL induced autophagy and regulated BECN1 though miR1290 in melanoma cells.
Conclusion
POL promoted the expression of BECN1 by inhibiting miR1290 levels, thus inducing melanoma-cell autophagy. Understanding the effect and regulatory mechanism of POL in melanoma could lead to the identification of novel potential antineoplastic agents for melanoma. Future studies to assess the role of POL in a clinical context are warranted.
Acknowledgments
This work was supported by the Social Development and Technology Support Foundation of Zhenjiang (SH2011057) and the Clinical Medical Science and Technology Development Fund of Jiangsu University (JLY20160002).
Disclosure
The authors report no conflicts of interest in this work.
References
- HaassNKSchumacherUMelanoma never says dieExp Dermatol20142347147224684560
- MilletAMartinARRoncoCRocchiSBenhidaRMetastatic melanoma: insights into the evolution of the treatments and future challengesMed Res Rev2016379814827569556
- LuanWLiLShiYLong non-coding RNA MALAT1 acts as a competing endogenous RNA to promote malignant melanoma growth and metastasis by sponging miR-22Oncotarget20167639016391227564100
- LittleEGEideMJUpdate on the current state of melanoma incidenceDermatol Clin20123035536122800543
- FerlayJShinHRBrayFFormanDMathersCParkinDMEstimates of worldwide burden of cancer in 2008: GLOBOCAN 2008Int J Cancer20101272893291721351269
- MathewRKarantza-WadsworthVWhiteERole of autophagy in cancerNat Rev Cancer2007796196717972889
- RubinsteinADKimchiALife in the balance: a mechanistic view of the crosstalk between autophagy and apoptosisJ Cell Sci20121255259526823377657
- ShiZLiCYZhaoSA systems biology analysis of autophagy in cancer therapyCancer Lett201333714916023791881
- SharonNLectins: carbohydrate-specific reagents and biological recognition moleculesJ Biol Chem20072822753276417145746
- SharonNLisHLectins as cell recognition moleculesScience19892462272342552581
- SilvaFOSantosPNFigueirôaEOAntiproliferative effect of Canavalia brasiliensis lectin on B16F10 cellsRes Vet Sci20149627628224565003
- LiuBBianHJBaoJKPlant lectins: potential antineoplastic drugs from bench to clinicCancer Lett201028711219487073
- YangYXuHLZhangZTCharacterization, molecular cloning, and in silico analysis of a novel mannose-binding lectin from Polygonatum odoratum (Mill.) with anti-HSV-II and apoptosis-inducing activitiesPhytomedicine20111874875521146383
- LiCChenJLuBMolecular switch role of Akt in Polygonatum odoratum lectin-induced apoptosis and autophagy in human non-small cell lung cancer A549 cellsPLoS One20149e10152624992302
- LiuBZhangBMinMWInduction of apoptosis by Polygonatum odoratum lectin and its molecular mechanisms in murine fibrosarcoma L929 cellsBiochim Biophys Acta2009179084084419414060
- HeCLevineBThe beclin 1 interactomeCurr Opin Cell Biol20102214014920097051
- ShiYWangYLuanWLong non-coding RNA H19 promotes glioma cell invasion by deriving miR-675PLoS One20149e8629524466011
- LuanWWangYChenXPKM2 promotes glucose metabolism and cell growth in gliomas through a mechanism involving a let-7a/c-Myc/hnRNPA1 feedback loopOncotarget20156130061301825948776
- LevineBKlionskyDJDevelopment by self-digestion: molecular mechanisms and biological functions of autophagyDev Cell2004646347715068787
- GozuacikDKimchiAAutophagy as a cell death and tumor suppressor mechanismOncogene2004232891290615077152
- LiuHHeZvon RütteTYousefiSHungerRESimonHUDown-regulation of autophagy-related protein 5 (ATG5) contributes to the pathogenesis of early-stage cutaneous melanomaSci Transl Med20135202ra123
- WangWJWangYChenHZOrphan nuclear receptor TR3 acts in autophagic cell death via mitochondrial signaling pathwayNat Chem Biol20141013314024316735
- WuLLiuTXiaoYPolygonatum odoratum lectin induces apoptosis and autophagy by regulation of microRNA-1290 and microRNA-15a-3p in human lung adenocarcinoma A549 cellsInt J Biol Macromol20168521722626562549
- BartelDPMicroRNAs: genomics, biogenesis, mechanism, and functionCell200411628129714744438
- YangCHWangYSimsMmiRNA203 suppresses the expression of protumorigenic STAT1 in glioblastoma to inhibit tumorigenesisOncotarget20167840178402927705947
- MaoYLiuJZhangDLiBMiR-1290 promotes cancer progression by targeting nuclear factor I/X(NFIX) in esophageal squamous cell carcinoma (ESCC)Biomed Pharmacother201576829326653554
- ZhangWCChinTMYangHTumour-initiating cell-specific miR-1246 and miR-1290 expression converge to promote non-small cell lung cancer progressionNat Commun201671170227325363
- LinMShiCLinXsMicroRNA-1290 inhibits cells proliferation and migration by targeting FOXA1 in gastric cancer cellsGene201658213714226851540
