Abstract
Objective
To evaluate the effect of CYP2D6 *10 polymorphism (C 100C>T, rs1065852) on clinical outcomes of female Asian breast cancer patients with tamoxifen adjuvant treatment.
Methods
Meta-analysis of retrospective cohort studies published in July 2017 was performed. Fifteen studies with 1,794 Asian breast cancer patients were included, using strict eligibility requirements. Associations of disease-free survival (DFS), overall survival (OS) and recurrence rate after tamoxifen intake, with CYP2D6 *10 polymorphism were investigated through random effects models.
Results
CYP2D6 *10 polymorphism was found to have effect on DFS and recurrence rate in various comparison models, but not on overall survival in the female Asian breast cancer patients.
Conclusion
In conclusion, our meta-analysis suggests that significant association of *10/*10 (TT) genotype with poorer DFS and recurrence exists in female Asian breast cancer patients with tamoxifen 20 mg/day adjuvant treatment. In the future, large and well-designed studies are required to illustrate the interactions of CYP2D6 genetic variants, including *10 polymorphism and tamoxifen response on female breast cancer patients.
Introduction
Tamoxifen has been widely used for >30 years as adjuvant treatment for women operated for estrogen receptor-positive breast cancer. It is reported that postoperative 5-year tamoxifen therapy effectively reduces the risk of breast cancer recurrence by 39%Citation1 and breast cancer mortality.Citation1
The clinical efficacy of tamoxifen adjuvant treatment varies among individuals. Tamoxifen itself has weak affinity for the estrogen receptor and undergoes considerable first-pass oxidative metabolism to more potent metabolites, such as 4-hydroxytamoxifen and 4-hydroxy-N-desmethyl tamoxifen (endoxifen), which is the main active tamoxifen, and has up to a 33-fold higher affinity for the estrogen receptor than tamoxifen itself.Citation2 The rate-limiting step in tamoxifen metabolism is mediated primarily by the highly polymorphic CYP2D6.Citation3
Several studies have reported that CYP2D6 genetic mutations leading to the absence of functional enzyme are related to the decrease in 4-hydroxytamoxifen and endoxifen levels.Citation4 However, it is still highly controversial on the clinical association of CYP2D6 genetic variants as predictors of tamoxifen efficacy at the standard dose of 20 mg/day. Nonfunctional allele variants of CYP2D6 gene were reported to be associated with increased risk of breast cancer relapse in female breast cancer patientsCitation4–Citation8 and increased risk of breast cancer occurrence in unaffected women.Citation9 Meanwhile, other studies do not support such an association.Citation10–Citation13 The causes of these conflicting results might be clarified by some newly emerged evidence,Citation14,Citation15 such as inclusion criteria for patients, source of deoxyribonucleic acid and genotyping method, coverage of genetic mutations and polymorphisms, as well as serological concentrations of endoxifen.
The classical CYP2D6-predicted phenotype is commonly divided into 4 levels of enzyme activity: poor metabolizers (PMs), intermediate metabolizers (IMs), extensive metabolizers and ultrarapid metabolizers.Citation16 To date, more than 100 allelic variants have been described for CYP2D6 gene.Citation17 CYP2D6 *10 is a major variant and produces an unstable CYP2D6 enzyme, with an allele frequency of ~50% in AsiansCitation18 but only 2% in Caucasians.Citation19 Mutation of CYP2D6 *10 generates a 188 C to T transition in exon 1, leading to a Proline 34 to Serine amino acid substitution and resulting in an unstable enzyme with lower activity and metabolic conversion rate.Citation20
It would be of great interest to investigate whether CYP2D6 *10 genotype affects the efficacy of tamoxifen in female Asian breast cancer patients. To test this hypothesis, we investigated whether the CYP2D6 *10 genotype is associated with the clinical outcome in a particular population subgroup of female Asian breast cancer patients by meta-analysis.
Methods
Literature and search strategy
The PubMed, MEDLINE, Cochrane, Embase, Wan Fang and Chinese National Knowledge Infrastructure database searches were conducted for all the pertinent trials. The search terms were: “CYP2D6” and “polymorphism” and “tamoxifen” and “breast cancer”. Manual scrutiny for eligible articles was conducted according to the bibliography of the original and review reports. The literature search was updated in July 2017.
Study selection
Retrieved studies were deemed eligible provided that they met all the following criteria: 1) studies on human beings; 2) in a cohort design or randomized controlled trial design; 3) investigated the effect of CYP2D6 *10 polymorphism with outcome of breast cancer patients taking tamoxifen; 4) in Asian population; 5) treated with 20 mg/day adjuvant tamoxifen for 2–5 years; 6) detailed outcomes could be obtained or calculated on the outcome of disease-free survival (DFS), overall survival (OS) or recurrence rate; 7) the number of women with breast cancer was >30; 8) and received more than 4 points in the Newcastle–Ottawa Scale (NOS), which was considered to be of high quality.
Data extraction
Three reviewers screened titles and abstracts for relevance to the topic independently (Junjun Lu, He Li and Peng Guo). All potential publications were independently evaluated in full text. The following information was sought for reaching consensus on all of the items: the first author’s name, year of publication, design of trial, comparison, number of patients, and the endpoints of clinical prognosis. Any disagreements were resolved by the fourth investigator (Zhu Weikang).
Statistical analysis
Five genic comparison models (TT vs CC, CT vs CC, TT vs CT/CC, TT/CT vs CC, and TT vs CT) for 3 endpoints (DFS, OS and reference) were analyzed. A χ2-test based on the Q test and I2 metric were used to quantify the heterogeneity.Citation21 When I2>50% and p<0.1, the between-study heterogeneity was considered to be significant and the random-effects model was used. Otherwise, for homogeneous studies, the fixed effects model was used. The mean hazard ratios (HRs) and 95% CIs were obtained from the original article by using survival analysis.Citation22,Citation23 If a publication did not provide HR values, Engauge Digitizer version 4.1 was used to obtain the HR values and 95% CIs.Citation23 The pooled HR was initially demonstrated using forest plots graphically. Begg’s Funnel plot and Egger’s test were conducted to evaluate the potential publication bias.Citation24 Sensitivity analysis was performed to assess the stability of our outcomes by omitting each study sequentially. Hardy–Weinberg equilibrium was examined with goodness-of-fit χ2-test. Two-sided p<0.05 was considered to be statistically significant. STATA version 12.0 (StataCorp LLC, College Station, TX, USA) was used for all the data analyses and graphs.
Results
Characteristics of studies included in this meta-analysis
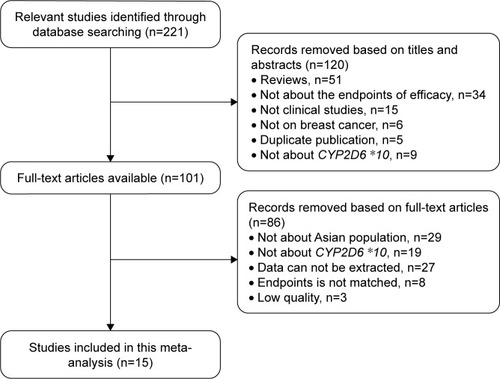
Initial search yielded a total of 221 potentially relevant citations. Finally, 15 studiesCitation7,Citation25–Citation38 were included in our meta-analysis to meet the inclusion criteria (). Publication years ranged from January 2008 to July 2017; the number of breast cancer patients varied from 39 to 296 (). As a result, data for our meta-analysis were available from 15 studies with a total of 1,794 Asian breast cancer patients. The eligible studies were assessed by the NOS. Each of the studies scored >4, which suggested that all the research was of high quality ().
Table 1 Characteristics and quality assessment of the included studies
Meta-analysis results
The pooled effect of CYP2D6 *10 genotype on the DFS of breast cancer patients treated with tamoxifen is shown in and . CYP2D6 *10 polymorphism (C 100C>T, rs1065852) significantly influences DFS in various comparison models (TT vs CC: HR =1.79, 95% CI =1.14–2.80, p=0.011; CT vs CC: HR =2.02, 95% CI =1.04–3.19, p=0.037; TT vs CC: HR =1.79, 95% CI =1.14–2.80, p=0.011; TT vs CT: HR =2.03, 95% CI =1.41–2.93, p<0.001; TT vs CT/CC: HR =2.19, 95% CI =1.07–4.50, p=0.033) except the dominant model (TT/CT vs CC: HR =1.80, 95% CI =0.81–4.00, p=0.147).
Figure 2 Forest plot to estimate the effect of CYP2D6 *10 polymorphism on the DFS of breast cancer patients taking tamoxifen.
Abbreviations: DFS, disease-free survival; HR, hazard ratio.
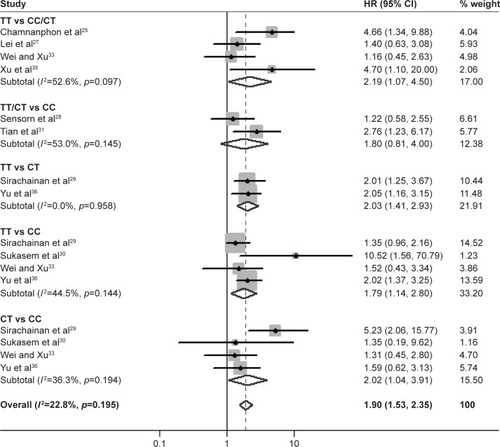
Table 2 Results of the effect of CYP2D6 *10 polymorphism on the DFS of breast cancer patients taking tamoxifen
Table 3 Results of the effect of CYP2D6 *10 polymorphism on the OS of female breast cancer patients taking tamoxifen
Figure 3 Forest plot to estimate the effect of CYP2D6 *10 polymorphism on the recurrence of breast cancer in patients taking tamoxifen in the comparison of (A) TT vs CC; (B) CT/TT vs CC; (C) TT vs CT/TT and (D) T vs C allele.
Abbreviations: CI, confidence interval; OR, odds ratio.
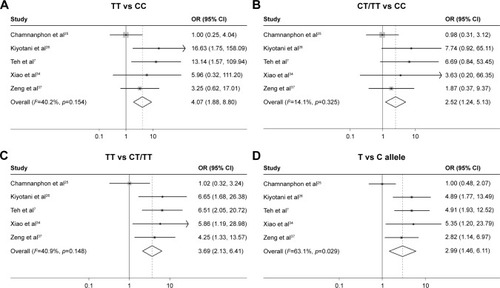
Table 4 Results of the effect of CYP2D6 *10 polymorphism on the recurrence of breast cancer in patients taking tamoxifen
Heterogeneity
There was no significant between-study heterogeneity for the endpoint of DFS in the comparison model of TT vs CC (I2=44.5%, p=0.114), CT vs CC (I2=36.3%, p=0.194) and TT vs CT (I2=0%, p=0.958). Meanwhile, for the dominant model and recessive model, heterogeneity was detected to be significant. Thus, random-effects estimates would be more appropriate for data analysis. Similarly, the random-effect model was conducted for the comparison of TT vs CC for OS and T allele vs C allele for recurrence ( and ).
Publication bias and sensitivity analysis
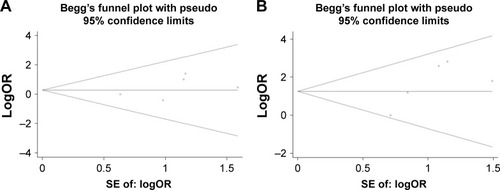
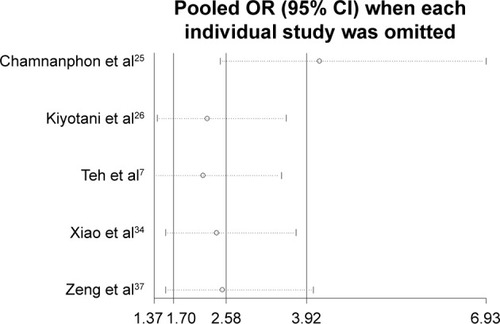
The publication bias of literature was assessed with both funnel plots and Egger’s test. As shown in – and , our results did not reveal any obvious asymmetry in the funnel plots. Moreover, the Egger’s test, which was used to provide statistical evidence of publication bias, suggested that no evidence of publication bias existed in the overall analysis (p>0.05 for all the comparisons). Sensitivity analyses showed that omitting each individual study from all the analyses did not affect the pooled odds ratios significantly, and no substantial change was detected, indicating that our results were statistically robust ().
Discussion
The CYP2D6 gene has earned special attention because an increasing number of studies have revealed that polymorphisms of the CYP2D6 gene were associated with the outcomes following tamoxifen treatment for breast cancer.Citation39 However, the results across studies have been equivocal.Citation40,Citation41 Previous meta-analyses were performed by Jung and Lim,Citation17 Province et alCitation42 and Johansson et al.Citation43 These meta-analyses were designed to evaluate the effect of CYP2D6 metabolizer types on outcome of breast cancer patients with tamoxifen treatment, for all the ethnicities. Different from these previous studies, our investigation aimed to assess the effect in a certain population and for a certain polymorphism, and was conducted in Asian breast cancer patients for CYP2D6 *10 polymorphism. Therefore, to our knowledge, it would be the first meta-analysis to report the unique association between CYP2D6 *10 and efficacy of tamoxifen in Asian breast cancer patients.
Ethnic difference in genetic background is one of the most important biological factors that might influence the patients’ clinical response and efficacy.Citation44 CYP2D6 *10 variant is commonly reported in Asian population, but not in other ethnicities.Citation19 The genetic discrepancies might be one of the reasons for the dispute on effect of CYP2D6 variants on tamoxifen response. Besides, the coverage of CYP2D6 mutations in the different studies might also play a role in the dispute.Citation20 Most research studies reported CYP2D6 metabolizer types with their effect of tamoxifen efficacy, however, CYP2D6-predicted phenotype classified system was too variable to reach a consensus. Therefore, it is of great clinical significance to conduct this meta-analysis to reveal the effect of CYP2D6 *10 polymorphism on the tamoxifen efficacy in Asian patients. It might provide a new insight on a range of outcomes, investigating a potential gene dose-response relationship, and an assessment of data from trials that investigated effect modification of tamoxifen by CYP2D6 genotype status.
In the process of study inclusion, we selected several indicators, including DFS, OS, and recurrence rate as the endpoints of our meta-analysis. These indicators were commonly reported by various studies and were suitable to be pooled. In fact, 2 of the studies includedCitation26,Citation45 reported the outcome of relapse-free survival (RFS). However, we failed to pool the results of RFS because these studies were with a different genetic comparison model. Similarly, data of disease-specific survival, time to progression, recurrence-free time were also reported by some studies but not available to be pooled.
Tamoxifen will still be commonly used in both premenopausal and postmenopausal women in the foreseeable future. Therefore, analysis of CYP2D6 *10 genotype may be useful in identifying patients who are more likely to benefit from tamoxifen treatment. In fact, men with breast cancer are also an important subgroup who are treated in adjuvant setting preferentially with tamoxifen. CYP2D6 *4 polymorphism was reported to be associated with a probability of recurrence in male breast cancer.Citation46 Therefore, analysis of CYP2D6 *10 variant in male breast cancer would also be of great clinical significance. Our meta-analysis indicated the relationship of CYP2D6 *10 polymorphism with tamoxifen response existed both on the endpoints of DFS and recurrence rate in female Asian breast cancer patients, with tamoxifen 20 mg/day adjuvant therapy. It is consistent in direction with the hypothesis that reduced tamoxifen metabolism is associated with poorer outcome.Citation5,Citation11,Citation30 In the genetic models of TT/CT vs CC in DFS and CT vs CC in recurrence, pooled results were negative for revealing the association of CYP2D6 *10 and tamoxifen efficacy. The former might be invalid, because this comparison contains only 2 studies, with significant heterogeneity (I2=53%, p=0.145), and is in contradiction to the other comparison, especially in TT vs CC and CT vs CC. For the CT vs CC comparison in recurrence, the results are without any between-study heterogeneity, and considered to be reliable. Our results suggests the decrease in enzyme activity caused by heterozygous mutation of CYP2D6 *10 does not translate into a significant poorer clinical outcome. Meanwhile, CYP2D6 *10 polymorphism was not associated with OS after pooled analysis, which was not consistent with the hypothesis. Given that OS is defined as the time from randomization to all-cause mortality, which is different from DFS, it might include in some death records that is irrelevant to cancer. Besides, for the endpoint of OS, 3 studies and 2 studies were pooled in different comparisons, which is relatively few and may have underpowered the results.
The heterogeneity between the studies was very low in our analysis. This suggested that the results from these studies were suitable to be pooled. The sensitivity analysis indicated that studies contributing to the heterogeneity did not significantly alter the pooled results. This suggested that our results were statistically robust. Taken together, our results might provide insights into the individualized therapy of breast cancer in Asian women. In patients with *10/*10 (TT) genotype, CYP2D6 enzyme activity and metabolic conversion rate was low. It is reported that increasing the regular tamoxifen dose could significantly increase endoxifen exposure in patients with CYP2D6 PMs or IMs.Citation47 Therefore, female breast cancer patients with *10 variant should increase their tamoxifen dose to >20 mg/day. However, the optimal dose of tamoxifen for *10 carriers needs to be explored.
Several limitations in our meta-analysis should be acknowledged. First, several studies with small sample size included in our analysis might be underpowered to detect the relationship. Second, our results are based on the unadjusted parameters. A more accurate analysis should be performed, in which the outcomes should be adjusted by some related parameters, including age, menopause status, and other important lifestyle factors.
In summary, our meta-analysis might be the first meta-analysis to estimate the association of CYP2D6 *10 polymorphism with the efficacy of tamoxifen treatment in female Asian breast cancer patients. Significant association of *10/*10 (TT) genotype with poorer DFS and recurrence was found in the Asian population. In the future, large and well-designed studies are required to illustrate the interactions of CYP2D6 genetic variants, including *10 polymorphism and tamoxifen response in female breast cancer patients.
Acknowledgments
The studies included obtained informed consent from all individual participants included in these studies.
Disclosure
The authors report no conflicts of interest in this work.
References
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG)DaviesCGodwinJRelevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trialsLancet2011378979377178421802721
- FabianCTilzerLSternsonLComparative binding affinities of tamoxifen, 4-hydroxytamoxifen, and desmethyltamoxifen for estrogen receptors isolated from human breast carcinoma: correlation with blood levels in patients with metastatic breast cancerBiopharm Drug Dispos1981243813907317574
- KleinDJThornCFDestaZFlockhartDAAltmanRBKleinTEPharmGKB summary: tamoxifen pathway, pharmacokineticsPharmacogenet Genomics2013231164364723962908
- KiyotaniKMushirodaTImamuraCKSignificant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patientsJ Clin Oncol20102881287129320124171
- SchrothWGoetzMPHamannUAssociation between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifenJAMA2009302131429143619809024
- MargolinSLindhJDThorenLCYP2D6 and adjuvant tamoxifen: possible differences of outcome in pre- and post-menopausal patientsPharmacogenomics201314661362223570465
- TehLKMohamedNISallehMZThe risk of recurrence in breast cancer patients treated with tamoxifen: polymorphisms of CYP2D6 and ABCB1AAPS J2012141525922183189
- GoetzMPSumanVJHoskinTLCYP2D6 metabolism and patient outcome in the Austrian Breast and Colorectal Cancer Study Group trial (ABCSG) 8Clin Cancer Res201319250050723213055
- BonanniBMacisDMaisonneuvePPolymorphism in the CYP2D6 tamoxifen-metabolizing gene influences clinical effect but not hot flashes: data from the Italian Tamoxifen TrialJ Clin Oncol200624223708370916877740
- GoetzMPSchaidDJWickerhamDLEvaluation of CYP2D6 and efficacy of tamoxifen and raloxifene in women treated for breast cancer chemoprevention: results from the NSABP P1 and P2 clinical trialsClin Cancer Res201117216944695121880792
- RaeJMDrurySHayesDFCYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patientsJ Natl Cancer Inst2012104645246022395643
- ReganMMLeyland-JonesBBouzykMBreast International Group (BIG) 1–98 Collaborative GroupCYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the breast international group 1–98 trialJ Natl Cancer Inst2012104644145122395644
- SestakIKealyRNikoloffMRelationships between CYP2D6 phenotype, breast cancer and hot flushes in women at high risk of breast cancer receiving prophylactic tamoxifen: results from the IBIS-I trialBr J Cancer2012107223023322735900
- KiyotaniKMushirodaTZembutsuHNakamuraYImportant and critical scientific aspects in pharmacogenomics analysis: lessons from controversial results of tamoxifen and CYP2D6 studiesJ Hum Genet201358632733323657426
- LienEASoilandHLundgrenSSerum concentrations of tamoxifen and its metabolites increase with age during steady-state treatmentBreast Cancer Res Treat2013141224324823996142
- ZangerUMRaimundoSEichelbaumMCytochrome P450 2D6: overview and update on pharmacology, genetics, biochemistryNaunyn Schmiedebergs Arch Pharmacol20043691233714618296
- JungJALimHSAssociation between CYP2D6 genotypes and the clinical outcomes of adjuvant tamoxifen for breast cancer: a meta-analysisPharmacogenomics2014151496024329190
- SakuyamaKSasakiTUjiieSFunctional characterization of 17 CYP2D6 allelic variants (CYP2D6.2, 10, 14A–B, 18, 27, 36, 39, 47–51, 53–55, and 57)Drug Metab Dispos200836122460246718784265
- GaedigkAGotschallRRForbesNSSimonSDKearnsGLLeederJSOptimization of cytochrome P4502D6 (CYP2D6) phenotype assignment using a genotyping algorithm based on allele frequency dataPharmacogenetics19999666968210634130
- JohanssonIOscarsonMYueQYBertilssonLSjöqvistFIngelman-SundbergMGenetic analysis of the Chinese cytochrome P4502D locus: characterization of variant CYP2D6 genes present in subjects with diminished capacity for debrisoquine hydroxylationMol Pharmacol19944634524597935325
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analyzesBMJ2003327741455756012958120
- BohningDMeta-analysis: a unifying meta-likelihood approach framing unobserved heterogeneity, study covariates, publication bias, and study qualityMethods Inf Med200544112713515778804
- TierneyJFStewartLAGhersiDBurdettSSydesMRPractical methods for incorporating summary time-to-event data into meta-analysisTrials200781617555582
- HarbordRMEggerMSterneJAA modified test for small-study effects in meta-analyzes of controlled trials with binary endpointsStat Med200625203443345716345038
- ChamnanphonMPechatananKSirachainanEAssociation of CYP2D6 and CYP2C19 polymorphisms and disease-free survival of Thai post-menopausal breast cancer patients who received adjuvant tamoxifenPharmgenomics Pers Med20136374823776391
- KiyotaniKMushirodaTSasaMImpact of CYP2D6*10 on recurrence-free survival in breast cancer patients receiving adjuvant tamoxifen therapyCancer Sci200899599599918294285
- LeiLWangXWuXDAssociation of CYP2D6*10 (c.100C>T) polymorphisms with clinical outcome of breast cancer after tamoxifen adjuvant endocrine therapy in Chinese populationAm J Transl Res2016883585359227648149
- SensornISukasemCSirachainanEABCB1 and ABCC2 and the risk of distant metastasis in Thai breast cancer patients treated with tamoxifenOnco Targets Ther201692121212927110128
- SirachainanEJaruhathaiSTrachuNCYP2D6 polymorphisms influence the efficacy of adjuvant tamoxifen in Thai breast cancer patientsPharmgenomics Pers Med2012514915323226070
- SukasemCSirachainanEChamnanphonMImpact of CYP2D6 polymorphisms on tamoxifen responses of women with breast cancer: a microarray-based study in ThailandAsian Pac J Cancer Prev20121394549455323167378
- TianCYangYLiHCorrelation between CYP2D6* 10 gene polymorphism and prognosis of breast cancer patients treated with tamoxifenChin J Breast Dis201376406411
- WangYYChengGHPuZCPolymorphisms of CYP2D6*10 and CYP2C19*2 and its effect on breast cancer patients with tamoxifen treatmentChin Clin Pharmacol Ther2015205552556
- WeiYXuZAssociation between CYP2D6* 10 genotype and survival of breast cancer patients receiving tamoxifen treatmentAnhui Med Pharm J2014185951954
- XiaoGLiDLiuLXClinical snalyisi of CYP2D6 gene polymorphism and Tamoxifen efficacy in post-menopause breast cancer patientsJ Ningxia Med Univ2014363315318
- XuYSunYYaoLAssociation between CYP2D6 *10 genotype and survival of breast cancer patients receiving tamoxifen treatmentAnn Oncol20081981423142918407954
- YuYYouWLiangDEfects of CYP2 D6 gene polymorphism on prognosis of ER-positive breast cancer patients treated with tamoxifen therapyShandong Med201656421720
- ZengYHuangKHuangWThe effect analysis of CYP2D6 gene polymorphism in the toremifene and tamoxifen treatment in patient with breast cancerPak J Pharm Sci2017303(Special)1095109828671087
- ZhangXPuZGeJShenJYuanXXieHAssociation of CYP2D6*10, OATP1B1 A388G, and OATP1B1 T521C polymorphisms and overall survival of breast cancer patients after tamoxifen therapyMed Sci Monit20152156356925701109
- NovilloARomero-LorcaAGaibarMRubioMFernández-SantanderATamoxifen metabolism in breast cancer treatment: taking the focus off the CYP2D6 genePharmacogenomics J201717210911127698402
- AreepiumNPanomvanaDRungwanonchaiPSathapornSVoravudNEffects of CYP2D6 and UGT2B7 polymorphisms on pharmacokinetics of tamoxifen in Thai breast cancer patientsBreast Cancer (Dove Med Press)20135737824648760
- Del ReMRofiECitiVShould CYP2D6 be genotyped when treating with tamoxifen?Pharmacogenomics201718875575628593824
- ProvinceMAGoetzMPBrauchHInternational Tamoxifen Pharmacogenomics ConsortiumCYP2D6 genotype and adjuvant tamoxifen: meta-analysis of heterogeneous study populationsClin Pharmacol Ther201495221622724060820
- JohanssonHGandiniSSerranoDA pooled analysis of CYP2D6 genotype in breast cancer prevention trials of low-dose tamoxifenBreast Cancer Res Treat201615919710827484880
- LimHSJu LeeHSeok LeeKSook LeeEJangIJRoJClinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancerJ Clin Oncol200725253837384517761971
- OkishiroMTaguchiTJin KimSShimazuKTamakiYNoguchiSGenetic polymorphisms of CYP2D6 10 and CYP2C19 2, 3 are not associated with prognosis, endometrial thickness, or bone mineral density in Japanese breast cancer patients treated with adjuvant tamoxifenCancer2009115595296119156902
- AbreuMHGomesMMenezesFCYP2D6*4 polymorphism: a new marker of response to hormonotherapy in male breast cancer?Breast201524448148625963137
- DezentjeVOvan SchaikRHVletter-BogaartzJMCYP2D6 genotype in relation to tamoxifen efficacy in a Dutch cohort of the tamoxifen exemestane adjuvant multinational (TEAM) trialBreast Cancer Res Treat2013140236337323842856