Abstract
The aim of this work was to examine the expression of cancerous inhibitor of protein phosphatase 2A (CIP2A) in non-small cell lung cancer (NSCLC) and analyze its correlation with clinical outcomes. CIP2A protein levels were detected by immunohistochemistry (IHC). One hundred and eighty-four of 209 (88.3%) primary stage I–III NSCLC specimens and 4 of 38 (10.5%) adjacent normal lung tissue specimens expressed CIP2A protein. High expression of CIP2A was detected in 38.8% (81/209) of the NSCLC specimens. Patients diagnosed histologically with late-stage NSCLC (p<0.001) and malignant nodes (p=0.001) exhibited high CIP2A expression. Univariate analysis using the log-rank test identified CIP2A expression as a prognostic predictor for overall survival (p=0.005). In multivariate analyses using the Cox regression test, CIP2A expression, T stage, N stage, histological type, and chemotherapy were identified as independent prognostic factors (p=0.007, 0.001, 0.003, <0.001, and <0.001, respectively). Furthermore, Kaplan–Meier survival curves demonstrated that high CIP2A expression indicated poor prognosis in the subgroup of patients with squamous cell carcinoma (p=0.008). Similar results were noted in the subgroup of patients with adenocarcinoma, but the results did not reach statistical significance (p=0.084). We also used univariate analysis and multivariate analysis to assess the prognostic factors for overall survival in the subgroup of patients who received postoperative chemotherapy. CIP2A expression was also an independent prognostic factor in NSCLC patients who received postoperative chemotherapy (p=0.009), along with histological type (p=0.001) and N stage (p=0.034). In conclusion, adding to the accumulating evidence, our research suggested that the CIP2A expression is associated with aggressiveness and correlates with poor prognosis in NSCLC. Our findings also indicated that CIP2A might be a potential therapeutic target against NSCLC.
Introduction
Lung cancer is the leading cause of cancer death worldwide,Citation1 and 80% of cases involve non-small cell lung cancer (NSCLC). Despite the numerous different treatment options available, including surgery, chemotherapy, radiation therapy, and targeted therapy, lung cancer cure rates have increased slowly over recent decades. As an indicator of cure rates, median 5-year survival rate ranges from ~50% for stage IA disease to <30% for stage IIIA NSCLC.Citation2 Novel predictive biomarkers and therapeutic targets are required to improve survival rate.
As a human oncoprotein, cancerous inhibitor of protein phosphatase 2A (CIP2A) was recently identified as a PP2A (protein phosphatase 2A) inhibitor and MYC (Transcription factor p64) stabilizer in human malignancies.Citation3 Furthermore, there is a positive-feedback loop between CIP2A and MYC that may account for the pan expression and activation of MYC protein in cancer cells.Citation4,Citation5 In addition, CIP2A promotes the phosphorylation and activity of other oncoproteins, such as E2F1 (E2F transcription factor 1) and AKT (protein kinase B).Citation6,Citation7 Various independent studies have validated CIP2A’s role in promoting tumor growth and resistance to apoptosis and senescence-inducing therapies. In clinical studies, CIP2A overexpression has been confirmed in solid and hematological cancers.Citation3,Citation4,Citation8–Citation10 Moreover, CIP2A overexpression predicts poor prognosis in several human cancers, such as gastric cancer, ovarian cancer, and tongue cancer.Citation4,Citation8,Citation10
By examining CIP2A expression in human NSCLC specimens using immunohistochemistry (IHC), this study aimed not only to investigate its relationship with clinicopathological variables and prognostic factors but also sought to assess its correlation with prognosis in different histological types. We also explored the influence of CIP2A expression on postoperative chemotherapeutic or radiotherapeutic clinical treatments.
Materials and methods
Patients and tissue specimens
This retrospective study was approved by the Medical Ethical Committee of Harbin Medical University Cancer Hospital, Harbin, China. All patients provided written informed consent for participation in this study. Two hundred and nine primary NSCLC specimens and 38 paired adjacent normal lung tissue specimens were used in this research. The specimens were obtained from patients diagnosed with stage IA–IIIB NSCLC who underwent surgeries at the Harbin Medical University Cancer Hospital between March 2005 and December 2007. These patients did not receive any therapy before operation. The median age of the cohort was 58 years. The TNM staging system for lung cancer was used to classify patients as stages I (n=59), II (n=81), and III (n=69).Citation11 In pathological diagnosis, 116 tumors were squamous cell carcinomas and 93 were adenocarcinomas. A total of 151 patients received three to four cycles of adjuvant platinum doublet chemotherapy, and 58 patients received postoperative radiotherapy at a total dose that ranged from 50 to 66 Gy with 2 Gy per fraction administered 5 days per week. Complete clinical and follow-up data were available for all patients.
Immunohistochemistry
Immunohistochemical staining was performed according to the manufacturer’s protocol of the PV-6001 kit (Zhong Shan Golden Bridge Biological Technology Inc., Beijing, China). Briefly, 4-μm sections were deparaffinized with xylene, rehydrated in a decreasing ethanol gradient, and rinsed in phosphate-buffered saline. The slides were immersed in hydrogen peroxide (3%) to block endogenous peroxide activity for 15 min at room temperature. Antigen retrieval was performed by placing the slides in citrate buffer (0.01 M, pH 6.0) heated in a pressure cooker for 8 min. Then, the specimens were incubated with the CIP2A primary antibody (1:50, rabbit polyclonal; ab84547; Abcam, Cambridge, UK) at 4°C overnight. After incubation with biotin-labeled secondary antibodies at room temperature for 30 min, positive staining was detected by diaminobenzidine (DAB) reagent sets. Then, the specimens were counterstained with hematoxylin. Finally, each section was dehydrated by gradient alcohol and covered with a coverslip.
Immunohistochemical staining evaluation
All slides were examined by two experienced pathologists in a double-blinded fashion. The CIP2A expression level was classified by the product of intensity and proportion of positively stained cells. Positive staining presented as brown-yellow particles. The staining intensity was classified based on the following criteria: 0 (no staining), 1 (light yellow/weak staining), 2 (yellow-brown/moderate staining), or 3 (brown/strong staining). The proportion of positively stained tumor cells was scored as follows: 1 (positive cells 1%–25%), 2 (positive cells 26%–50%), 3 (positive cells 51%–75%), or 4 (positive cells 75%–100%).Citation12 The scores of each tumor sample were multiplied to yield a final score of 0–12, and the median value of score 3 was used to distinguish low versus high CIP2A expression. The specimen with a final score of ≤3 was classified as having low expression. Otherwise, the specimen was classified as having high expression.
Statistical analysis
Statistical analysis was performed using the SPSS 21.0 software (IBM Corp., Armonk, NY, USA). Categorical variables were analyzed by Pearson’s chi-square test or Fisher’s exact test. Survival analyses were calculated by the Kaplan–Meier method with log-rank test and Cox regression method. The p-values <0.05 were considered statistically significant.
Results
CIP2A expression profile in NSCLC
CIP2A protein expression in NSCLC samples and adjacent normal tissues was analyzed by IHC. Positive CIP2A staining was predominantly localized within the cytoplasm and detected in 184 (88.3%) out of the 209 tumors and 4 (10.5%) out of 38 adjacent normal lung tissues. Based on IHC scoring, 128 (61.2%) tumor specimens exhibited low expression and 81 (38.8%) tumor specimens exhibited high CIP2A expression and all the adjacent normal lung tissue specimens were characterized with CIP2A low expression. Overall, CIP2A was highly detectable in tumor cells compared with adjacent normal lung tissues ( and ).
Figure 1 Immunohistochemical staining of CIP2A in NSCLC specimens.
Abbreviations: CIP2A, cancerous inhibitor of protein phosphatase 2A; NSCLC, non-small cell lung cancer.
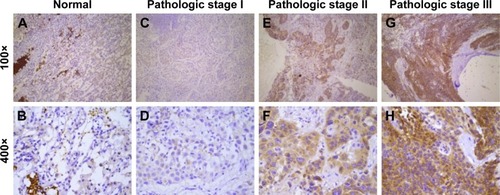
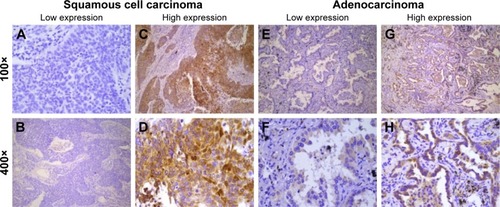
Figure 2 Immunohistochemical staining of CIP2A in squamous cell carcinoma and adenocarcinoma.
Abbreviation: CIP2A, cancerous inhibitor of protein phosphatase 2A.
Correlation between CIP2A expression and clinicopathological variables
Next, we assessed whether CIP2A expression correlated with clinicopathological parameters in NSCLC. demonstrates that CIP2A expression was significantly associated with high pathologic stage and N status. Patients diagnosed with a higher pathologic stage (p<0.001) and N stage (p=0.001) exhibited increased CIP2A expression. However, no significant differences were observed regarding diagnosis, age, gender, smoking, histological type, differentiation grade, and tumor status (all p>0.05).
Table 1 Clinicopathological variables of patient samples and expression of CIP2A in NSCLC
Clinical impact of CIP2A expression on survival
In survival analyses, the median time of overall survival (OS) was 50.0 months (95% CI, 35.83–64.17 months). The univariate analysis identified six variables that exhibit statistically significant associations with OS in all patients: CIP2A expression (p=0.005), pathologic stage (p=0.005), N stage (p=0.001), histological type (p=0.003), postoperative chemotherapy (p=0.037), and postoperative radiotherapy (p=0.006) (). Kaplan–Meier survival curves demonstrated that high CIP2A expression was a predictor of poor prognosis in NSCLC patients (p=0.005, ). The median OS of patients with high CIP2A expression and low CIP2A expression were 31.0 months (95% CI, 23.79–38.21 months) and 68.0 months (95% CI, 48.20–87.80 months), respectively.
Figure 3 Kaplan–Meier curves of OS based on CIP2A expression in NSCLC patients.
Abbreviations: CIP2A, cancerous inhibitor of protein phosphatase 2A; NSCLC, non-small cell lung cancer; OS, overall survival.
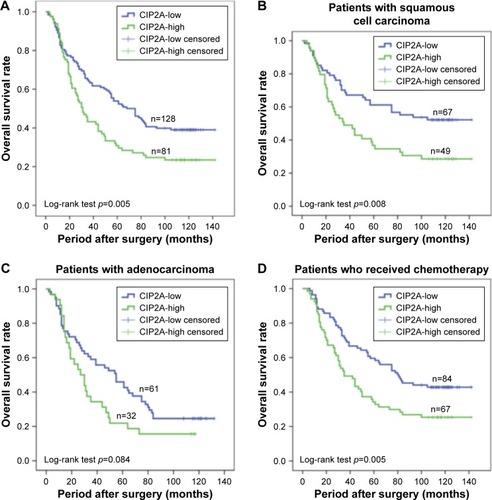
Table 2 Univariate and multivariate analyses of the prognostic factors in 209 patients with NSCLC
In addition, multivariate Cox proportional hazard analysis demonstrated that high CIP2A expression (p=0.007) was an independent prognostic factor along with T stage (p=0.001), N stage (p=0.003), histological type (p<0.001), and chemotherapy (p<0.001) (). Overall, high CIP2A expression is a poor and independent prognostic factor for postoperative stage I–III NSCLC patients.
Clinical impact of CIP2A expression on survival in subgroups
Furthermore, we analyzed the relationship between OS and CIP2A expression in different subgroups. For patients diagnosed with squamous cell carcinoma (n=116; 49 high CIP2A expression and 67 low CIP2A expression), Kaplan–Meier survival curves demonstrated that high CIP2A expression indicated poor prognosis in NSCLC patients with squamous cell carcinoma (p=0.008, ). A trend toward worse OS was noted in patients with high CIP2A expression and adenocarcinoma (n=93; 32 high CIP2A expression and 61 low CIP2A expression), but the trend did not achieve statistical significance (p=0.084, ).
The median OS of patients who received postoperative chemotherapy with high (n=67) and low (n=84) expressions of CIP2A were 35.0 months (95% CI, 21.97–48.03 months) and 80.0 months (95% CI, 54.85–105.15 months), respectively (p=0.005, ). Multivariate analysis revealed that CIP2A expression (p=0.009), histological type (p=0.001), and N stage (p=0.034) were independent prognostic factors for OS in patients who received postoperative chemotherapy (). Overall, high CIP2A expression was a poor and independent prognostic factor for stage I–III NSCLC patients who received postoperative chemotherapy. In the subgroup of patients who received postoperative radiotherapy, a log-rank test demonstrated that high CIP2A expression correlated with poor OS (p=0.165). The median OS values of patients who received postoperative radiotherapy with high (n=31) and low (n=27) CIP2A expressions were 21.0 months (95% CI, 10.87–31.13 months) and 36.0 months (95% CI, 24.13–47.87 months), respectively. However, the results were not statistically significant.
Table 3 Multivariate analysis of the prognostic factors using Cox regression test in 151 NSCLC patients who received postoperative chemotherapy
Discussion
In this study, we demonstrated that CIP2A protein expression in lung cancer tissues was dramatically increased compared with normal lung tissues. As determined by IHC, CIP2A expression was detected in 184 (88.3%) stage I–III NSCLC specimens and in 4 (10.5%) adjacent normal lung tissues. Junttila et al demonstrated that CIP2A mRNA levels were very low in most nonmalignant samples, except testis, bone marrow, brain, cerebellum, and prostate.Citation3 They also confirmed high CIP2A protein expression in head and neck squamous cell carcinoma (HNSCC) and negative staining of CIP2A in all nine nonmalignant tissues. This finding indicated that CIP2A expression was significantly increased in tumor tissues compared with adjacent normal tissues, which is consistent with other studies.Citation3,Citation13
Given that CIP2A was identified as a new oncoprotein that stabilizes the function of c-MYC and is overexpressed in HNSCC and colon cancer,Citation3 CIP2A overexpression has been detected in a variety of tumor types.Citation3,Citation8,Citation14–Citation16 In NSCLC, Ma et al reported that CIP2A is detected in 63.3% (by Western blotting) and 67.2% (at the mRNA level) of tumor specimens.Citation17 Dong et al reported that CIP2A is expressed in 82.7% of cases at the mRNA level and in 72.2% of cases at the protein level (detected by IHC).Citation12 In our research, CIP2A protein levels were detected in a higher proportion (88.3%) of the cohort compared with previous studies. Liu et al reported that CIP2A protein was detected in 90.3% of nasopharyngeal cancer samples analyzed.Citation16 In the review by Khanna et alCitation14 several presumed oncogenic signaling mechanisms that drive the expression of CIP2A were summarized, including MYC, the EGFR–MEK–ETS1 pathway, p53 inactivation, and E2F1 and ATF-2 (activating transcription factor 2) overexpression. Khanna recently identified CHK1 (checkpoint kinase 1), a DNA-damage kinase, as a stimulator of CIP2A transcription in cancer cells. CHK1 is constitutively phosphorylated by another DNA-damage kinase DNA-PK.Citation18 The above results may explain the widespread overexpression of CIP2A across multiple human cancer types due to the numerous factors driving CIP2A expression.
Furthermore, we found that high CIP2A expression is associated with late pathologic stage and metastatic lymph node status in NSCLC. Several studies also demonstrated similar phenomena in breast cancer,Citation15,Citation19 gastric cancer, tongue cancer, ovarian cancer, and renal cell cancer.Citation4,Citation8,Citation10,Citation20 In our study, the correlation between CIP2A protein expression and pathologic stage (p<0.001) in NSCLC is consistent with Xu et al’s research.Citation21 We demonstrated for the first time that CIP2A overexpression is associated with lymph node status in NSCLC, further supporting a role for CIP2A in promoting cancer aggressiveness. The possible explanations for the discrepancies in the outcomes between our research and the other studies that failed to identify the association between CIP2A expression and tumor aggressiveness in NSCLC could be the difference in sample size and the use of the median score as the cutoff point. Alternatively, the use of different antibodies for CIP2A sample staining could further explain the disparity. However, the mechanism of CIP2A in tumor migration is unclear. Ren et al reported that CIP2A depletion by siRNA resulted in both inhibition of c-MYC protein expression and migration in renal cells.Citation20 Based on the role of CIP2A in stabilizing and upregulating the MYC oncoprotein, it is likely that MYC is involved in CIP2A-stimulated tumor cell invasion. Sung et al demonstrated that IL-10-mediated tumor aggressiveness in vivo and in vitro occurs through increased CIP2A expression via the PI3K signaling pathway.Citation22
In addition, we confirmed that high CIP2A expression predicted poor prognosis in stage I–III NSCLC. CIP2A overexpression has been established as a prognostic biomarker in various cancers, including gastric cancer, bladder cancer, ovarian cancer, tongue cancer, colon cancer, chronic myelogenous leukemia, and NSCLC.Citation4,Citation8–Citation10,Citation12,Citation23 Dong et al found that patients diagnosed with CIP2A-positive NSCLC exhibited reduced survival compared with patients diagnosed with CIP2A-negative NSCLC.Citation12 This result is consistent with Xu et al’s and our study.Citation21
We also performed survival analyses on CIP2A expression in patients with different histological types and found that high CIP2A expression is a prognosis factor in squamous cell carcinoma (p=0.008, ). High expression of CIP2A might be a prognostic factor even in adenocarcinoma; however, the result did not achieve statistical significance (p=0.084, ). High CIP2A expression indicated poor prognosis in other squamous cell cancers, such as tongue cancer and nasopharyngeal cancer.Citation10,Citation16 However, a study of colorectal cancer by Bockelman et al demonstrated that CIP2A expression was not associated with patient prognosis.Citation24 Khanna et al demonstrated that the prognostic role of CIP2A was established only in particular subgroups of gastric cancer patients.Citation4 Of course, the change in sample size might account for the discrepancies between all patients and subgroups of adenocarcinoma patients. Differences in the role of CIP2A carcinogenesis in squamous cell cancer and adenocarcinoma should be validated by further investigations.
CIP2A is defined as an oncoprotein, based on its relatively low expression in normal lung tissues and its correlation with oncogenic pathways, and it serves as a promising molecular target for cancer therapy. In multivariate survival analysis, CIP2A overexpression predicts poor outcomes in the cohort undergoing postoperative chemotherapy. Previous studies indicate that high CIP2A expression is related to chemotherapy resistance.Citation6,Citation25 Laine et al demonstrated that CIP2A confers resistance to vinorelbine in HER2-negative breast cancers.Citation6 Moreover, Wei et al reported that CIP2A overexpression reduced NSCLC cell chemosensitivity to cisplatin by activating the Akt pathway.Citation26 Vinorelbine and platinum were both used broadly in NSCLC chemotherapy. Based on all the aforementioned results, a combination of chemotherapy and targeted therapy against CIP2A in NSCLC may be more effective. A recent study identified CIP2A as a target gene of Oct4 (a stem cell transcription factor) and provided evidence that these genes cooperate in radioresistance in HNSCC.Citation27 We also analyzed CIP2A protein expression and the prognosis in the subgroup of NSCLC patients who received postoperative radiotherapy, but we did not identify a correlation between CIP2A expression and OS. The limited number of patients who received postoperative radiotherapy in our study (n=58) might explain this finding. Additional data and further research are required to make a final conclusion.
Conclusion
In summary, our research demonstrates that high CIP2A expression is associated with cancer aggressiveness and poor prognosis in NSCLC, especially in squamous cell carcinoma and in patients who received postoperative chemotherapy. Our results indicated that CIP2A might be a promising predictive biomarker and therapeutic target in NSCLC. Further studies are required to understand the underlying molecular and cellular mechanisms of CIP2A in NSCLC.
Acknowledgments
We thank all the patients and their families who participated in this study. We are also grateful to Xia Bingshu and Zhang Siliang for their technical help and fruitful discussions.
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- GroomePABolejackVCrowleyJJThe IASLC lung cancer staging project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumoursJ Thorac Oncol20072869470517762335
- JunttilaMRPuustinenPNiemeläMCIP2A Inhibits PP2A in human malignanciesCell20071301516217632056
- KhannaABöckelmanCHemmesAMYC-dependent regulation and prognostic role of CIP2A in gastric cancerJ Natl Cancer Inst20091011179380519470954
- MathiasenDPEgebjergCAndersenSHIdentification of a c-Jun N-terminal kinase-2-dependent signal amplification cascade that regulates c-Myc levels in ras transformationOncogene201131339040121706057
- LaineASihtoHComeCSenescence sensitivity of breast cancer cells is defined by positive feedback loop between CIP2A and E2F1Cancer Discov20133218219723306062
- ChenK-FLiuC-YLinY-CCIP2A mediates effects of bortezomib on phospho-Akt and apoptosis in hepatocellular carcinoma cellsOncogene201029476257626620729919
- BockelmanCLassusHHemmesAPrognostic role of CIP2A expression in serous ovarian cancerBr J Cancer2011105798999521897396
- LucasCMHarrisRJGiannoudisACoplandMSlupskyJRClarkRECancerous inhibitor of PP2A (CIP2A) at diagnosis of chronic myeloid leukemia is a critical determinant of disease progressionBlood2011117246660666821490338
- BockelmanCHagstromJMakinenLKHigh CIP2A immunoreactivity is an independent prognostic indicator in early-stage tongue cancerBr J Cancer2011104121890189521610708
- DetterbeckFCBoffaDJTanoueLTThe new lung cancer staging systemChest2009136126027119584208
- DongQ-ZWangYDongX-JCIP2A is overexpressed in non-small cell lung cancer and correlates with poor prognosisAnn Surg Oncol201118385786520842459
- LiWGeZLiuCCIP2A is overexpressed in gastric cancer and its depletion leads to impaired clonogenicity, senescence, or differentiation of tumor cellsClin Cancer Res200814123722372818559589
- KhannaAPimandaJEWestermarckJCancerous inhibitor of protein phosphatase 2A, an emerging human oncoprotein and a potential cancer therapy targetCancer Res201373226548655324204027
- ComeCLaineAChanrionMCIP2A is associated with human breast cancer aggressivityClin Cancer Res200915165092510019671842
- LiuNHeQ-MChenJ-WOverexpression of CIP2A is an independent prognostic indicator in nasopharyngeal carcinoma and its depletion suppresses cell proliferation and tumor growthMol Cancer20141311924387052
- MaLWenZ-SLiuZOverexpression and small molecule-triggered downregulation of CIP2A in lung cancerPLoS One201165e2015921655278
- KhannaAKaukoOBockelmanCChk1 targeting reactivates PP2A tumor suppressor activity in cancer cellsCancer Res201373226757676924072747
- YuGLiuGDongJJinYClinical implications of CIP2A protein expression in breast cancerMed Oncol201330252423471718
- RenJLiWYanLExpression of CIP2A in renal cell carcinomas correlates with tumour invasion, metastasis and patients’ survivalBr J Cancer2011105121905191122075943
- XuPXuX-LHuangQZhangZ-HZhangY-BCIP2A with survivin protein expressions in human non-small-cell lung cancer correlates with prognosisMed Oncol20112931643164721874565
- SungW-WWangY-CLinP-LIL-10 promotes tumor aggressiveness via upregulation of CIP2A transcription in lung adenocarcinomaClin Cancer Res201319154092410323743567
- XueYWuGWangXCIP2A is a predictor of survival and a novel therapeutic target in bladder urothelial cell carcinomaMed Oncol20123011011
- BöckelmanCKoskensaloSHagströmJLundinMRistimäkiAHaglundCCIP2A overexpression is associated with c-Myc expression in colorectal cancerCancer Biol Ther201213528929522310977
- ChoiYAParkJSParkMYIncrease in CIP2A expression is associated with doxorubicin resistanceFEBS Lett2011585575576021241697
- WeiLQuWSunJKnockdown of cancerous inhibitor of protein phosphatase 2A may sensitize NSCLC cells to cisplatinCancer Gene Ther201421519419924874844
- VenteläSSittigEMannermaaLCIP2A is an Oct4 target gene involved in head and neck squamous cell cancer oncogenicity and radioresistanceOncotarget20156114415825474139
