Abstract
Aim
The absence of reliable single serum biomarkers for oral premalignant lesion (OPL) and oral squamous cell carcinoma (OSCC) limits early diagnosis, monitoring of advanced disease, and prediction of prognosis.
Methods
In this prospective study, serum levels of matrix metalloproteinase (MMP)-2, MMP-3, MMP-13, insulin-like growth factor (IGF)-1, and IGF-binding protein (IGFBP)-3 were measured in 81 untreated OSCC patients, 49 healthy subjects, and 75 individuals with OPLs, and correlated with clinicopathological parameters.
Results
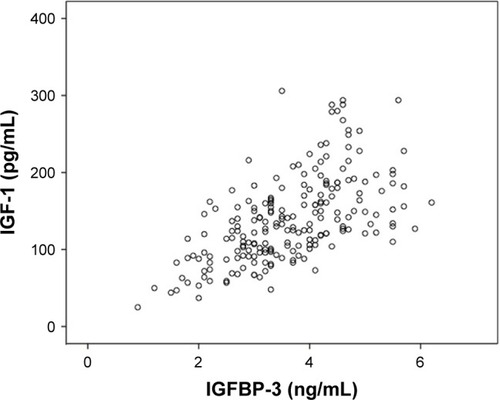
Serum levels of MMP-3 were significantly higher in OSCC patients compared to healthy subjects (p=0.004). Mean IGF-1 and IGFBP-3 levels in OSCC patients were significantly lower in healthy subjects (p=0.001 and p<0.001). OSCC patients with an IGF-1 serum value <130 ng/mL (median) showed a significantly lower survival rate compared to ≥130 ng/mL (p=0.049). Combined use of IGF-1 (<130 ng/mL) and IGFBP-3 (<3.1 μg/mL) resulted in a significantly lower 12-month cumulative survival compared to the complementary set (78.5% vs 93.8%; p=0.031). There was a significantly positive correlation between IGF-1 and IGFBP-3 serum values (rs =0.625, p<0.001).
Conclusion
This study shows that IGF-1 and IGFBP-3 have a vital role in the pathogenesis of OSCC and indicates for the first time that IGF-1 and IGFBP-3 in combination may be applied as potential tools for prognosis of OSCC.
Introduction
Oral squamous cell carcinoma (OSCC) poses a major health problem in the world and ~600,000 cases are diagnosed every year.Citation1 Although advancements in surgery techniques, radiation, and chemotherapy protocols have increased the local control of OSCC, the overall survival rates have not improved significantly over the last 3 decades and remain at a low level of ~50%–60%.Citation2 However, when oral cancer is diagnosed early in stages I–II, the survival of the patients rises from 60% to 80%.Citation3 Therefore, early detection is of vital importance for the patients. There is clear evidence that the annual rate of malignant transformation of oral premalignant lesions (OPLs) differs from 0% to 20% in 1–30 years.Citation4,Citation5 Tumorigenesis and the malignant transformation from OPL to OSCC is a highly complex regulated process and depends on multiple steps.Citation6 In this context, angiogenesis is described as one of the most important mechanisms and has been identified to play a crucial role in progression and invasiveness of most tumors.Citation7–Citation9 Angiogenesis depends on pro- and anti-angiogenic molecules, which are expressed by normal and malignant cells. Recently described pro- and anti-angiogenic factors were matrix metalloproteinases (MMPs) and insulin-like growth factors (IGFs).Citation10 Analysis of these molecules could be highly useful for the early detection as well as for the determination of the individual prognosis of (pre-)malignant lesions in the oral cavity.Citation11
MMPs belong to a zinc-dependent endopeptidase superfamilyCitation12,Citation13 that is distinct from other proteinases by the presence of a histidine sequence pattern.Citation14 The MMP family consists of 23 currently known zinc-containing enzymes and is classified into five groups based on their substrate and molecular structure: collagenases, gelatinases, stromelysins, membrane type MMPs and others.Citation13–Citation15 MMPs are multifunctional proteolytic enzymes, able to cleave extracellular matrix components like collagens and fibronectin, growth factors, cytokines, and cell receptors.Citation14 In cancer, these MMPs regulate cancer cell growth, differentiation, apoptosis, migration, invasion, and regulation of tumor angiogenesis and immune surveillance.Citation14,Citation16 With regard to gene expression and immunohistochemistry level, an involvement of MMP-1, MMP-2, MMP-9, and MMP-10 in OSCC oncogenesis was seen.Citation17–Citation20 As serum markers in OSCC, MMP-2, MMP-3, and MMP-13 were previously described.Citation13,Citation21–Citation23
The IGF system comprises a multifaceted network of ligands (IGF-1 and -2), their related receptors (IGFR-1 and -2), IGF-binding proteins (IGFBP1–6), and IGFBP proteases.Citation10,Citation24 Regarding carcinogenesis, the IGF family members regulate proliferation and inhibition of cell apoptosis, influence cell transformation through the synthesis of numerous regulatory proteins and control clonal expansion and metastases of cells.Citation25 IGF-binding proteins (IGFBPs) modulate the bioavailability of IGFs and regulate the interaction between IGFs and cell-surface receptors. In this context, IGFBP-3 seems to have the principal role in OSCC carcinogenesis.Citation26,Citation27
There are numerous studies indicating an important role of MMP-2, MMP-3, MMP-13, IGF-1, and IGFBP-3 in different malignancies. However, to the best of our knowledge, there is no report available on serum status of these proteins in patients with OPL and OSCC. Therefore, the aim of this study was to evaluate the serum level of these proteins in a prospective multi-biomarker approach and correlate it with clinicopathological parameters.
Materials and methods
Subjects and sample preparation
This prospective clinical study includes 81 patients with histopathologically proven primary OSCC, 75 noncancerous patients with OPLs, and 49 healthy individuals. The patients were treated in the Department of Oral and Maxillofacial Surgery (University Medical Centre Mainz, Germany). The OPL group consisted of noncancerous patients with oral leukoplakia and/or erythroplakia and lichen. All sera were collected from patients at the time of diagnosis and prior to surgery or radiation therapy. The control group was recruited from patients undergoing routine dentoalveolar surgery. Main inclusion criterion for the control group was the absence of any history of malignancy and infectious disease. All patients provided informed consent before participating in the study. The study was conducted in accordance with the Helsinki Declaration of 1975 as revised in 2000. The protocol was approved by the local ethics committee (Landesärztekammer Rheinland-Pfalz, Ethik-Komission, number 837.524.15 (10292)). The mean ages of OSCC patients, OPL patients, and healthy controls were 68±11.9 years (range 34–90), 61±12.8 years (range 24–81), and 58±14.0 years (range 26–93), respectively. Sites of tumor were buccal mucosa (14.8%), maxilla (11.1%), mandible (29.6%), tongue (14.8%), floor of mouth (16.8%), and palate (9.9%). According to T-Grade, 33.3% were T1, 24.7% were T2, 9.9% were T3, and 32.1% were T4 tumors; 58% of the cases had no lymph node metastasis (N0); 13.6% were graded N1; and 28.4 % were graded N2. One patient (1.2%) showed distant metastases (M1).
Assay
Sandwich enzyme-linked immunosorbent assay (ELISA) was used for the quantification of MMP-2, MMP-3, and MMP-13 levels in blood samples with the DuoSets ELISA for humans (R&D Systems, Minneapolis, MN, USA) following the manufacturer’s protocol. IGF-1 and IGFBP-3 were measured using a Chemiluminescence Immunoassay (IMMULITE® 1000; Siemens Healthineers, Erlangen, Germany). Venous blood was allowed to clot for 30 minutes at room temperature and was then centrifuged for 10 minutes at 3,000× g. Serum samples were stored at −80°C until further processing. All measurements were made in triplicates. Examiners were blinded to clinical results.
Statistical analysis
Statistical analysis was performed with univariate tests by using the software package of IBM SPSS Statistics 20.0 for Windows (IBM Corporation, Armonk, NY, USA). As serum levels of the investigated biomarkers were skewed to the right, logarithmic transformed values were analyzed. Survival curves were plotted by using the Kaplan–Meier method and compared using the univariate log rank test as described earlier.Citation28 For analysis of diagnostic accuracy, receiver operating characteristic (ROC) curves were used. For examination of the correlation between serum values, Spearman’s rank-order correlation was performed. P-values <0.05 are described as “statistically significant,” although no adjustment for multiple tests has been applied.
Results
Serum levels in association with clinicopathological data
The serum levels of MMP-2, MMP-3, MMP-13, IGF-1, and IGFBP-3 in association with clinicopathological data are described in . Serum levels of MMP-3 were significantly higher in OSCC patients compared to healthy controls (p=0.004). Serum levels of IGF-1 in OPL and OSCC patients were significantly lower than IGF-1 serum levels of healthy controls (p=0.018 and p=0.001). Serum IGFBP-3 levels were significantly lower in OSCC patients compared to healthy controls (p<0.001). Serum levels in relation to T-Grade (T1/T2 vs T3/T4) and lymph node involvement (N0 vs N+) showed the following p-values (T-Grade: MMP-2: p=0.002; MMP-3: p=0.953, MMP-13: p=0.234, IGF-1: p=0.732, and IGFBP-3: p=0.131; lymph node involvement: MMP-2: p=0.638; MMP-3: p=0.299, MMP-13: p=0.326, IGF-1: p=0.385, and IGFBP-3: p=0.103).
Table 1 Clinicopathological features and biomarker serum levels of the included subjects
Serum levels in association with tumor recurrence, metastasis, and prognosis
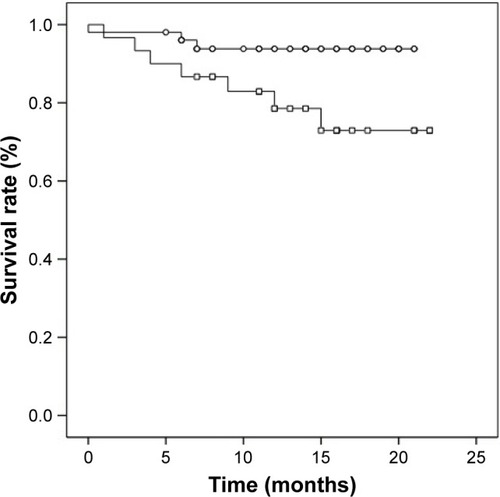
In our examination of the relationship between serum levels of MMP-2, MMP-3, MMP-13, IGF-1, and IGFBP-3 and tumor recurrence, metastasis, or prognosis, the serum concentrations were divided into two groups. We chose the median of all OSCC serum values as cutoff. None of the investigated biomarkers had a significant influence on tumor recurrence or metastasis. OSCC patients with an IGF-1 serum value <130 ng/mL showed a significant lower survival rate compared to OSCC patients with an IGF-1 serum value ≥ 130 ng/mL (p=0.043; ). Cumulative survival after 12 months was 80.8% for patients with IGF-1 serum value <130 ng/mL and 94.9% for patients with IGF-1 serum value ≥ 130 ng/mL. OSCC patients with IGFBP-3 serum value <3.1 μg/mL had a lower survival rate compared to OSCC patients with IGFBP-3 serum value ≥3.1 μg/mL, however not statistically significant (p=0.157; ). Twelve-months cumulative survival was 82.9% for patients with IGFBP-3 <3.1 μg/mL and 92.7% for patients with IGFBP-3 ≥ 3.1 μg/mL. Combined use of IGF-1 (<130 ng/mL) and IGFBP-3 (<3.1 μg/mL) resulted in a significant lower 12-month cumulative survival compared to the complementary set (78.5% vs 93.8%; p=0.031; ).
Figure 1 Survival rate of OSCC patients with IGF-1 serum values ≥ 130 ng/mL (circles, n=41) and with IGF-1 serum values <130 ng/mL (squares, n=40; p=0.043).
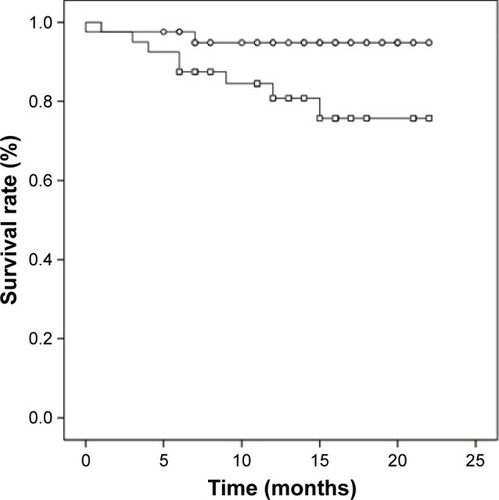
Figure 2 Survival rate of OSCC patients with IGFBP-3 serum values ≥ 3.1 μg/mL (circles, n=42) and with IGFBP-3 serum values <3.1 μg/mL (squares, n=39; p=0.149).
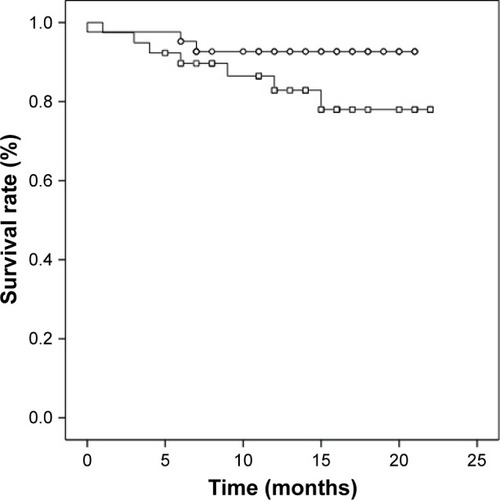
Figure 3 Survival rate of OSCC patients with IGF-1 (≥ 130 ng/mL) and IGFBP-3 (≥ 3.1 μg/mL) serum values (circles, n=51) and with IGF-1 (<130 ng/mL) and IGFBP-3 (<3.1 μg/mL) serum values (squares, n=30; p=0.031).
Determination of the diagnostic accuracy
To examine the performance of the investigated biomarkers for diagnosis of OSCC versus healthy controls and OPL patients, ROC curves were determined by plotting sensitivity versus specificity. The respective area under the ROC curve (AUC) as a measure of diagnostic performance in the discrimination of OSCC patients versus healthy controls is displayed in . For single biomarker analysis, only IGFBP-3 showed a good diagnostic accuracy with an AUC of 0.724. AUC for the discrimination of OSCC patients versus OPL patients is indicated in . None of the investigated biomarkers showed a good diagnostic accuracy in this context.
Table 2 Area under the curve for the discrimination of OSCC patients versus healthy controls
Table 3 Area under the curve for the discrimination of OSCC patients versus OPL patients
Correlation of serum values
A Spearman’s rank-order correlation was performed to determine the relationship between the serum values of the different biomarkers. There was a strong, positive correlation between IGF-1 and IGFBP-3 values, which was statistically significant (rs =0.625, p<0.001; ).
Discussion
Many previous studies have demonstrated altered expression of cytokines and/or growth factors in tumor tissues or sera of patients with oral carcinoma.Citation29,Citation30 However, relevance of this altered expression on tumor diagnosis and influence on tumor recurrence and patient prognosis remain unclear. The aim of this prospective study was to investigate serum levels of MMP-2, MMP-3, MMP-13, IGF-1, and IGFBP-3 in healthy controls, patients with OPL, and patients with OSCC and correlate them with clinicopathological parameters.
MMP-2 is a gelatinase A, and in contrast to most MMP family members, activation of this protein can occur on the cell membrane.Citation31 MMP-2 is correlated with poor prognosis in many cancer types and was associated with tumor invasion and lymph node metastasis by mediating the degradation of extracellular matrix components.Citation31,Citation32 In our patient collective, higher MMP-2 serum values were found in OSCC patients compared to healthy controls, however not statistically significant. Lotfi et al showed significantly increased serum levels of MMP-2 in 20 OSCC patients compared to 20 healthy subjects.Citation23 In a study by Patel et al, serum MMP-2 values failed to show any correlation with disease status during follow-up period.Citation33
MMP-3 is able to degrade a multitude of molecules including collagen type II, IV, IX, X, and XI, fibronectin, gelatins, elastin, proteoglycanase, E-cadherin, and osteopontin.Citation15,Citation34,Citation35 Previous studies described the role of MMP-3 in apoptosis induction, angiogenesis regulation, invasion, and metastasis in cancer.Citation15 In our study, serum levels of MMP-3 were significantly higher in OSCC patients compared to healthy controls. Furthermore, Agha-Hosseini et al showed increased MMP-3 serum concentrations from the reticular form of OPL to erosive form of OPL, and further to low stage of OSCC and advanced stage of OSCC.Citation22 Andisheh-Tadbir et alCitation36 indicated significantly elevated serum MMP-3 level in OSCC patients versus healthy controls. However, a correlation of serum MMP-3 concentration with clinicopathologic features such as tumor stage, tumor size, nodal status, and histological grade was not seen.
MMP-13 is known as collagenase-3, and increased expression of MMP-13 has been associated with tumor behavior and prognosis.Citation37 Studies indicated that MMP-13 is secreted directly by cancer tissue and indirectly promotes tumor angiogenesis.Citation12,Citation38 In the investigated subjects, higher MMP-13 values were seen in OPL and OSCC patients compared to healthy controls, however not statistically significant. Serum values in OPL and OSCC patients were comparable as described before.Citation21
IGF-1 and its principal binding protein IGFBP-3 are essentially involved in normal somatic growth by supporting cellular proliferation and inhibiting apoptosis.Citation26 In addition, there is clear evidence that the IGF signaling pathway has a key role in the onset and progress of various malignant tumors and can be targeted for therapeutic intervention.Citation10,Citation25 To the best of our knowledge, this is the first study that shows significant lower IGF-1 serum values in OPL patients compared to healthy controls. Regarding lower levels in OSCC patients compared to healthy controls, two further clinical studies supported our results.Citation26,Citation39 In addition, our results indicated significant lower IGFBP-3 values in OSCC patients compared to healthy controls. Brady et al showed similar findings.Citation26 These results implicate that IGF-1 and IGFBP-3 may help in the early detection of OSCC and could be a potential tool for screening. However, the diagnostic accuracy in ROC analysis was not sufficient. These low ROC values may be overcome in the future, if joint effects of multiple biomarkers with a cumulated ROC value are applied, as described before.Citation40 In this way, the ROC values and diagnostic accuracy may be increased. IGF-1 and IGFBP-3 have practical advantages over other cytokines for diagnostic use. For example, no specific collection of protocols are needed, as IGF-1 is not expressed by blood cells. A possible disadvantage is that IGF-1 is also expressed in several other types of cancer. Therefore, a multibiomarker approach may be reasonable. The shown lower IGF-1 and IGFBP-3 values in OSCC seem to contradict the accepted role of IGF-1 in tumorigenesis, as an increased risk of cancer is associated with higher circulating levels of IGF-1 and IGFBP-3 in different cancers.Citation26,Citation41,Citation42 This could be explained with the hypothesis that OSCC is regulated by systemic IGF-1 levels at the onset of growth. With tumor progress, an independent autocrine stimulation of the tumor occurs with low IGF-1 systemic concentrations.Citation42 Another explanation may be that there is actually no relationship between serum values in cancer patients and healthy controls and that current technology in the detection of the molecules is insufficiently reliable or sensitive to levels of the active molecules of interest.Citation26,Citation42 Our results describe both IGF-1 and IGFBP-3 as new marker of prognosis of OSCC. The combination of both the factors showed the highest accuracy predicting survival. Therefore, clinical implementation of these factors in preoperative diagnosis seems to be promising and may result in a more aggressive surgical and adjuvant therapeutic approach. In our study, IGF-1 and IGFBP-3 levels correlated strongly with each other. These results indicate that these molecules have a strong functional interaction. The pathophysiology behind these results merits further investigation.
Conclusion
In conclusion, high MMP-3 and low IGF-1 and IGFBP-3 serum values are associated with OSCC. IGF-1 and IGFBP-3 and especially the combination of both the factors represent a novel potential prognostic tool of important clinical interest for a better risk assessment of patient survival. The promising results of these biomarkers should now be confirmed in a clinical setting with larger patient numbers, and it should be determined whether the diagnostic and prognostic utility could be further improved by combining the investigated molecules with other serum markers.
Acknowledgments
The data from this study are part of the dissertation work submitted to Johannes Gutenberg University of Mainz as part of doctoral theses of Holger Schön and Christoph Gülle. Both authors collected and processed the samples.
Disclosure
The authors report no conflicts of interest in this work.
References
- ParkinDMBrayFFerlayJPisaniPGlobal cancer statistics, 2002CA Cancer J Clin20055527410815761078
- CantoMTDevesaSSOral cavity and pharynx cancer incidence rates in the United States, 1975–1998Oral Oncol200238661061712167440
- MehrotraRGuptaDKExciting new advances in oral cancer diagnosis: avenues to early detectionHead Neck Oncol201133321798030
- NapierSSSpeightPMNatural history of potentially malignant oral lesions and conditions: an overview of the literatureJ Oral Pathol Med200837111018154571
- GhallabNAShakerOGSerum and salivary levels of chemerin and MMP-9 in oral squamous cell carcinoma and oral premalignant lesionsClin Oral Invest2017213937947
- ThiemDGSchneiderSVenkatramanNTSemiquantifiable angiogenesis parameters in association with the malignant transformation of oral leukoplakiaJ Oral Pathol Med Epub20161230
- KammererPWAl-NawasBKalkanSAngiogenesis-related prognosis in patients with oral squamous cell carcinoma-role of the VEGF +936 C/T polymorphismJ Oral Pathol Med201544642943625213013
- KammererPWToyoshimaTEletrSSingle nucleotide polymorphisms of the vascular endothelial growth factor gene associated with incidence of oral squamous cell carcinomaJ Oral Pathol Med2010391078679220618614
- MohtashamNBabakoohiSSalehinejadJMast cell density and angiogenesis in oral dysplastic epithelium and low- and high-grade oral squamous cell carcinomaActa Odontol Scand201068530030420586672
- van BeijnumJRPietersWNowak-SliwinskaPGriffioenAWInsulin-like growth factor axis targeting in cancer and tumour angiogenesis – the missing linkBiol Rev Camb Philos Soc20179231755176827779364
- SchiegnitzEKammererPWRodeKSchornTBriegerJAl-NawasBGrowth differentiation factor 15 as a radiation-induced marker in oral carcinoma increasing radiation resistanceJ Oral Pathol Med2016451636925880686
- KudoYIizukaSYoshidaMMatrix metalloproteinase-13 (MMP-13) directly and indirectly promotes tumor angiogenesisJ Biol Chem201228746387163872822992737
- BakerEALeaperDJHayterJPDickensonAJThe matrix metalloproteinase system in oral squamous cell carcinomaBr J Oral Maxillofac Surg200644648248616338034
- CieplakPStronginAYMatrix metalloproteinases – from the cleavage data to the prediction tools and beyondBiochim Biophys Acta2017186411 Pt A1952196328347746
- TadbirAAPurshahidiSEbrahimiHSerum level of MMP-3 in patients with oral squamous cell carcinoma – lack of association with clinico-pathological featuresAsian Pac J Cancer Prev20121394545454823167377
- EgebladMWerbZNew functions for the matrix metalloproteinases in cancer progressionNat Rev Cancer20022316117411990853
- ShresthaBBajracharyaDByatnalAAKamathARadhakrishnanRMay high MMP-2 and TIMP-2 expressions increase or decrease the aggressivity of oral cancer?Pathol Oncol Res201723119720627853937
- LeeCHLiuSYLinMHUpregulation of matrix metalloproteinase-1 (MMP-1) expression in oral carcinomas of betel quid (BQ) users: roles of BQ ingredients in the acceleration of tumour cell motility through MMP-1Arch Oral Biol200853981081818571622
- MonteiroLSDelgadoMLRicardoSPrognostic significance of CD44v6, p63, podoplanin and MMP-9 in oral squamous cell carcinomasOral Dis201622430331226788715
- YenCYChenCHChangCHMatrix metalloproteinases (MMP) 1 and MMP10 but not MMP12 are potential oral cancer markersBiomarkers200914424424919489686
- Agha-HosseiniFMirzaii-DizgahISerum and saliva collagenase-3 (MMP-13) in patients with oral lichen planus and oral squamous cell carcinomaMed J Islam Repub Iran20152921826478876
- Agha-HosseiniFMirzaii-DizgahIMahboobiNShirazianSHarirchiISerum and saliva MMP-3 in patients with OLP and oral SCCJ Contemp Dent Pract201516210711125906800
- LotfiAMohammadiGTavassoliAMousaviagdasMChavoshiHSanieeLSerum levels of MMP9 and MMP2 in patients with oral squamous cell carcinomaAsian Pac J Cancer Prev20151641327133025743793
- FengXLinJXingSLiuWZhangGHigher IGFBP-1 to IGF-1 serum ratio predicts unfavourable survival in patients with nasopharyngeal carcinomaBMC Cancer20171719028143425
- KasprzakAKwasniewskiWAdamekAGozdzicka-JozefiakAInsulin-like growth factor (IGF) axis in cancerogenesisMutat Res201777278104
- BradyGO’ReganEMillerIOgungbowaleAKapasSCreanSJSerum levels of insulin-like growth factors (IGFs) and their binding proteins (IGFBPs), -1, -2, -3, in oral cancerInt J Oral Maxillofac Surg200736325926217113753
- MarimuthuAChavanSSatheGIdentification of head and neck squamous cell carcinoma biomarker candidates through proteomic analysis of cancer cell secretomeBiochim Biophys Acta20131834112308231623665456
- SchiegnitzEKammererPWKochFPKrugerMBerresMAl-NawasBGDF 15 as an anti-apoptotic, diagnostic and prognostic marker in oral squamous cell carcinomaOral Oncol201248760861422341306
- BlattSKrugerMZiebartTBiomarkers in diagnosis and therapy of oral squamous cell carcinoma: a review of the literatureJ Craniomaxillofac Surg201745572273028318929
- SchiegnitzEKammererPAl-NawasBQuality assessment of systematic reviews and meta-analyses on biomarkers in oral squamous cell carcinomaOral Health Prev Dent2017151132128232970
- ShenWXiHWeiBChenLThe prognostic role of matrix metalloproteinase 2 in gastric cancer: a systematic review with meta-analysisJ Cancer Res Clin Oncol201414061003100924610446
- WangGWangWZhouJYangXCorrelation between telomerase activity and matrix metalloproteinases 2 expression in gastric cancerCancer Biomark2013131212823736018
- PatelBPShahSVShuklaSNShahPMPatelPSClinical significance of MMP-2 and MMP-9 in patients with oral cancerHead Neck200729656457217252594
- AgnihotriRCrawfordHCHaroHMatrisianLMHavrdaMCLiawLOsteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin)J Biol Chem200127630282612826711375993
- AmalineiCCaruntuIDBalanRABiology of metalloproteinasesRom J Morphol Embryol200748432333418060181
- Andisheh-TadbirAKhademiBKamaliFFattahiMJMalekzadehMTaghvaMUpregulation of serum vascular endothelial growth factor and matrix metalloproteinase-3 in patients with oral squamous cell carcinomaTumour Biol20143565689569324577893
- NielsenBSRankFLopezJMCollagenase-3 expression in breast myofibroblasts as a molecular marker of transition of ductal carcinoma in situ lesions to invasive ductal carcinomasCancer Res200161197091710011585740
- LederleWHartensteinBMeidesAMMP13 as a stromal mediator in controlling persistent angiogenesis in skin carcinomaCarcinogenesis20103171175118419892798
- BhatavdekarJMPatelDDVoraHHBalarDBCirculating markers and growth factors as prognosticators in men with advanced tongue cancerTumour Biol199314155588493451
- El-mezayenHAMetwallyFMDarwishHA novel discriminant score based on tumor-associated trypsin inhibitor for accurate diagnosis of metastasis in patients with breast cancerTumour Biol20143532759276724222329
- VasanRSSullivanLMD’AgostinoRBSerum insulin-like growth factor I and risk for heart failure in elderly individuals without a previous myocardial infarction: the Framingham Heart StudyAnn Intern Med2003139864264814568852
- PollakMNSchernhammerESHankinsonSEInsulin-like growth factors and neoplasiaNat Rev Cancer20044750551815229476