Abstract
There are very few treatments for musculoskeletal tumors, compared to other cancers; thus, novel therapeutic drugs are needed. Pristimerin (PM) is a triterpene compound isolated from plant extracts that reportedly has antitumor effects on various cancers, such as of the breast and prostate. The purpose of this study was to evaluate the antitumor effects of PM on human osteosarcoma cells. Treatment of the human osteosarcoma cell lines, MNNG and 143B, with PM led to a dose-dependent decrease in cell viability. The effects of PM on apoptosis were evaluated with the Annexin V/propidium iodide assay and analysis of caspases 3, 8, and 9 activities. Western blot analysis showed that PM caused a decrease in the expression of Akt, mTOR, and NF-κB. The volumes and weights of human osteosarcoma xenografts decreased significantly with PM treatment. The results of this study revealed that PM can inhibit human osteosarcoma growth in vitro and in vivo, and may be a novel therapeutic agent for the disease.
Introduction
Osteosarcoma is the most common primary malignant bone tumor, accounting for approximately 20% of all malignant bone tumors.Citation1 Although surgery was the only treatment until the 1970s, the survival rate dramatically improved with the introduction of chemotherapy in the 1980s.Citation2 However, there have been no significant advances in osteosarcoma treatment for osteosarcoma for almost 40 years, despite extensive research.Citation3,Citation4 Although the development of several novel molecular targeted drugs for the treatment of soft-tissue sarcomas is well advanced and molecular targeted drugs, novel therapeutic drugs, have been developed for soft-tissue sarcomas, molecular targeted drugs for osteosarcoma are still in the preclinical or early clinical trial phase.Citation5,Citation6
In recent years, natural anticancer drugs have received much attention, including Yondelis (trabectedin), a natural compound from the tunicate, Ecteinascidia turbinata, which has been used to treat soft-tissue sarcoma.Citation7,Citation8 Terpenoids are a large and diverse class of natural compounds that occur widely in nature as a secondary metabolite of organisms, and are derived biosynthetically from units of isoprene. Among the terpenoids, triterpenes reportedly possess anti-inflammatory, antipyretic, and antitumor effects.Citation9,Citation10 Pristimerin (PM) is a quinoid-type triterpene isolated from members of the Celastraceae and Hippocrateaceae plant families that was recently shown to have antitumor effects against glioma, cervical cancer, and prostate cancer.Citation11–Citation13 In addition, PM induces cell death via various mechanisms such as caspase activation, changes in mitochondrial membrane potential, and inhibition of anti-apoptotic factors, including nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and Akt.Citation12,Citation14 Furthermore, some studies have shown that PM can inhibit tumor angiogenesis and the development of drug resistance.Citation15,Citation16 However, the effects of PM on osteosarcoma cells have not been reported.
The purpose of this study was to evaluate the antitumor effects of PM in osteosarcoma cells and its mechanism of action, both in vitro and in vivo.
Materials and methods
Cell lines and culture conditions
The human osteosarcoma cells MNNG (CRL1547) and 143B (CRL1427) were obtained from the American Type Culture Collection (Rockville, MD, USA). The Normal Human Osteoblast (NHOst) cell line was obtained from Lonza Biosciences (Walkersville, MD, USA). All cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Nacalai Tesque, Kyoto, Japan) supplemented with 10% fetal bovine serum (FBS; Equitech-Bio, Kerrville, TX, USA), 100 units/mL penicillin, and 100 mg/mL streptomycin (complete DMEM; Nacalai Tesque) at 37°C in 5% CO2/95% humidified air. Cells were trypsinized with trypsin/ethylene-diaminetetraacetic acid (Nacalai Tesque) and subcultured.
Reagents
PM was purchased from Sigma (St Louis, MO, USA), dissolved in dimethyl sulfoxide (Nacalai Tesque) to yield a 10 mM stock solution, and stored at −20°C. PM was diluted to the required concentrations in cell culture medium.
Antibodies
Anti-phospho-Akt (Ser473), anti-Akt (C67E7), anti-Bax, anti-phospho-mTOR (Ser2448), anti-mTOR (7C10), anti-phospho-4E-BP1, anti-NF-κB p65, anti-phospho-NF-κB p65 (Ser536), and anti-Cox2 antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-β-actin, anti-mouse IgG, and anti-rabbit IgG were purchased from Sigma.
Measurement of cell viability
Cell viability was determined using the RealTime-Glo MT Cell Viability Assay Kit (Promega; Madison, WI, USA). Cells were plated in a Poly-L-Lysine (PLL)-coated 96-well plate at a density of 1×103 cells per well in 100 μL medium containing 10% FBS. After 24 h, MT cell viability substrate and Nanoluc® Enzyme were added to the medium, and the cells were exposed to various concentrations of PM. The CentroXS3 LB 960 System (Berthold Technologies; Oak Ridge, TN, USA) was used to measure light emission.
Measurement of caspase activity
The activity of caspases 3/7, 8, and 9 was assessed using the Caspase-Glo 3/7, 8, and 9 Assay (Promega). Experiments were conducted according to Ma et al.Citation17 Caspase 3 activity was measured 12 h after PM treatment. Caspases 8 and 9 most likely act upstream of caspase 3; therefore, their activity was measured 8 h after PM treatment. Caspase activity was measured in luminescence units.
Annexin-V/propidium iodide assay
Cells were harvested following treatment with PM for 24 h, and stained with 5 μL Annexin V-FITC and 5 μL PI solution (Nacalai Tesque) for 15 min at room temperature in the dark. Following incubation, binding buffer was added and the cells were analyzed by flow cytometry using a FACS Vantage Flow Cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). For each sample, 10,000 events were recorded.
Western blotting
These procedures were conducted as described previously.Citation18
Human osteosarcoma xenograft mouse model
Animal experiments were conducted with approval from the Experimental Animals Committee, Kyoto Prefectural University of Medicine (Code No M29-137).
BALB/C-nu/nu mice (age 5 weeks, females) were purchased from Shimizu Laboratory Supplies (Kyoto, Japan). All procedures were undertaken in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Cells (5.0×106) re-suspended in 100 μL phosphate-buffered saline were injected into the hypodermis of the back of each mouse. After tumors grew to approximately 100 mm3, the mice were injected intraperitoneally (ip,) with PM at a dose of 1 mg/kg, every other day for 14 days. Untreated mice were used as controls. Tumor size was measured with a caliper (calculated volume = shortest diameter2 × longest diameter/2) at 2-day intervals. All mice were sacrificed, and the tumors were weighed on Day 14. Liver toxicity was checked by blood sample liver enzyme assay on Day 14.
Statistical analysis
All duplicate and triplicated experiments yielded almost identical results. All experimental data are expressed as means ± SD. Parametric one-way analysis of variance (ANOVA) was used to test for any differences among the groups. If the result was significant, the Tukey–Kramer test was used to determine specific differences between the groups. In all analyses, p<0.05 was defined as statistically significant.
Results
PM inhibits the viability of osteosarcoma cells
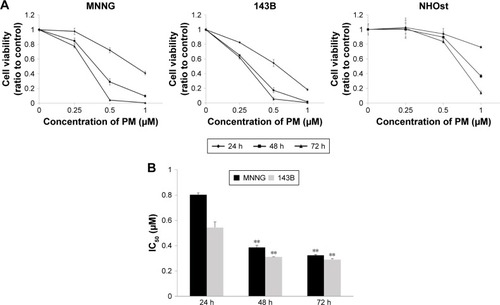
To investigate the effects of PM, a cell viability assay was conducted in osteosarcoma cell lines (MNNG and 143B). The cell lines were treated with various concentrations of PM (0.25, 0.5, 1.0 μM) for 24, 48, and 72 h. As shown in , PM inhibited the growth of osteosarcoma cells in a dose- and time-dependent manner. The IC50 values of PM in MNNG were 0.80±0.02, 0.39±0.02, and 0.32±0.01 μM at 24, 48, and 72 h, respectively. For 143B, the values were 0.54±0.04, 0.31±0.002, and 0.29±0.004 μM, respectively (). Thus, 143B cells were more sensitive to PM than MNNG cells. The normal human osteoblast cell line, NHOst, was assessed for comparison with osteosarcoma cells. At a PM concentration of 1 μM, viability decreased; however, the decrease in viability at 0.25 and 0.5 μM was slight compared with the osteosarcoma cell lines.
Figure 1 Effects of PM on osteosarcoma cell viability.
PM induces apoptosis in osteosarcoma cells
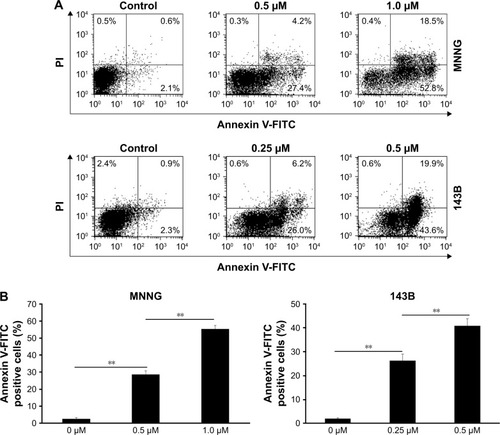
Next, we used the Annexin V-FITC/PI binding assay to determine whether PM could induce apoptosis in osteosarcoma cells. MNNG and 143B cells were treated with PM (0–1 μM) for 24 h, and the binding of Annexin V-FITC was determined by flow cytometry (). The percentage of apoptotic cells (AnnexinV+/PI−) in both cell lines increased in a dose-dependent manner (). These results demonstrate the proapoptotic effects of PM in osteosarcoma cells.
Figure 2 Annexin V-FITC/PI staining of osteosarcoma cells treated with PM.
PM increased the activities of caspases 3/7, 8, and 9 in osteosarcoma cells
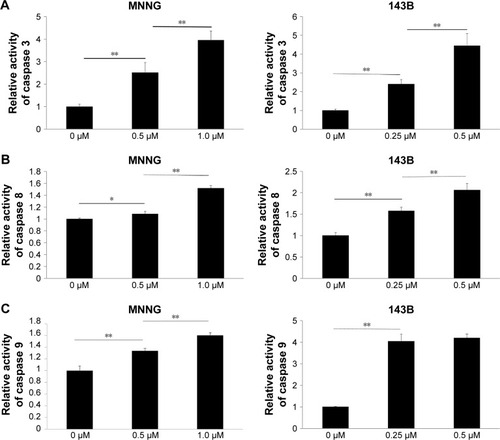
The Caspase-Glo Assay was used to assess the effects of PM on the activities of the main caspases (caspases 3/7 [effectors], 8, and 9 [initiators]) in osteosarcoma cells. Caspase 3/7 activity was measured 12 h after PM treatment, whereas caspase 8 and 9 activities were measured 8 h post treatment. As shown in , the activities of caspases 3, 8 and 9 increased significantly after treatment with PM. These results demonstrate that apoptosis induced by PM in osteosarcoma cells may involve both intrinsic and extrinsic pathways.
Figure 3 Effects of PM on the activity of caspases 3, 8, and 9 in osteosarcoma cells.
PM inhibits protein expression of Akt, mTOR, and NF-κB in osteosarcoma cells
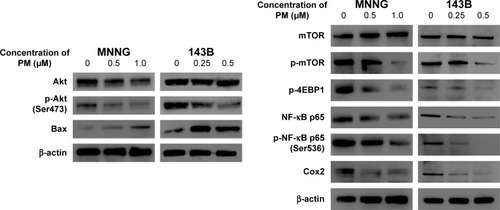
mTOR and NF-κB are downstream factors of Akt, and Akt activation is involved in the resistance to apoptosis. Therefore, we used Western blot analysis to examine the protein expression levels of Akt, mTOR, NF-κB, and downstream mediators of these signaling molecules (). Phosphorylation of Akt and mTOR was inhibited by PM in both osteosarcoma cell lines, whereas total expression levels of Akt and mTOR were unchanged. Moreover, PM reduced the expression of several downstream mediators of Akt, mTOR, and NF-κB. Expression of Bax – proapoptotic effector suppressed by Akt – was elevated by PM treatment.
Figure 4 PM inhibits the Akt/mTOR signaling pathway and NF-κB.
Abbreviation: p, phospho.
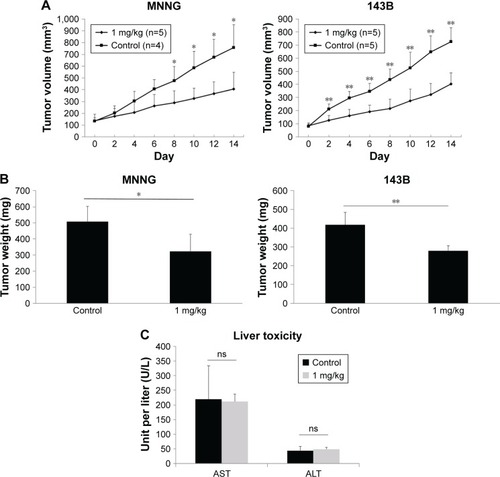
PM inhibited tumor growth in vivo
The antitumor effects of PM in vivo were investigated using a xenograft human osteosarcoma model. A concentration of 1 mg/kg PM applied every other day reduced both tumor volume and tumor weight (). The initial administration was set to Day 0, and administration was done a total of seven times on alternate days. A significant tumor-suppressing effect was observed from Day 8 in MNNG cells and from Day 2 in 143B cells. These results show that PM has antitumor effects in vivo. Liver enzymes, aspartate aminotransferase (AST) and alanine aminotransferase (ALT), were measured as parameters of liver toxicity for both the control and 1 mg/kg–PM-treated group of nude mice. As shown in , there were no significant differences in the levels of AST and ALT.
Figure 5 Inhibition of tumor growth by PM in an osteosarcoma xenograft model.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ns, not significant.
Discussion
PM is extracted as an active ingredient of traditional medicinal plants, and has anti-inflammatory, antiviral, and antibacterial properties.Citation9,Citation10 Recently, there have been reports that PM has antitumor effects against several cancers;Citation11–Citation13 however, its effects on osteosarcoma cells have not been elucidated. In this study, we demonstrated for the first time that PM has antitumor effects on human osteosarcoma cells in a time- and dose-dependent manner. Moreover, PM showed tumor growth inhibitory effects in vivo. Wu et alCitation19 evaluated the antitumor effects of PM on A-549 (lung), MCF-7 (breast), HepG2, and Hep3B (liver) cells, and reported IC50 values of 0.42–0.61 μM at 72 h. In this study, the IC50 value in MNNG and 143B cells was approximately 0.3 μM at 72 h. These results suggest that PM has antitumor effects on osteosarcoma, similar to its observed effects in other cancers. Importantly, although PM treatment, at higher concentration, reduced the viability of normal osteoblasts, the effects were not significant compared to PM in osteosarcoma cells.
Cell survival and death are regulated by cell growth and apoptosis signals, and deregulation of either process can lead to cancer.Citation20 Thus, the induction of apoptosis is one mechanism by which antitumor drugs can kill cancer cells.Citation21 In this study, PM induced apoptosis in a dose-dependent manner, as determined by the Annexin V-PI assay.
Apoptosis is induced as a result of caspase activation.Citation22 There are two major caspase cascades: the intrinsic (mitochondrial) pathway that activates caspases 9 and 3 via cytochrome C release from mitochondria, and the extrinsic (death receptor) pathway that activates caspases 8 and 10 through receptors (so-called “death receptor”) present on the cell membrane surface.Citation23 In this study, PM induced elevation of caspase 3 and activation of upstream caspases 8 and 9. These results indicate that apoptosis was induced via both intrinsic and extrinsic pathways.
Activation of the PI3K/Akt/mTOR pathway is one of the drivers of cancer cell growth, and is closely involved in cancer initiation, progression, and treatment resistance. Thus, it is considered an important target in cancer treatment.Citation24 Currently, drug development aimed at suppressing PI3K/Akt pathway is progressing, and inhibitors of mTOR are already used in clinical practice.Citation25,Citation26 In this study, the expression of phosphorylated Akt and mTOR, indicative of activation, was reduced by PM, suggesting that PM inhibits the growth of osteosarcoma cells by inhibiting the Akt/mTOR pathway.
NF-κB is the most important transcription factor involved in inflammation induction, and PM decreased its expression.Citation27,Citation28 It was revealed that PM, which has long been used as an anti-inflammatory drug, had anti-inflammatory effects by inhibiting NF-κB, which is not only associated with inflammation but also inhibits apoptosis.Citation29 The Akt/mTOR pathway and NF-κB cross-talk and induce apoptosis.Citation30,Citation31 This study revealed that PM induces apoptosis by inhibiting Akt, mTOR, and NF-κB. Moreover, inhibition of Akt/mTOR signaling is strongly linked to autophagy and is reported to cross-talk with the apoptotic pathway.Citation32 Although a role for PM in the induction of autophagy has not been reported to date, this possibility warrants further investigation in the context of osteosarcoma. Inhibition of the Akt/mTOR pathway and NF-κB in various cancers enhances the effects of anticancer drugs and radiation and suppresses tumor angiogenesis.Citation33–Citation35 Thus, PM has potential as an adjuvant therapy and metastasis inhibitor for osteosarcoma treatment.
The antitumor effects of PM in vivo have been reported for breast cancer, glioma, and colorectal cancer.Citation11,Citation16,Citation36 In this study, administration of PM in xenograft mouse models of osteosarcoma resulted in a decrease in tumor volume compared with the untreated group, demonstrating that PM has tumor growth inhibitory effects on osteosarcoma in vivo.
A comprehensive evaluation of PM toxicity is yet to be undertaken. Yousef et alCitation36 reported that no significant change in body weight was observed after treatment of mice with 1 mg/kg PM every other day, and minimal toxicity has reported in another study.Citation37 However, in this work, weight loss of approximately 1.5 g was observed after administration of the first dose (data not shown), although no obvious liver toxicity was observed as with the result of Wang et al.Citation37 PM-associated bone marrow suppression has been reported.Citation38 Additional investigation is needed to determine the safest and most effective dosing and administration methods of PM.
In conclusion, PM exhibited antitumor effects on human osteosarcoma cells in vitro and in vivo, and may be a novel therapeutic agent for osteosarcoma.
Acknowledgments
This work was supported by the JSPS KAKENHI (grant nos 17K10975 and 15K10454).
Disclosure
The authors report no conflicts of interest in this work.
References
- DamronTAWardWGStewartAOsteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National Cancer Data Base ReportClin Orthop Relat Res2007459404717414166
- FerrariSPalmeriniEAdjuvant and neoadjuvant combination chemotherapy for osteogenic sarcomaCurr Opin Oncol200719434134617545797
- AndersonMEUpdate on Survival in OsteosarcomaOrthop Clin North Am201647128329226614941
- FerrariSSerraMAn update on chemotherapy for osteosarcomaExpert Opin Pharmacother201516182727273626512909
- RatanRPatelSRChemotherapy for soft tissue sarcomaCancer2016122192952296027434055
- ShaikhABLiFLiMPresent advances and future perspectives of molecular targeted therapy for osteosarcomaInt J Mol Sci201617450627058531
- NewmanDJCraggGMNatural products as sources of new drugs from 1981 to 2014J Nat Prod201679362966126852623
- De SanctisRMarrariASantoroATrabectedin for the treatment of soft tissue sarcomasExpert Opin Pharmacother201617111569157727328277
- AkihisaTTokudaHIchiishiEAnti-tumor promoting effects of multiflorane-type triterpenoids and cytotoxic activity of karounidiol against human cancer cell linesCancer Lett2001173191411578803
- BannoNAkihisaTYasukawaKAnti-inflammatory activities of the triterpene acids from the resin of Boswellia carteriJ Ethnopharmacol2006107224925316621377
- YanYYBaiJPXieYYuJZMaCGThe triterpenoid pristimerin induces U87 glioma cell apoptosis through reactive oxygen species-mediated mitochondrial dysfunctionOncol Lett20135124224823255929
- ByunJYKimMJEumDYReactive oxygen species-dependent activation of Bax and poly(ADP-ribose) polymerase-1 is required for mitochondrial cell death induced by triterpenoid pristimerin in human cervical cancer cellsMol Pharmacol200976473474419574249
- YangHLandis-PiwowarKRLuDPristimerin induces apoptosis by targeting the proteasome in prostate cancer cellsJ Cell Biochem2008103123424417541980
- GaoXLiuYDeebDArbabASGautamSCAnticancer activity of pristimerin in ovarian carcinoma cells is mediated through the inhibition of prosurvival Akt/NF-kappaB/mTOR signalingJ Exp Ther Oncol201410427528325509983
- MuXShiWSunLLiHJiangZZhangLPristimerin, a triterpenoid, inhibits tumor angiogenesis by targeting VEGFR2 activationMolecules20121766854686822669041
- XieGYuXLiangHPristimerin overcomes adriamycin resistance in breast cancer cells through suppressing Akt signalingOncol Lett20161153111311627123073
- MaSLiuXYaoYEffect of temozolomide on cell viability in gonadotroph adenoma cell linesOncol Rep201126354355021617871
- MoriYTerauchiRShiraiTSuppression of heat shock protein 70 by siRNA enhances the antitumor effects of cisplatin in cultured human osteosarcoma cellsCell Stress Chaperones Epub201752
- WuCCChanMLChenWYTsaiCYChangFRWuYCPristimerin induces caspase-dependent apoptosis in MDA-MB-231 cells via direct effects on mitochondriaMol Cancer Ther2005481277128516093444
- HanahanDWeinbergRAThe hallmarks of cancerCell20001001577010647931
- DasariSTchounwouPBCisplatin in cancer therapy: molecular mechanisms of actionEur J Pharmacol201474036437825058905
- DenaultJBSalvesenGSCaspases: keys in the ignition of cell deathChem Rev2002102124489450012475198
- WongRSApoptosis in cancer: from pathogenesis to treatmentJ Exp Clin Cancer Res2011308721943236
- PolivkaJJrJankuFMolecular targets for cancer therapy in the PI3K/AKT/mTOR pathwayPharmacol Ther2014142216417524333502
- LiuPChengHRobertsTMZhaoJJTargeting the phosphoinositide 3-kinase pathway in cancerNat Rev Drug Discov20098862764419644473
- BrownJSBanerjiUMaximising the potential of AKT inhibitors as anti-cancer treatmentsPharmacol Ther201717210111527919797
- BrasierARThe NF-kappaB regulatory networkCardiovasc Toxicol20066211113017303919
- CoussensLMWerbZInflammation and cancerNature2002420691786086712490959
- KarinMNuclear factor-kappaB in cancer development and progressionNature2006441709243143616724054
- HussainARAhmedSOAhmedMCross-talk between NFkB and the PI3-kinase/AKT pathway can be targeted in primary effusion lymphoma (PEL) cell lines for efficient apoptosisPLoS One201276e3994522768179
- DeebDGaoXLiuYThe inhibition of cell proliferation and induction of apoptosis in pancreatic ductal adenocarcinoma cells by verrucarin A, a macrocyclic trichothecene, is associated with the inhibition of Akt/NF-κB/mTOR prosurvival signalingInt J Oncol20164931139114727573873
- Eisenberg-LernerABialikSSimonHUKimchiALife and death partners: apoptosis, autophagy and the cross-talk between themCell Death Differ200916796697519325568
- BurrisHA3rdOvercoming acquired resistance to anticancer therapy: focus on the PI3K/AKT/mTOR pathwayCancer Chemother Pharmacol201371482984223377372
- HeLWuYLinLHispidulin, a small flavonoid molecule, suppresses the angiogenesis and growth of human pancreatic cancer by targeting vascular endothelial growth factor receptor 2-mediated PI3K/Akt/mTOR signaling pathwayCancer Sci2011102121922521087351
- TangQLXieXBWangJGlycogen synthase kinase-3β, NF-κB signaling, and tumorigenesis of human osteosarcomaJ Natl Cancer Inst20121041074976322534782
- YousefBAHassanHMGuerramMPristimerin inhibits proliferation, migration and invasion, and induces apoptosis in HCT-116 colorectal cancer cellsBiomed Pharmacother20167911211927044819
- WangLNWangYLuYPristimerin enhances recombinant adeno-associated virus vector-mediated transgene expression in human cell lines in vitro and murine hepatocytes in vivoJ Integr Med2014121203424461592
- LuZJinYChenCLiJCaoQPanJPristimerin induces apoptosis in imatinib-resistant chronic myelogenous leukemia cells harboring T315I mutation by blocking NF-kappaB signaling and depleting Bcr-AblMol Cancer2010911220482842
