Abstract
Objective
To investigate the effect of aristolochic acids (AA) exposure, including exposure duration and years since last exposure, on oncologic outcomes of patients with upper tract urothelial carcinoma (UTUC) after radical nephroureterectomy (RNU).
Methods
We retrospectively collected clinicopathologic and AA exposure variables for 942 UTUC patients treated with RNU between 1999 and 2014 in a high-volume center of China. AA exposure duration was categorized as (>3 vs ≤3 years) and time since last AA exposure to surgery as (>5 vs ≤5 years).
Results
A total of 856 patients (90.9%) had none or possible AA exposure and 86 patients (9.1%) had credible AA exposure history. Among the 86 patients, 57 (66.3%) had AA exposure for ≤3 years and 29 (33.7%) had exposure for >3 years. The median follow-up duration was 60 months. By multivariate analysis, AA exposure history was significantly associated with cancer specific survival (hazard ratio [HR]: 0.43, p=0.02), intravesical recurrence (IVR) (HR: 2.25, p<0.001) and contralateral UTUC recurrence (HR: 2.71, p=0.001). After adjusted for the effects of standard clinicopathologic characteristics, exposure duration was independent risk factor for subsequent IVR (exposure duration ≤3 years vs none/possible AA, HR: 1.87, p=0.009; exposure duration >3 years vs none/possible AA, HR: 3.07, p<0.001), but not for cancer-specific survival (p=0.06). Also, of those patients who had AA exposure, those having exposure within 5 years prior to RNU did not differ from patients having last exposure >5 years ago regarding cancer specific mortality (p=0.67) and IVR (p=0.54).
Conclusion
AA exposure was associated with worse cancer-specific survival, higher rate of IVR and contralateral UTUC recurrence of UTUC treated with RNU. The association between AA exposure and IVR seems to be time-dependent. Exposure cessation >5 years prior to RNU cannot mitigate the impact of AA on the UTUC prognosis.
Introduction
Upper tract urothelial carcinoma (UTUC) accounts for 5%–10% of all urothelial carcinomas, with an estimated annual incidence of ~2 in 100,000 individuals in western countries.Citation1,Citation2 Radical nephroureterectomy (RNU) with excision of the bladder cuff is the gold standard treatment for UTUC at present.Citation3 Aristolochic acids (AA), a compound found in the plants of genus Aristolochia, is a powerful nephrotoxin and carcinogen associated with chronic kidney disease and urothelial malignancy.Citation4 It was classified as a class I human carcinogen by the International Agency for Research on Cancer of the World Health Organization in 2002.Citation5
AA exposure, normally pertaining to the history of intake of traditional Chinese medicine among Chinese population, is a well-established risk factor for developing urothelial carcinoma.Citation6 Preclinical studies suggest that AA reacts with DNA to form covalent aristolactam (AL)-DNA adducts, which activate oncogenes or inactivate tumor suppressor genes throughout the urothelium.Citation7–Citation9 One population-based case-control study in Taiwan showed that lifetime AA consumption was associated with the risk of developing urinary tract cancer in a dose-dependent manner, even when controlling for the effects of age, gender and arsenic exposure.Citation10 In one retrospective study on UTUC patients in the Balkans, endemic nephropathy patients were found to be more likely to develop lower stage and lower grade UTUC, which suggests that AA-associated UTUC may have a lower potential for malignancy.Citation11 Until now, only one study focused on the prognosis of patients with AA-associated UTUC and indicated that it is associated with a higher risk of contralateral UTUC recurrence after RNU.Citation12
In this study, we aim to evaluate the association between AA exposure and tumor characteristics in a large cohort of Chinese UTUC patients. Furthermore, we aim to investigate the effect of duration of exposure and time since last exposure to AA on the oncologic outcomes of UTUC patients.
Materials and methods
Patient selection
Following the approval of the institutional review board of Peking University First Hospital (Beijing, China), clinicopathologic data were collected from 1,031 consecutive UTUC patients who had undergone RNU without any neoadjuvant therapy at Peking University First Hospital from 1999 to 2014. We excluded 65 patients who had missing information regarding AA exposure history and 24 patients who were diagnosed with metastatic disease. Finally, 942 patients were enrolled for evaluation. Signed written informed consent was obtained from all patients.
RNU was performed according to standardized techniques with the excision of bladder cuff. The decision to perform lymph node dissection was made by the surgeon based on clinical presentation and radiological image.
Exposure assessment
According to Pharmacopeia of The People’s Republic of China,Citation13 aside from Guan Mu Tong (Aristolochia manshuriensis), there are still 6 herbs containing AA: Guang Fangchi (Aristolochia fangchi), Qing Mu Xiang (Radix Aristolochiae), Ma Dou Ling (Fructus Aristolochiae), Tian Xian Teng (Caulis Aristolochiae), Xun Gu Feng (herba Aristolochiae mollissimae), and Zhu Sha Lian (Kaempfer Dutchmanspipe Root). These herbs were taken as single products or were components of mixed herbal formulas (eg, Guan Mu Tong in the Long Dan Xie Gan mixture). Moreover, according to the policy of China Food and Drug Administration, Chinese herbal products produced before June 2003 might include AA-containing herb Guan Mu Tong,Citation14 and those produced before September 2004 might include Guang Fangchi and Qing Mu Xiang.Citation15
Self-reported data on AA-containing herb intake were routinely assessed during the clinical visit of patients prior to RNU. Data on AA exposure included the duration of exposure and time since last AA exposure to surgery. In our study, patients with a history of long-term exposure (>3 months) of intermittent intake of regular doses of AA-containing TCM were assigned to the AA group; other patients were classified into the none/possible AA group. Patients in the AA group were further classified according to the exposure duration and time since last AA exposure to surgery.
Pathological evaluation
All surgical specimens were processed according to standard pathological procedures. Tumor stage was determined according to the 2002 Tumor Node Metastasis staging system of the Union for International Cancer Control, and tumor grading was performed according to the 1973 World Health Organization classification. Tumor location was defined as renal pelvic or ureteric. For tumors involving both sites, the location was assigned based on the largest lesion. Tumor multifocality was described as the simultaneous existence of 2 or more pathologically validated tumors from any location.
Follow-up
Follow-up was performed according to institutional protocols.Citation16 The follow-up interval was every 3 months during the first 2 years and once a year thereafter. Routine follow-up consisted of history, physical examination, urinalysis, serum creatinine measurement, ultrasonography, chest radiography, computed tomography (and/or magnetic resonance imaging), urine cytology (or urine fluorescence in situ hybridization) and cystoscopy. Survival time was calculated as the duration from surgery to death or last follow-up.
Statistical analysis
Statistical analyses were performed using SPSS software version 20.0 (SPSS, Chicago, IL, USA). Categorical data were analyzed using Pearson’s chi-squared test. Continuous data were analyzed using the Mann–Whitney U-test. The Kaplan–Meier survival method was used to estimate cancer-specific survival (CSS), intravesical recurrence (IVR)-free survival, and contralateral UTUC recurrence, and log-rank tests were used to test significant differences. Multivariate Cox regression analyses were performed to evaluate the prognostic significance of each variable with respect to CSS, IVR and contralateral UTUC recurrence. A 2-sided p<0.05 was considered statistically significant.
Results
Patient demographics
After the exclusion of 22 patients who were diagnosed with metastatic disease and 18 patients who had missing information regarding AA exposure history, a total of 942 patients were included for final analysis. The mean age of the entire cohort was 66.9 years (range 20–92 years), with 424 males (45.0%) and 518 females (55.0%). Overall, 86 patients (9.1%) who had a credible history of AA-containing herb intake due to the consumption of TCM were classified into the AA group, and the rest of the patients were classified into the none/possible AA group. In the AA group, the mean AA exposure duration was 74 months (3–481 months), and the mean time from last exposure was 62 months (0–240 months). Therefore, we subdivided the AA group based on exposure duration (>6 and ≤6 years) and time since last AA exposure to surgery (>5 and ≤5 years).
Association of AA exposure with clinicopathological characteristics
shows the patient clinicopathological characteristics and their association with AA exposure. Patients with AA exposure tended to be predominantly female (70.9%, p=0.002), had a history of infrequent smoking (p=0.03) and showed poorer renal function (p<0.001). Renal pelvic tumors were more commonly noted among the patients in the AA group (p=0.001). Lower tumor stage (p=0.04) and lower lymph node status (p=0.009) were observed in patients with a history of AA exposure.
Table 1 AA exposure by clinical and pathological characteristics in 942 patients treated with RNU for UTUC
Of the 86 patients in the AA group, 57 (66.3%) had <6 years of AA exposure, and 29 (33.7%) had >6 years of AA exposure. Patients with an exposure duration of >6 years were more likely to be female than patients in the none/possible AA group (p=0.002). Patients with exposure duration of >6 years were significantly more likely to have smaller tumors than patients in the none/possible AA and ≤6-year AA groups (p=0.03 and p=0.01, respectively). There was no association between exposure duration and pathological tumor characteristics.
Association of AA exposure with survival outcomes
At the median follow-up of 60 months (interquartile range 36–100 months), 209 patients died of UTUC. The mean actuarial estimates of 3- and 5-year CSS were 83% and 77% in the none/possible AA group and 92% and 90% in the AA group, respectively (, p<0.001). In the multivariate Cox regression analysis, gender (p=0.006), tumor grade (p=0.004), tumor stage (p<0.001) and AA exposure history (p=0.02) were identified as independent risk factors of CSS ().
Figure 1 Estimated Kaplan–Meier cancer-specific survival curves (A), intravesical recurrence-free survival curves (B) and contralateral UTUC recurrence-free survival curves (C) stratified by AA exposure history.
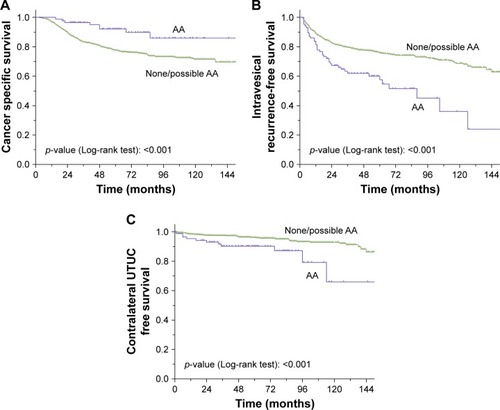
Table 2 Multivariate Cox regression analysis predicting time to intravesical recurrence and cancer-specific mortality in 942 patients treated with RNU for UTUC
During the follow-up period, 252 (26.8%) and 55 (5.8%) patients experienced IVR and contralateral UTUC recurrence, respectively. Twenty-three (2.4%) patients presented with both IVR and contralateral UTUC recurrence. The 3- and 5-year IVR-free survival rates were 61.9%±5%, and 57.7%±5% in the AA group and 79.6%±1% and 75.7%±2% in the none/possible AA group (, p<0.001), respectively. In addition, the 3-year contralateral UTUC-free survival rates were 90.2%±3% in the AA group and 97.5%±1% in the none/possible AA group (, p<0.001). In the multivariate Cox regression analysis, primary tumor size (p=0.004), primary tumor location (p=0.025), tumor grade (p=0.049), lymph node status (p=0.001) and AA exposure history (p<0.001) were identified as prognostic factors for IVR (), while primary tumor size (p=0.01) and exposure history (p=0.007) were found to be prognostic factors for contralateral UTUC recurrence.
We further subdivided the AA group based on exposure duration and time since last exposure. Patients with AA exposure duration >6 years had a higher rate of CSS compared with none/possible AA exposure (p=0.02). However, there was no difference between patients in AA exposure duration >6 years and exposure duration ≤6 years (p=0.22) and, interestingly, patients in AA exposure duration ≤6 years and none/possible AA exposure (, p=0.06). Exposure duration was associated with bladder tumor recurrence (, p=0.001). Patients with an AA exposure of >6 years had the lowest IVR-free survival rates (exposure duration >6 years vs none/possible AA exposure, p<0.001; exposure duration >6 years vs exposure duration ≤6 years, p=0.03). In multivariate Cox regression analyses adjusted for the effects of standard clinicopathologic characteristics, exposure duration was found to be an independent risk factor for subsequent IVR (p=0.001) but not for CSS (p=0.07). With regards to IVR, the odds increased with exposure duration (hazard ratio [HR] =1.699 for ≤6-year and HR =2.990 for >6-year AA group).
Figure 2 Estimated Kaplan–Meier cancer-specific survival curves (A), intravesical recurrence-free survival curves (B) stratified by the exposure duration, and estimated Kaplan–Meier cancer-specific survival curves (C), intravesical recurrence-free survival curves (D) stratified by time since last AA exposure.
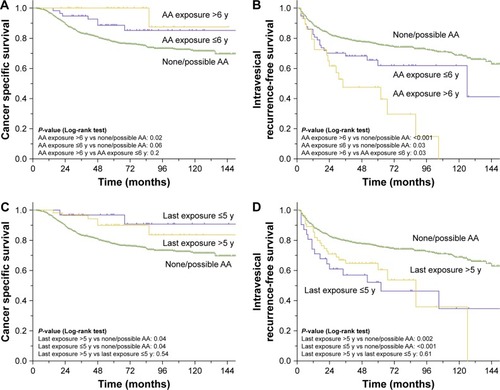
In terms of the time since last exposure, both patients with >5 years since exposure and patients with exposure within 5 years displayed lower cancer-specific mortality (, p=0.04 and p=0.04, respectively) and higher IVR (, p<0.001 and p=0.002, respectively) rates, while the cancer-specific mortality (p=0.64) or IVR (p=0.61) were comparable between the groups classified based on the time since last AA exposure.
Discussion
To the best of our knowledge, this is the first study investigating the association between AA exposure history and oncologic outcomes of UTUC in a large Chinese cohort, with further stratification analyses based on exposure duration and time since last exposure. In the present study, we found that there is a time-dependent relationship between AA exposure and the oncologic outcomes. Long-term AA exposure can significantly increase the risk of bladder tumor recurrence of UTUC. Meanwhile, the effect of AA exposure on UTUC seems to be persistent, as long-term cessation does not abrogate the influence of AA exposure on disease progression and outcomes.
Prior studies have demonstrated that patients with AA-associated UTUC are predominantly female, have poor renal function and present with lower grade of disease.Citation11,Citation12,Citation17 In one retrospective study in a Balkan patient cohort with endemic nephropathy, Cukuranovic et alCitation11 found that patients from endemic regions had lower stage UTUC during the period from 1957 to 1986. Consistent with prior findings, two-thirds of the patients in our AA group were female, and lower tumor stage and lower lymph node status were observed within that group. We have previously reported that unlike Western populations, Chinese UTUC populations are predominantly female.Citation18,Citation19 It seems that patients in the AA exposure might share some of the features of Chinese UTUC populations. Considering that the true incidence of AA exposure was likely underestimated due to the high rate of TCM intake and lack of awareness regarding AA-containing herbs in these patients, exposure to AA-containing herbs might be the key factor responsible for the distinctive features of Chinese UTUC populations.
The only other study to investigate the oncologic outcomes of AA-associated UTUC was conducted by Chen et al in a cohort of 152 patients.Citation12 They found that patients with AA-associated UTUC had significant shorter contralateral UTUC recurrence-free survival and demonstrated the predictive value of TP53 mutations in the prognosis of UTUC patients.Citation20 Within our cohort, we found that patients with AA exposure not only had higher contralateral UTUC recurrence rates but also had greater CSS and IVR rates. AA exposure history was an independent prognostic factor for CSS, IVR and contralateral UTUC recurrence. It is evident that the lower grade of malignancy in AA-associated UTUC may lead to the increased rate of CSS. However, the higher rates of bladder and contralateral upper tract tumor recurrence may be explained by the field cancerization hypothesis, suggesting that the exposure to carcinogenic toxins in AA-containing herbs might induce neoplasms along the entire urothelial field.Citation21,Citation22
A previous study has demonstrated that a longer duration of AA exposure might increase the risk of developing urothelial carcinoma.Citation23 However, there is a lack of evidence regarding the effect of AA exposure duration on UTUC progression and patient outcomes. In the present study, a strong time-dependent correlation between the duration of AA exposure and risk of bladder tumor recurrence was identified, whereby the risk continued to increase through 6 years of AA exposure, and individuals who took AA-containing herbs for 6 or more years had a 3-fold risk of bladder tumor recurrence compared with patients with none/possible AA exposure. Notably, during the follow-up period, bladder tumor recurrence was observed in ~90% of patients (19/22) with an AA exposure history of more than 10 years. Currently, the exact mechanisms underlying the effect of AA exposure on UTUC patient outcomes are still not fully understood. Continuous AA exposure may lead to cumulative molecular changes that result in a high risk of bladder tumor recurrence. Unfortunately, patients usually lack the knowledge regarding the association between AA and UTUC. Therefore, we should do our best to make individuals understand the harmful effects of AA exposure and, more importantly, prevent further exposure of these individuals to AA-containing herbs.
A recent molecular epidemiologic study reported that AL-DNA adducts and TP53 mutational signature can persist for years after AA exposure.Citation8 In a small cohort, Lemy et alCitation17 reported that despite the cessation of AA exposure for >68 months, 12 of 17 UTUC patients (71%) experienced IVR. In our study, we found that the cessation of exposure >5 years before RNU did not influence CSS and IVR compared with AA exposure within 5 years. Taken together, AA exposure seemed to have a long-term impact on oncologic outcomes. For this reason, irrespective of the time since last exposure, patients with a history of AA exposure should be closely examined by urologists due to their high risk of IVR after surgery.
There are several limitations of our study. First and foremost are the limitations inherent due to its single-center and retrospective design, which is subject to selection and recall bias. In the present study, the association between AA exposure and oncologic outcomes was evaluated based on the history of AA intake. Although it is a practical and commonly used method in clinical practice, inaccurate data on intake history may affect the accuracy of the study. Recently, AL-DNA adducts and TP53 mutational signature have been identified as robust molecular biomarkers for evaluating AA-associated UTUC.Citation8,Citation20 We are currently conducting another study to classify AA-associated UTUC using gene sequencing and trying to verify our findings at the gene level.
Furthermore, the data used for analyses included AA exposure history before RNU, and we had no data on AA exposure after surgery. Some patients with or without AA exposure before surgery could have taken AA-containing TCM after RNU, which would reduce the strength of this study. Additionally, we were unable to obtain information on the cumulative dose of AA due to the lack of prescription data for most patients, and therefore, we cannot evaluate the potential relationship between cumulative AA exposure and UTUC prognosis. Other limitations of this study include the limited sample size of the AA group and lack of data regarding other potential environmental factors, tumor architecture, and the presence of adjuvant therapy.
Conclusion
A credible history of exposure to AA-containing Chinese herbal products was associated with lower malignancies, lower cancer-specific mortality, higher risk of IVR and contralateral UTUC recurrence in patients. Moreover, AA exposure was associated with an increased risk of IVR in a time-dependent manner. However, the cessation of exposure >5 years prior to RNU did not mitigate the impact of AA exposure. Although our results need to be further verified at the gene level, these findings should help urologists to educate patients about the risk of AA exposure and encourage timely follow-up for UTUC patients with a history of AA exposure.
Disclosure
The authors report no conflicts of interest in this work.
References
- RoupretMBabjukMComperatEEuropean association of urology guidelines on upper urinary tract urothelial cell carcinoma: 2015 updateEur Urol201568586887926188393
- SiegelRLMillerKDJemalACancer statistics, 2016CA Cancer J Clin201666173026742998
- MargulisVShariatSFMatinSFOutcomes of radical nephroureterectomy: a series from the upper tract urothelial carcinoma collaborationCancer200911561224123319156917
- NortierJLMartinezMCSchmeiserHHUrothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi)N Engl J Med2000342231686169210841870
- IARC Working Group on the Evaluation of Carcinogenic Risks to HumansSome traditional herbal medicines, some mycotoxins, naphthalene and styreneIARC Monogr Eval Carcinog Risks Hum200282155612687954
- WuFWangTRisk assessment of upper tract urothelial carcinoma related to aristolochic acidCancer Epidemiol2013225812820
- DebelleFDVanherweghemJLNortierJLAristolochic acid nephropathy: a worldwide problemKidney Int200874215816918418355
- JelakovicBKaranovicSVukovic-LelaIAristolactam-DNA adducts are a biomarker of environmental exposure to aristolochic acidKidney Int201281655956722071594
- ChenCHDickmanKGMoriyaMAristolochic acid-associated urothelial cancer in TaiwanProc Natl Acad Sci U S A2012109218241824622493262
- LaiMNWangSMChenPCChenYYWangJDPopulation-based case-control study of Chinese herbal products containing aristolochic acid and urinary tract cancer riskJ Natl Cancer Inst2010102317918620026811
- CukuranovicRIgnjatovicIVisnjicMCharacteristics of upper urothelial carcinoma in an area of Balkan endemic nephropathy in south Serbia. A fifty-year retrospective studyTumori201096567467921302610
- ChenCHDickmanKGHuangCYAristolochic acid-induced upper tract urothelial carcinoma in Taiwan: clinical characteristics and outcomesInt J Cancer2013133114s20s
- National Pharmacopoeia CommitteeThe Pharmacopoeia of People’s Republic of China1st edBeijingChemical Industry Press2000
- China Food and Drug AdministrationNotice on the cancellation of the medicinal use of Guan Mu Tong, No. 121 [in Chinese] Available from: http://www.sda.gov.cn/WS01/CL0844/9977.htmlAccessed April 1, 2003
- China Food and Drug AdministrationNotice on Strengthening the Supervision and Management on the Use and Production of Guang Fangchi and Five Other Herbs, No. 379 [in Chinese] Available from: http://www.sda.gov.cn/WS01/CL0844/10242.htmlAccessed August 5, 2004
- ZhangLXiongGFangDContralateral upper tract urothelial carcinoma after nephroureterectomy: the predictive role of DNA methylationJ Exp Clin Cancer Res201534525613404
- LemyAWissingKMRoriveSLate onset of bladder urothelial carcinoma after kidney transplantation for end-stage aristolochic acid nephropathy: a case series with 15-year follow-upAm J Kidney Dis200851347147718295063
- LughezzaniGSunMPerrottePGender-related differences in patients with stage I to III upper tract urothelial carcinoma: results from the Surveillance, Epidemiology, and End Results databaseUrology201075232132719962727
- ChenXPXiongGYLiXSPredictive factors for worse pathological outcomes of upper tract urothelial carcinoma: experience from a nationwide high-volume centre in ChinaBJU Int2013112791792423905945
- ChenCHDickmanKGHuangCYRecurrence pattern and TP53 mutation in upper urinary tract urothelial carcinomaOncotarget2016729452254523627286260
- MiyakeHHaraIKamidonoSEtoHMultifocal transitional cell carcinoma of the bladder and upper urinary tract: molecular screening of clonal origin by characterizing CD44 alternative splicing patternsJ Urol200417231127112915311055
- FangDLiuPLiXCharacteristics and treatment outcomes of pan-urothelial cell carcinoma: a descriptive analysis of 45 patientsSci Rep201551801426657777
- LiWHYangLSuTSongYLiXMInfluence of taking aristolochic acid-containing Chinese drugs on occurrence of urinary transitional cell cancer in uremic uremic patients undergoing dialysisZhonghua Yi Xue Za Zhi2005853524872491 Chinese16321276
