Abstract
Background
Ovarian cancer is one of the most fatal gynecologic malignancies, with most patients diagnosed at the late stage due to insidious onset and lack of early onset specific symptoms. Previous studies have implied that isoliquiritigenin (ILQ) is a promising chemopreventive agent against oral cancer.
Aim
This study aimed to investigate effects of ILQ and elucidate the related mechanism.
Materials and methods
Ovarian cancer cell lines, SKOV3 and OVCAR3, were treated with various concentrations of ILQ to detect the dose-dependent effects of ILQ and select the suitable concentration. CCK8 assay and clone formation efficiency assays were used to detect viability and proliferation. The cell migration, invasion, and apoptosis were evaluated by wound healing assays, transwell, and flow cytometry assays. The expression of apoptosis-related proteins (Caspase-3, Caspase3-p17, Bcl-2, Bax, and Bim) and related-signaling pathway proteins were also detected by Western blot.
Results
It was observed that the treatment of ILQ inhibited the survival and proliferation of SKOV3 and OVCAR3 cells. ILQ treatment inhibited migration and invasion, and induced apoptosis in SKOV3 and OVCAR3 cells. Also, the ILQ treatment increased the Bax/Bcl-2 ratio in SKOV3 and OVCAR3 cells, suggesting that a mitochondrial apoptotic pathway was triggered. It was also observed that, after treated with ILQ, the phosphorylated form of Akt and mTOR decreased and the expression of GSK3β increased, while P70/S6K decreased. ILQ treatment also decreased the expression of Wnt3a and, therefore, caused the decrease of phosphorylated ERK. ILQ also suppressed the PI3K/Akt/mTOR pathway by reduced the expression level of p-Akt, p-mTOR, P70/S6K and Cyclin D1 in Ishikawa and ES-2 cells.
Conclusion
The data suggested that ILQ inhibited viability, proliferation, and invasion, and induced apoptosis of SKOV3 and OVCAR3 cells through the PI3K/Akt/mTOR pathway. Together, the data revealed that ILQ treatment may be used as a novel strategy for ovarian cancer therapy.
Introduction
Ovarian cancer is one of the most fatal gynecologic malignancies, with most patients diagnosed at the late stage due to insidious onset and lack of early specific symptoms.Citation1 About two thirds of diagnoses are in the advanced stage. The 5-year survival rate after standard therapy is only 30%.Citation2 Management of these patients with advanced ovarian cancer includes a primary cytoreductive surgery, followed by a combination chemotherapy.Citation3 Unfortunately, the majority of patients are chemo-resistant and suffer relapse later.Citation4 Moreover, endocrine therapy, such as blocking interactions between estrogen and classical nuclear estrogen receptors by tamoxifen, shows a low response rate in patients with chemoresistance.Citation5 New treatment strategies for ovarian cancer are needed.
SKOV3 and OVCAR3 are human ovarian cancer cell lines with epithelial-like morphology. These cells are resistant to tumor necrosis factors and many other cytotoxic drugs such as diphtheria toxin, cisplatin, and adriamycin. Herein, we used SKOV3 and OVCAR3 to investigate the proliferation, migration, and invasion of ovarian cancer in vitro.
Isoliquiritigenin (ILQ) mainly presents in roots of licorice and many other plants, foods, beverages, and tobaccos. ILQ is a flavonoid with a chalcone structure.Citation6,Citation7 ILQ has various potent biological and pharmacological activities, including anti-inflammatory,Citation8 antivirus,Citation9 antioxidative,Citation8 antiaging,Citation10 and antidiabetic activities.Citation11 ILQ prevents skin papilloma formation,Citation12 colonic tumorigenesis,Citation13 and lung metastasis of murine renal carcinoma cells.Citation14 Some in vitro studies reveal that ILQ has antiproliferation activities in skin,Citation15 pulmonary,Citation14 breast,Citation16 prostate,Citation17 and gastric cancer cells.Citation18 Previous studies have shown that ILQ induces human oral squamous cell carcinoma cell cycle G2/M phase arrest, apoptosis, and DNA damage,Citation19 and ILQ could inhibit the growth of prostate cancer cells.Citation17 Implying that ILQ is a promising chemopreventive agent against oral cancer.
In the present study, we aimed to investigate the effects of ILQ on human ovarian cancer cell lines, SKOV3 and OVCAR3, and elucidate the underlying mechanisms. Herein, we reported that ILQ inhibited viability and induced apoptosis of SKOV3 and OVCAR3 cells through the PI3K/Akt/ mTOR pathway. Our data suggest that ILQ treatment may be a novel strategy for ovarian cancer therapy.
Materials and methods
Agents
RPMI-1640 medium was purchased from HyClone Company (Cat#SH30809.01; Logan, UT, USA). Fetal goat serum was purchased from Gibco (Cat#10099-133; Thermo Fisher Scientific, Waltham, MA, USA). Antibiotics, 0.25% trypsin, Cell Counting Kit-8 (CCK-8), and dimethyl sulfoxide (DMSO) were purchased from Beijing Solarbio Science & Technology Co., Ltd. (Cat#T1302, CA1210, D8371; Beijing, China). Annexin V-FITC/PI apoptosis kit was ordered from 4A Biotech Company (Cat#FXP018-100, Beijing, China). Primary antibodies, including anti-Caspas3-P17 (Cat#ab90437), anti-Bim (Cat#ab7888), anti-Bcl-2 (Cat#ab32124), anti-Bax (Cat#ab32503), anti-p-AKT (Cat#ab81283), anti-AKT (Cat#ab32505), anti-mTOR (Cat#ab2732), anti-p-mTOR (Cat#ab131538), anti-p-ERK (Cat#ab214362), anti-GSK3β (Cat#ab75745), anti-P70/S6K (Cat#ab32529), anti-Cyclin D1 (Cat#ab40754), and anti-WNT3a (Cat#ab28472), were purchased from Abcam (Cambridge, UK). Anti-GAPDH (Cat#60004-1-lg) and all secondary antibodies were ordered from PTG Company (Rosemont, IL, USA). ILQ was purchased from MCE Company (Monmouth Junction, NJ, USA). The cell culture stuffs were ordered from Eppendorf (Hamburg, Germany).
Cell culture
Human ovarian cancer cell lines SKOV3 and OVCAR3 were obtained from the cell bank of the Chinese Academy of Sciences (Shanghai, China). Human endometrial cancer cell line Ishikawa and human ovarian cancer clear cell line ES-2 were obtained from ATCC (American Type Culture Collection, Rockville, MD, USA). Cells were incubated in RPMI-1640 medium containing 10% fetal bovine serum (FBS), 100 U/mL Penicillin, and 0.1 mg/mL streptomycin at 37°C with 5% CO2.
Western blot
Cells were cultured on a 6-well plate to 95% confluent. Twenty-four hours after treatment with 30 μM ILQ or 0.1% DMSO as negative control (NC), cells were changed to serum-free medium for 24 h starvation. Cells were lysed with ice-cold radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 1.0% NP-40, 0.1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCl, pH 8.0, and protease inhibitors), and the protein concentration was detected by bicinchoninic acid (BCA) method. Protein samples were denatured by heating at 95°C for 5 min, and about 20 μg were loaded to each lane on 10% SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) gel. After transferring proteins to polyvinylidene difluoride (PVDF) membrane, it was blocked with 5% non-fat milk for 1 h at room temperature, and incubated with primary antibodies in blocking solution at 4°C overnight. We then washed the membrane with tris buffered saline with Tween 20 (TBST) three times and incubated the membrane with secondary antibody in blocking buffer at room temperature for 1 h. After washing, enhanced chemiluminescence (ECL) substrate was applied to the membrane with ECL substrate for signal development. Images were acquired by using darkroom development techniques for chemiluminescence.
Cell viability and proliferation assays
Normal cultured cells were trypsinized and counted to make suspension. Approximately 1,000 cells were seeded to each well of 96-well plates. Cells were then treated with 30 μM ILQ, and 0.1% DMSO was used as a control. Cell vitality was detected every 24 h by adding 10 μL of CCK-8 reagent. After incubation at 37°C for 90 min, the optical density (OD) value of excitation light was detected by using enzyme standard instrument with 450 nm. A proliferation curve was drawn using the OD values.
Clone formation efficiency assays
Normal cultured cells were trypsinized to produce a single-cell suspension and count the cells. About 500 cells were seeded to each 10 cm dish containing 5 mL medium. Cells were treated with 30 μM ILQ, and 0.1% DMSO was used as a negative control. The dishes were placed in an incubator at 37°C with 5% CO2 and were left there until cells had formed sufficiently large clones. We removed the medium above the cells, and rinsed them carefully with phosphate-buffered saline (PBS). Cells were fixed with 5 mL 4% paraformaldehyde for 30 min, followed by staining with 0.1% crystal violet for 30 min. The crystal violet was removed carefully and rinsed with tap water. The dishes were left with colonies to air-dry at room temperature. The number of colonies was counted and compared to the colony size and number of the control group.
Wound healing assays
Wound healing assays were carried out to estimate the rates of migration and proliferation of SKOV3 and OVCAR3 cells under the isoliquiritigenin treatment. Cells were plated on a 6-well plate at 5×105 per well and incubated at 37°C overnight to get a 100% confluent cell monolayer. ILQ was added to cells at a final concentration of 30 μM, and DMSO was used as control. Twenty-four hours after ILQ treatment, a line was scratched with a tip to destroy a small area of the cell layer. After washing with PBS, cells were incubated in serum-free medium. The open gaps were then detected by microscope over times of 0, 24, or 48 h, and the size of gaps were measured with ImageJ at six to eight different points.
Cell invasion assays
Coating buffer (containing 0.01 M Tris pH 8.0, 0.7% NaCl, and filtered by 0.2 μm sterile filter unit) was prepared before the experiment. Any pipets, syringes, or containers that will come into contact with Matrigel must be chilled prior to use. The Matrigel Matrix aliquot was thawed on ice at 4°C overnight. Matrigel was diluted with serum-free 1640 medium at 1:6, and 100 μL were applied to each permeable support well of 24-well plates, incubated at 37°C for 4 h. The remaining coating buffer was carefully removed from the permeable support membrane without disturbing the layer of Matrigel on the membrane. Then 100 μL and 600 μL serum-free 1640 medium were added to the inside and outside of the support well, respectively, and incubated at 37°C for 30 min. The coated invasion chambers were now ready to use. SKOV3 and OVCAR3 cells treated by ILQ for 24 h were trypsinized and resuspended in serum-free medium; 100 μL cell suspension containing 1×104 cells was added to each 24 well invasion chamber and 600 μL medium containing 10% FBS was added to the outside of the chamber. Twenty-four hours after incubation at 37°C, non-invading cells were removed by using a cotton swab, and both sides of the chambers were washed with 1×PBS twice. Cells were fixed with 4% paraformaldehyde at room temperature for 15 min and stained with 0.1% crystal violet for 5 min. After washing with 1×PBS, the filters were cut off and mounted on slides. The invaded cells were finally observed, imaged, and counted under the microscope.
Cell apoptosis analysis with flow cytometer
Cells were treated with 30 μM ILQ for 48 h and 0.1% DMSO was used as a control. After 24 more hours incubation in serum-free medium for starvation, cells were trypsinized with EDTA-free trypsin. After washing with PBS, cells were resuspended in 1× binding buffer (10 mM HEPES/NaOH [pH 7.4], 140 mM NaCl, 2.5 mM CaCl2). Cell intensity was adjusted to 3–5×105 cells/mL; 5 μL of Annexin V-FITC was added to 100 μL cell suspension, incubated at room temperature in dark, and then added 10 μL of 20 μg/mL PI for double staining for another 2 min. Results were analyzed with a flow cytometer (BD FACSCanto II; BD Biosciences, San Jose, CA, USA). Viable cells were negative for both PI and Annexin V, while apoptotic cells were positive for Annexin V and negative for PI. Late apoptotic dead cells showed both Annexin V and PI positivity. The apoptotic rate was calculated by BD FACSDiva software.
Statistical analysis
Results were expressed as mean ± standard deviation (SD) or mean ± standard error of mean (SEM), as indicated in the figure legends. The data were representative of three independent experiments performed in triplicate. Statistical analysis of the data was performed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). The Student’s t-test was used to determine the significance for all pairwise comparisons of interest. Differences were considered statistically significant when values of P<0.05.
Results
Isoliquiritigenin inhibits viability and proliferation of ovarian cancer cells
We first detected the dose-dependent effects of ILQ on ovarian cancer cell lines. Our results showed that ILQ had no effect on survival of SKOV3 cells at a concentration lower than 20 μM. At a concentration of 30 μM or higher, ILQ had a significant inhibitory effect on the viability of SKOV3 cells compared with the NC cells (). The similar results were also shown in OVCAR3 cells (). Since 30 μM of ILQ had a clear effect, and the higher concentration may cause toxic effects to cells, we used 30 μM concentrations in the rest of the experiments. We then detected the effects of ILQ on the viability of SKOV3 and OVCAR3 cells through the CCK-8 assay. A significant decrease of the OD value of excitation light was found in both SKOV3 and OVCAR3 cells, which were treated with 30 μM ILQ for 24 h, compared with the NC cells (P<0.05, ). The inhibitory effect of ILQ on the proliferation of SKOV3 and OVCAR3 cells was still significant with the treatment at 48 h or 72 h (P<0.05, ).
Figure 1 Isoliquiritigenin inhibits viability and proliferation of SKOV3 and OVCAR3 cells. (A) The dose-dependent effect of ILQ on ovarian cancer cells, SKOV3 and OVCAR3. ILQ has an inhibitory effect on the survival of SKOV3 and OVCAR3 cells at 20 μM. The time course effect of ILQ on the viability of SKOV3 (B) and OVCAR3 (C) cells. (D) ILQ decreases the clone formation efficiency of SKOV3 and OVCAR3 cells. *P<0.05 compared with NC; **P<0.01 compared with NC; ***P<0.001 compared with NC.
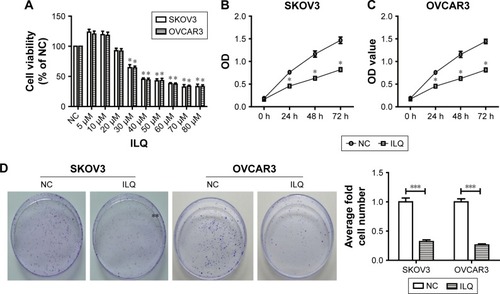
The clone formation efficiency of the ILQ treated SKOV3 and OVCAR3 cells was then detected. The cell clone formation rate of cells refers to the cell survival rate, which is used to evaluate the ability of the cells to live and form the number of clones. Not all the cells proliferated and formed clones; only those who form the clones stick to the walls and have the cells that proliferate. The clone formation rate reflects both the dependence and proliferation of cell populations. Two weeks after incubation with ILQ, we fixed and stained the colonies of cells. Compared with control cells, the number and size of colonies on ILQ treated dishes were numerically much less (<40%) and smaller both in SKOV3 and OVCAR3 cells (P<0.001, ).
These results together indicated that the treatment of ILQ significantly inhibited ovarian cancer cell survival and proliferation, thereby reflecting a potential pathologic role of this reagent in ovarian cancer.
Isoliquiritigenin decreases cell migration and invasion of ovarian cancer in vitro
Transwell and wound healing assays were used to determine SKVO3 and OVCAR3 cell migration and proliferation. The wound healing assay is a simple method to study directional cell migration in vitro, which mimics cell migration during wound healing in vivo. We treated the 100% confluent cell monolayer with 30 μM ILQ for 24 h, and scratched a line with a tip to destroy a small area of the cell layer. The open gap was then inspected microscopically over time as the cells moved in and filled the damaged area. The representative photos showed that the gap distances of ILQ treated SKVO3 and OVCAR3 cells were both bigger than the control cells at 24 and 48 h time points (P<0.01, ). The gap of the control cell was almost closed at 48 h, while the gap size of the ILQ treated SKVO3 cell was triple the control. Similar results were also shown in OVCAR3 cells (). The open gaps were then measured over times of 0, 24, or 48 h with ImageJ at six to eight different points. The time course curve showed that the migration rate of non-treated cells was much bigger than ILQ treated cells (P<0.01, ). This result suggested that the ILQ inhibited the migration of ovarian cancer cells.
Figure 2 Isoliquiritigen inhibits cell migration and invasion in SKOV3 and OVCAR3 cells. (A) Wound healing assay. The 100% confluent cell monolayer was treated with 30 μM ILQ for 24 h, and scratched a line with a tip to destroy a small area of the cell layer. Images were obtained at 0, 24, and 48 h after scratching. (B) Cell invasion in the ILQ-treated group decreased significantly compared with the control group (×100 magnification). **P<0.01 compared with NC; ***P<0.001 compared with NC.
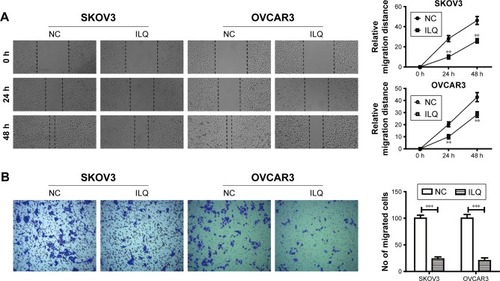
The invasion assay provides an in vitro system to study cell invasion of malignant and normal cells. Invasion chambers coated with Matrigel provide cells with the conditions that allow assessment of their invasive capacity in vitro. Matrigel serves as a reconstituted basement membrane in vitro, occluding the pores of the membrane and blocking non-invasive cells from migrating through the membrane, while invasive cells secrete proteases to degrade Matrigel and enable invasion through the membrane pores. shows that, compared with the control group, cell invasion in the ILQ-treated groups decreased significantly in both SKVO3 and OVCAR3 cells (P<0.001).
The results from both wound healing and the transwell assays were consistent, and ILQ treatment significantly inhibited migration and invasion of ovarian cancer cells.
Isoliquiritigen induces apoptosis in ovarian cancer cells and regulates expression of apoptosis-related proteins
Next, we investigated whether ILQ impaired apoptosis in SKOV3 and OVCAR3 cells. The apoptotic rate of ILQ treated cells was analyzed by flow cytometry. Cells were treated with 30 μM ILQ for 48 h and then double stained by Annexin V and PI. Early and late apoptotic cells were identified by Annexin V-positive and PI-negative staining (left upper and left lower). The ILQ treatment significantly increased the apoptotic rate of SKOV (79.69%) and OVCAR3 (59.20%) cells compared with control cells (42.89%, 35.90%, respectively) (P<0.05, ).
Figure 3 Isoliquiritigenin induces apoptosis in SKOV3 and OVCAR3 cells and regulates expression of apoptosis-related proteins. (A) Flow cytometry staining revealed a significant shift in annexin V-FITC-positive cells after ILQ treatment in both SKOV3 and OVCAR3 cells. (B) ILQ treatment affects the expression level of apoptosis-related proteins. *P<0.05 compared with negative control (NC); **P<0.01 compared with NC; ***P<0.001 compared with NC.
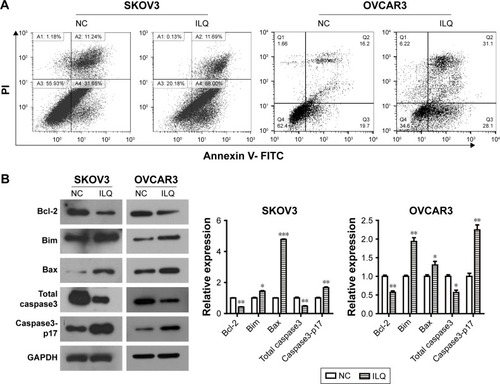
To determine the mechanism of apoptosis induction by ILQ, Western blot analysis was performed to investigate the effects of ILQ on the expression of apoptosis-related proteins. Previous studies have shown that the expressions of anti-apoptotic Bcl-2 and pro-apoptotic Bax are critical factors for initiating apoptosis via mitochondria.Citation17,Citation20 Therefore, we examined the expression of Bcl-2 and Bax in SKOV3 and OVCAR3 cells by Western blotting. Compared with the control group, ILQ treatment decreased Bcl-2 expression, while it simultaneously increased expression of Bax in both SKOV3 and OVCAR3 cells (P<0.05, ). These results indicated that ILQ treatment increased the Bax/Bcl-2 ratio in SKOV3 and OVCAR3 cells, and ILQ might trigger the mitochondrial apoptotic pathway in ovarian cancer cells. Additionally, we detected the expression of Caspase-3 and Caspase3-p17. After treatment with ILQ, the expression of Caspase-3 was significantly reduced relative to untreated control cells while the expression of Caspase3-p17 was drastically increased in both SKOV3 and OVCAR3 cells (P<0.05, ).
Isoliquiritigenin may affect the SKOV3 and OVCAR3 apoptosis by regulating the PI3K/Akt/mTOR pathway
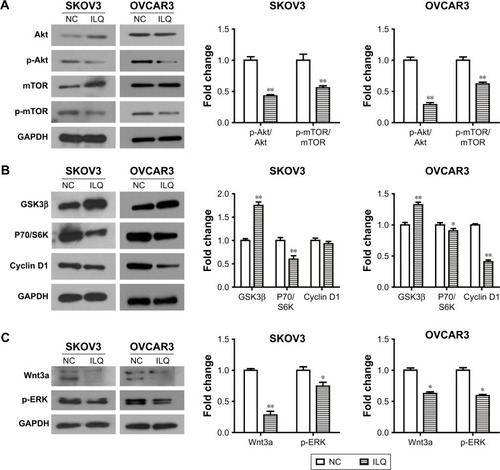
We detected the effect of ILQ on the PI3K/Akt/mTOR pathway. The PI3K/Akt/mTOR pathway is implicated in numerous cellular processes, ranging from cell growth and survival to the promotion of angiogenesis. PI3K can be active at the cell membrane, initiating the signaling cascade. Once activated, PI3K phosphorylates PIP2, leading to the accumulation of PIP3.Citation21 This lipid second messenger recruits Akt and PDK1 to the cell membrane, where Akt is phosphorylated by PDK1.Citation22 Phosphorylated Akt regulates cellular processes by phosphorylation of a number of substrates, including BclxL/Bcl-2 associated death promoter (BAD).Citation23 Another Akt substrate, mTOR, has the most significant role in tumorigenesis.Citation24 When activated, p-mTOR increases mRNA translation by phosphorylation of the downstream molecule P70/S6K. S6K phosphorylates the S6 component of the 40S ribosomal subunit, increasing translation of mRNA.Citation25 The PI3K/Akt/mTOR pathway can be negatively regulated by tumor suppressor PTEN.Citation26 We intended to investigate if ILQ could change the expression of key proteins of the PI3K/Akt/mTOR pathway.
Our results showed that the expression of Akt increased, while the phosphorylated form, p-Akt, decreased in the ILQ treated SKOV3 group (P<0.01, ). For mTOR, it had the same expression pattern with Akt in the ILQ treated SKOV3 group (P<0.01, ). In OVCAR3 cells, ILQ did not impact the expression level of total Akt and mTOR, while it significantly reduced the phosphorylated form p-Akt and p-mTOR (P<0.01, ). These data suggested that treatment of ILQ inhibited PI3K and caused a decrease of the phosphorylated form of Akt and mTOR. To further confirm this conclusion, we detected the expression levels of GSK3β, P70/S6K, and Cyclin D1. Our results showed that the expression of GSK3β increased, while P70/S6K decreased in both SKOV3 and OVCAR3 cells (P<0.05, ). There was no significant change of Cyclin D1 expression in SKOV3 cells, but it was significantly decreased in OVCAR3 cells (P<0.01, ). The decreased P70/S6K expression in the ILQ treated group was consistent with the inhibition of Akt and mTOR phosphorylation. As the PI3K activation inhibited GSK3β, the increased expression of GSK3β suggested that the PI3K was inhibited by ILQ treatment. In addition, Wnt3a increases PI3K/Akt activity and activates ERK1/2. Our results showed that ILQ treatment decreased the expression of Wnt3a and, as a result, further decreased the ERK phosphorylation level (P<0.05, ).
Figure 4 Isoliquiritigenin regulates the members of PI3K/Akt/mTOR pathway. (A) ILQ treatment significantly reduced the phosphorylated form p-Akt and p-mTOR in both SKOV3 and OVCAR3 cells. (B) ILQ treatment significantly reduced the expression level of downstream proteins. (C) ILQ treatment significantly reduced the expression level of Wnt3a and p-ERK. *P<0.05 compared with NC; **P<0.01 compared with NC.
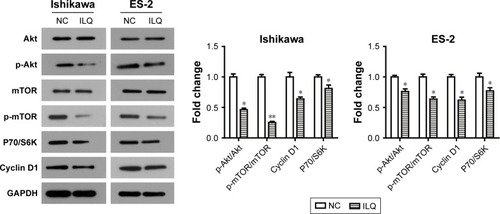
Isoliquiritigenin suppresses the PI3K/Akt/ mTOR pathway in Ishikawa and ES-2 cells
In order to further demonstrate the suppression of ILQ on the PI3K/Akt/mTOR pathway, the human endometrial cancer cell line, Ishikawa, and ovarian cancer clear cell line, ES-2, were performed, as it has been reported that the PI3K/Akt/mTOR pathway may possibly be activated in these cells.Citation27,Citation28 We observed similar results can be obtained by ILQ in Ishikawa and ES-2 cells. As shown in , ILQ significantly inhibited the phosphorylated form p-Akt and p-mTOR (P<0.05) both in Ishikawa and ES-2 cells. Moreover, the expression level of P70/S6K and Cyclin D1 were both decreased in ILQ treated cells (P<0.05, ).
Figure 5 Isoliquiritigenin regulates the members of PI3K/Akt/mTOR pathway in Ishikawa and ES-2 cells. ILQ treatment significantly reduced the expression level of p-Akt, p-mTOR, P70/S6K, and Cyclin D1 in both Ishikawa and ES-2 cells. *P<0.05 compared with NC; **P<0.01 compared with NC.
Discussion
Ovarian cancer is one of the most fatal gynecologic malignancies.Citation1 Because of the difficulty of diagnosis and a low survival rate,Citation2 new treatment strategies for ovarian cancer are needed. In our study, we used SKOV3 and OVCAR3 cell lines, the human ovarian cancer cell lines which are resistant to tumor necrosis factor and other cytotoxic drugs to investigate the proliferation, migration, and invasion of ovarian cancer. The anti-tumor activity of ILQ has recently been reported in various types of cancer, including breast, prostate, colon, oral, cervical, and leukemia.Citation29–Citation33 The discovered anticancer mechanisms of ILQ include cell proliferation inhibition, cell cycle arrest, inflammation suppression, apoptosis induction, and elevation of oxidative stress. In the present study, we reported the effects of ILQ on human ovarian cancer cell lines, SKOV3 and OVCAR3, and elucidated the underlying mechanisms. Our results showed that treatment of ILQ significantly inhibited cell survival, proliferation, migration and invasion, and induced apoptosis in both SKOV3 and OVCAR3 cells. These results reflected the potential pathologic role of this reagent in ovarian cancer.
BCL2 associated X (Bax), a member of the BCL2 family, is a pro-apoptotic protein. Overexpression of Bax triggers the release of mitochondrial proteins that cleave and thereby activate Caspase-3, resulting in apoptosis.Citation34 In our study we found that ILQ treatment increased the Bax/Bcl-2 ratio in SKOV3 and OVCAR3 cells. The expression of Caspase-3 decreased while Caspase3-p17 increased. These data suggested that ILQ triggered the apoptosis in a mitochondrial pathway.
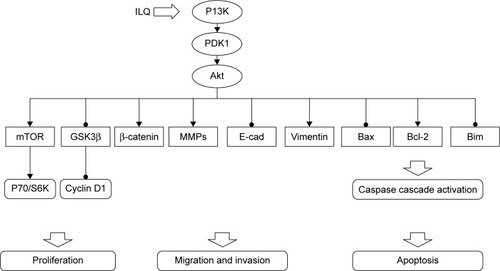
To elucidate the mechanism of ILQ on inhibiting ovarian cancer cells, we studied the effect of ILQ on PI3K/Akt/ mTOR pathway members. The PI3K/Akt/mTOR pathway is implicated in numerous cellular processes, ranging from cell growth and survival to the promotion of angiogenesis.Citation21,Citation35 The serine/threonine kinase Akt inhibits apoptosis and mediates cell survival by activating phosphatidylinositol 3-kinase.Citation36 Another kinase mTOR is expressed in most mammalian cells,Citation37 inhibiting autophagy and acting as a cellular sensor to nutrients and growth factors, as well as being an important effecter in the pathway of PI3K signaling.Citation38 GSK3β works as an inhibitor of the Wnt signaling pathway, which plays a role in resisting cancer development by inducing apoptosis for ovarian cancer cells.Citation39,Citation40 Wnt proteins belong to a family of secreted proteins that play important roles in the development and maintenance of many tissues. Wnt proteins also control kinase-signaling pathways. Notably, canonical Wnt3a increases PI3K/Akt activity, resulting in GSK3β phosphorylation and increased free β-catenin levels.Citation41 In addition, Wnt3a activates ERK1/2 by direct signaling and post-transcriptional activation via the β-catenin/Tcf4 complex,Citation42 indicating that these kinases may act as important mediators of Wnt signaling.Citation43 We found that ILQ treatment decreased the phosphorylated form p-Akt and p-mTOR, which happened at upstream PI3K, suggesting that ILQ treatment could inhibit the activation of the PI3K/Akt/ mTOR signaling pathway in ovarian cancer cells. Since the PI3K activation inhibited GSK3β, the increased expression of GSK3β and decreased P70/S6K further confirmed this conclusion. The suppression of ILQ on the PI3K/Akt/mTOR pathway was further confirmed by the similar results obtained by ILQ in Ishikawa and ES-2 cells that the PI3K/Akt/mTOR pathway may possibly be activated.Citation27,Citation28 Based on these results, ILQ has a significant suppression on the PI3K/Akt/mTOR pathway, which contributes to inhibit growth, metastasis, and survival of ovarian cancer. The inhibitory action of ILQ on PI3K/Akt/ mTOR pathway members is summarized in .
Conclusion
Our findings indicated that ILQ might be considered as a potent ovarian cancer inhibitor and be chronically used as a supplementary agent in ovarian cancer therapy. The interaction between ILQ and cancer conventional therapies also requires deep further study in the future.
Disclosure
The authors report no conflicts of interest in this work.
References
- EdwardsBKBrownMLWingoPAAnnual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatmentJ Natl Cancer Inst200597191407142716204691
- JemalASiegelRXuJWardECancer statistics, 2010CA Cancer J Clin201060527730020610543
- EltabbakhGHAwtreyCSCurrent treatment for ovarian cancerExpert Opin Pharmacother20012110912411336573
- YusufRZDuanZLamendolaDEPensonRTSeidenMVPaclitaxel resistance: molecular mechanisms and pharmacologic manipulationCurr Cancer Drug Targets20033111912570657
- HasanJTonNMullamithaSPhase II trial of tamoxifen and goserelin in recurrent epithelial ovarian cancerBr J Cancer200593664765116222310
- AslMNHosseinzadehHReview of pharmacological effects of Glycyrrhiza sp. and its bioactive compoundsPhytother Res200822670972418446848
- TakahashiTTakasukaNIigoMIsoliquiritigenin, a flavonoid from licorice, reduces prostaglandin E 2 and nitric oxide, causes apoptosis, and suppresses aberrant crypt foci developmentCancer Science200495544845315132774
- WuTYKhorTOSawCLAnti-inflammatory/anti-oxidative stress activities and differential regulation of Nrf2-mediated genes by non-polar fractions of tea Chrysanthemum zawadskii and licorice Glycyrrhiza uralensisAAPS J201113111320967519
- TraboulsiHCloutierABoyapellyKThe flavonoid isoliquiritigenin reduces lung inflammation and mouse morbidity during influenza virus infectionAntimicrob Agents Chemother201559106317632726248373
- CroninHDraelosZDTop 10 botanical ingredients in 2010 anti-aging creamsJ Cosmet Dermatol20109321822520883295
- MukhtarHMAnsariSHAliMBhatZANavedTEffect of aqueous extract of Pterocarpus marsupium wood on alloxan-induced diabetic ratsPharmazie200560647847915997844
- YamamotoSAizuEJiangHThe potent anti-tumor-promoting agent isoliquiritigeninCarcinogenesis19911223173231899810
- BabaMAsanoRTakigamiIStudies on cancer chemoprevention by traditional folk medicines XXV. Inhibitory effect of isoliquiritigenin on azoxymethane-induced murine colon aberrant crypt focus formation and carcinogenesisBiol Pharm Bull200225224725011853176
- YamazakiSMoritaTEndoHIsoliquiritigenin suppresses pulmonary metastasis of mouse renal cell carcinomaCancer Lett20021831233012049811
- IwashitaKKoboriMYamakiKTsushidaTFlavonoids inhibit cell growth and induce apoptosis in B16 melanoma 4A5 cellsBiosci Biotechnol Biochem20006491813182011055382
- MaggioliniMStattiGVivacquaAEstrogenic and antiproliferative activities of isoliquiritigenin in MCF7 breast cancer cellsJ Steroid Biochem Mol Biol2002824–531532212589938
- ZhangXYeungEDWangJIsoliquiritigenin, a natural anti-oxidant, selectively inhibits the proliferation of prostate cancer cellsClin Exp Pharmacol Physiol201037884184720456427
- MaJFuNYPangDBWuWYXuALApoptosis induced by isoliquiritigenin in human gastric cancer MGC-803 cellsPlanta Med200167875475711731922
- HsiaSMYuCCShihYHIsoliquiritigenin as a cause of DNA damage and inhibitor of ataxia-telangiectasia mutated expression leading to G2/M phase arrest and apoptosis in oral squamous cell carcinomaHead Neck201638Suppl 1E360E37125580586
- MartinouJCYouleRJMitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamicsDev Cell20112119210121763611
- RamehLECantleyLCThe role of phosphoinositide 3-kinase lipid products in cell functionJ Biol Chem1999274138347835010085060
- DownwardJMechanisms and consequences of activation of protein kinase B/AktCurr Opin Cell Biol19981022622679561851
- ShawRJCantleyLCRas, PI(3)K and mTOR signalling controls tumour cell growthNature2006441709242443016724053
- RaughtBGingrasACSonenbergNThe target of rapamycin (TOR) proteinsProc Natl Acad Sci U S A200198137037704411416184
- JefferiesHBFumagalliSDennisPBReinhardCPearsonRBThomasGRapamycin suppresses 5′TOP mRNA translation through inhibition of p70s6kEMBO J19971612369337049218810
- LiJYenCLiawDPTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancerScience19972755308194319479072974
- SlomovitzBMColemanRLThe PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancerClin Cancer Res201218215856586423082003
- TakaiMNakagawaTTanabeATeraiYOhmichiMAsahiMCrosstalk between PI3K and Ras pathways via protein phosphatase 2A in human ovarian clear cell carcinomaCancer Biol Ther201516232533525756515
- YeLGhoWMChanFLChenSLeungLKDietary administration of the licorice flavonoid isoliquiritigenin deters the growth of MCF-7 cells overexpressing aromataseInt J Cancer200912451028103619065667
- LeeYMLimDYChoiHJJungJIChungWYParkJHInduction of cell cycle arrest in prostate cancer cells by the dietary compound isoliquiritigeninJ Med Food200912181419298190
- LiDWangZChenHIsoliquiritigenin induces monocytic differentiation of HL-60 cellsFree Radic Biol Med200946673173619110051
- ParkIParkKKParkJHChungWYIsoliquiritigenin induces G2 and M phase arrest by inducing DNA damage and by inhibiting the metaphase/anaphase transitionCancer Lett2009277217418119167809
- TakahashiTTakasukaNIigoMIsoliquiritigenin, a flavonoid from licorice, reduces prostaglandin E2 and nitric oxide, causes apoptosis, and suppresses aberrant crypt foci developmentCancer Sci200495544845315132774
- EskesRAntonssonBOsen-SandABax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ionsJ Cell Biol199814312172249763433
- WangZWangNHanSDietary compound isoliquiritigenin inhibits breast cancer neoangiogenesis via VEGF/VEGFR-2 signaling pathwayPLoS One201387e6856623861918
- StalOPerez-TenorioGAkerbergLAkt kinases in breast cancer and the results of adjuvant therapyBreast Cancer Res200352R37R4412631397
- BrownEJAlbersMWShinTBA mammalian protein targeted by G1-arresting rapamycin-receptor complexNature199436964837567588008069
- ErsahinTTuncbagNCetin-AtalayRThe PI3K/AKT/mTOR interactive pathwayMol Biosyst20151171946195425924008
- CaoQLuXFengYJGlycogen synthase kinase-3beta positively regulates the proliferation of human ovarian cancer cellsCell Res200616767167716788573
- GaisinaINGallierFOugolkovAVFrom a natural product lead to the identification of potent and selective benzofuran-3-yl-(indol-3-yl) maleimides as glycogen synthase kinase 3beta inhibitors that suppress proliferation and survival of pancreatic cancer cellsJ Med Chem20095271853186319338355
- HuangTSLiLMoalim-NourLA regulatory network involving β-catenin, e-cadherin, PI3k/Akt, and slug balances self-renewal and differentiation of human pluripotent stem cells in response to Wnt signalingStem Cells20153351419143325538040
- GeorgopoulosNTKirkwoodLASouthgateJA novel bidirectional positive-feedback loop between Wnt-β-catenin and EGFR-ERK plays a role in context-specific modulation of epithelial tissue regenerationJ Cell Sci2014127132967298224816560
- HayENouraudAMariePJN-cadherin negatively regulates osteoblast proliferation and survival by antagonizing Wnt, ERK and PI3K/Akt signallingPLoS One2009412e828420011526