Abstract
Under normal conditions, the immune system responds effectively to both external and internal threats without damaging healthy tissues. Cells undergoing a neoplastic transformation are one such threat. An efficient activation of T cells is enabled by T-cell receptor (TCR) interactions with antigen-presenting class I and class II molecules of the major histocompatibility complex (MHC), co-stimulatory molecules, and cytokines. After threatening stimuli are removed from the body, the host’s immune response ceases, which prevents tissue damage or chronic inflammation. The recognition of foreign antigens is highly selective, which requires multistep regulation to avoid reactions against the antigens of healthy cells. This multistep regulation includes central and peripheral tolerance toward the body’s own antigens. Here, we discuss T-cell dysfunction, which leads to poor effector function against foreign antigens, including cancer. We describe selected cellular receptors implicated in T-cell dysfunction and discuss how immune-checkpoint inhibitors can help overcome T-cell dysfunction in cancer treatment.
Introduction
Complex immune mechanisms enable the differentiation between self and non-self so that the immune response can be effectively directed at foreign antigens, such as cancer cells, and does not damage the body’s own healthy tissues. The immune system recognizes certain antigens as own due to both central and peripheral tolerance. Similarly, full activation of the immune system against foreign antigens is precisely regulated and requires several signals.Citation1 An efficient activation of T cells is enabled by T-cell receptor (TCR) interactions with the antigen-presenting class I and class II molecules of the major histocompatibility complex (MHC) (signal 1), co-stimulatory molecules (signal 2), and cytokines (signal 3).Citation2 This multistep regulation enables termination of the immune response when threatening stimuli are removed from the body.
Here, we discuss the role and mechanisms of T-cell dysfunction in cancer, which leads to immune evasion by cancer cells and, thus, to cancer progression.Citation3,Citation4 We describe cellular receptors implicated in T-cell dysfunction and discuss how immune-checkpoint inhibitors can help overcome T-cell dysfunction in cancer treatment.
Immune evasion in cancer due to T-cell dysfunction
The complex cross talk between cancer cells, immune cells, and tumor microenvironment involves many mechanisms that lead to an inefficient immune response toward cancer cells. In cancer, T-cell dysfunction may be due to T-cell exhaustion, T-cell anergy, decreased phosphorylation of the CD3ζ chain, and inhibitory signaling within the tumor microenvironment.
Continuous TCR stimulation in effector T cells gradually leads to exhaustion of these cells, which occurs mostly in chronic viral infections and cancer.Citation5 Due to T-cell exhaustion, cytotoxic lymphocytes lose their effector function, which leads to an impaired immune response. T-cell exhaustion develops more likely when antigen levels are high or antigen exposure is prolonged.Citation6 Lymphocyte exhaustion manifests initially with decreased IL-2 secretion; however, subsequently, other cytokines, including tumor necrosis factor α (TNFα), are secreted in lower amounts.Citation7,Citation8 Moreover, T-cell exhaustion impairs antigen-stimulated lymphocyte proliferation, halts lymphocyte renewal (mediated by IL-7 and IL-15), causes abnormal expression and function of transcription factors, decreases cytokine production, and impairs the response of memory T cells.Citation9,Citation10 In particular, an accumulation of exhausted cells is observed in the tumor microenvironment, which resembles the microenvironment of chronic inflammation.Citation11 Functionally exhausted T cells have an increased expression of inhibitory molecules.Citation12 A high expression of inhibitory receptors impairs the effector and proliferative functions of immune cells, and it creates a state of immunosuppression. Thus, the immune response toward cancer cells is insufficient and causes therapeutic failure.Citation13
T-cell anergy – that is, tolerance of T cells toward specific antigens – may develop due to TCR stimulation without sufficient co-stimulatory signals or in the presence of inhibitory stimulation. This mechanism of T-cell activation is associated with reduced IL-2 production and a state of hyporesponsiveness of T cells. T-cell anergy may develop in patients with cancer because co-inhibitory signals prevail over co-stimulatory signals in the tumor microenvironment. For example, there is a greater expression of the inhibitory B7 family proteins over B7 stimulatory protein in the tumor microenvironment.Citation14
The CD3ζ chain is an intracellular element of the TCR complex. Phosphorylation of the CD3ζ chain is crucial for antigen-specific T-cell activation, and downregulation of the CD3ζ chain is associated with a reduced response of T cells. Notably, CD3ζ downregulation is observed in many cancers, particularly in tumor-infiltrating cells.Citation15 As the CD3ζ chain is crucial for T-cell activation, CD3ζ chain downregulation may be associated with T-cell exhaustion and T-cell apoptosis.Citation16 Moreover, there is evidence that T-cell activation without CD3ζ phosphorylation causes T-cell anergy.Citation17
Finally, inhibitory signaling – due to overexpression of inhibitory molecules in the tumor microenvironment – is important in the development of T-cell dysfunction in cancer. Below, we describe the most important inhibitory molecules implicated in immune evasion by cancer cells ().
Table 1 Immune-checkpoint molecules and their inhibitors
Inhibitory molecules related to T-cell dysfunction in cancer PD-1
PD-1 mRNA was first detected in the mouse thymus; after treatment with an anti-CD3 antibody, the thymocytes entering the path of the cell’s programmed death showed an increase in PD-1 expression.Citation18 Despite its name, PD-1 does not cause cell death, but it blocks the cell cycle.Citation19,Citation20 PD-1 is a transmembrane glycoprotein from the CD28:B7 family. It is mostly expressed on activated T and B cells, but is also expressed on activated monocytes, dendritic cells (DCs), and NK (natural killer) and NKT (natural killer T) cells.Citation7 Unlike other molecules from the CD28 superfamily, which are expressed only by T cells, PD-1 is expressed by many cell types. This suggests that PD-1 has a central place in the regulation of immune responses.Citation21 PD-1 is a receptor with a length of 288 amino acids, and it is encoded by the PDCD-1 gene on chromosome 2. PD-1 has an intracellular transmembrane domain and an extracellular immunoglobulin domain, which contains 21%–33% sequences that are identical to the sequences of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), CD28, and the inducible T-cell co-stimulator (ICOS).Citation22 The receptor functions of PD-1 are mediated by its cytoplasmic part, which contains two tyrosine motifs that bind phosphatases responsible for transmitting immunosuppressive signals. The two motifs include the immunoreceptor tyrosine-based inhibitory motif (ITIM), located proximally to the cell membrane, and the immunoreceptor tyrosine-based switch motif (ITSM), which is essential to the inhibitory function of PD-1 ().Citation23 PD-1 expression is induced by the signaling pathways of the TCR and the B-cell receptor (BCR), and it is maintained during antigen stimulation. Moreover, some cytokines (IL-2, IL-7, and IL-15), Toll-like receptors (TLRs; TLR-9), and interferons (IFNs) stimulate the expression of PD-1 in T cells.Citation24,Citation25 Moreover, the nuclear factor of activated T cells c1 (NFATc1) is important for PD-1 expression.Citation26
Figure 1 Signaling pathways of immune-checkpoint molecules.
Abbreviations: BTLA, B- and T-lymphocyte attenuator; CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; HVEM, herpesvirus entry mediator; ITIM, immunoreceptor tyrosine-based inhibition motif; ITSM, immunoreceptor tyrosine-based inhibition motif; LAG-3, lymphocyte-activation gene 3; MHC, major histocompatibility complex; P13K, phosphoinositide 3-kinase; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PD-L2, programmed death-ligand 2; PIP3, phosphatidylinositol (3,4,5)-trisphosphat; PP2A, protein phosphatase 2A; TCR, T-cell receptor; TIM-3, T-cell immunoglobulin and mucin domain 3.
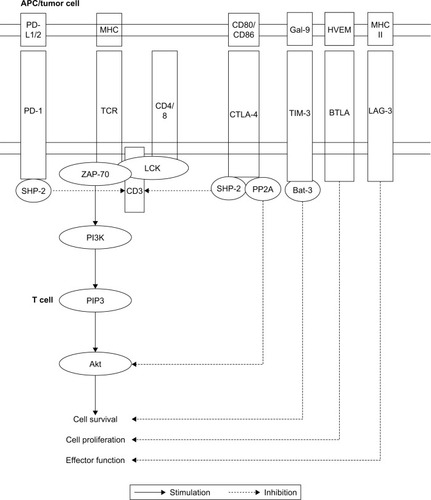
PD-L1 and PD-L2
Two PD-1 ligands that induce its inhibitory proprieties have been identified: PD-L1 (CD274 or B7-H1) and PD-L2 (CD273 or B7-DC). Both these ligands are type I transmembrane glycoproteins.Citation27 The constitutive expression of PD-L1 is substantially higher in mice than in humans, particularly in T and B cells, DCs, macrophages, and mesenchymal stem cells (MSCs); moreover, PD-L1 expression increases during activation of these cells.Citation28,Citation29 Besides hematopoietic cells, PD-L1 is expressed by other cell types, such as pancreatic cells, epithelial cells, endothelial cells, muscle cells, hepatocytes, astrocytes, spleen cells, kidney cells, and lung cells.Citation28–Citation31 PD-L2 is expressed only in the core layer of the thymus and, in lesser amounts, in the fetal myocardium and endothelial cells – particularly within the placenta.Citation32,Citation33 PD-L2 expression can be induced on DCs, peritoneal B1 lymphocytes, macrophages, medullary mast cells, and memory B cells.Citation34 Importantly, PD-L1 and PD-L2 are expressed by cancer cells, cancer-associated fibroblasts, and myeloid-derived stem cells. The expression of PD-L2 increases only slightly on stimulated CD8+ T cells, but it does not increase at all on CD4+ lymphocytes.Citation35 Binding of PD-1 to PD-L1 or PD-L2 during TCR activation suppresses the proliferation of both B and T cells, decreases cytokine secretion, inhibits cytolysis, and prolongs T-cell survival.Citation36 PD-L1- or PD-L2-mediated prolongation of T-cell survival and impairment of their function may occur both indirectly, through interference with the early activating signals induced by CD28, and directly, through interference with IL-2 secretion.Citation37 Furthermore, PD-L1 is essential for Treg induction by DCs.Citation38
CTLA-4
CTLA-4 is a transmembrane receptor protein that inhibits T-cell function, mostly by competing with the co-stimulatory molecule CD28 for CD80 and CD86 located on antigen-presenting cells (APCs). CTLA-4 is expressed on conventional CD4+ and CD8+ T cells after TCR stimulation, which prevents an excessive early immune reaction; moreover, CTLA-4 is essential for the suppressive function of regulatory T cells (Treg).Citation39,Citation40 CTLA-4 ligation causes lymphocyte anergy, which reduces the synthesis of IFNγ, IL-2, IL-3, and granulocyte-macrophage colony-stimulating factor (GM-CSF), and increases the production of transforming growth factor beta (TGFβ).Citation41 The synthesis of CTLA-4 mRNA increases within the first hours of lymphocyte stimulation, and peaks after 48–72 hours.Citation42 CTLA-4 stimulation makes lymphocytes more likely to remain in the G0/G1 phase of the cell cycle, which is due to a reduced synthesis of cyclin D3 and kinases cdk4/cdk6, degradation of the inhibitory protein p27, and increased expression of cyclin D2.Citation43 Anergic lymphocytes are not activated after antigen recognition even when they receive co-stimulatory signals sufficient to activate a naïve lymphocyte. CD86, like CD80, is a ligand for CD28 and CTLA-4, and it is important in the co-stimulation of T cells during the primary immune response. CD86 belongs to the superfamily of immunoglobulins, and it is expressed on monocytes, DCs, as well as activated T, B, and NK cells. The chromosome region encoding CD86 contains a series of genes involved in carcinogenesis.Citation44,Citation45 Blocking CTLA-4 by monoclonal antibodies (mAbs) maintains T cells in an activation state and improves the immune response against cancer cells. Thus, anti-CTLA-4 mAbs are effective in cancer immunotherapy.
LAG-3
LAG-3 (CD223) prevents an excessive immune activation. This receptor is expressed by T and NK cells after MHC class II ligation, and by cytotoxic T cells upon antigen stimulation.Citation22,Citation46 LAG-3 inhibits CD4+ cell activation and directly decreases the cytotoxic function of CD8+ cells.Citation46,Citation47 Blocking LAG-3 restores the function of cytotoxic T cells and simultaneously inhibits Tregs.Citation48 Preclinical studies showed that LAG-3, fused with immunoglobulin (LAG- 3-Ig), binds with a high affinity to MHC II of DCs, which stimulates DC maturation, and that, in turn, potentiates T helper 1 (Th1)-type responses.Citation49 In contrast, monomeric LAG-3, shed from the cell surface, does not bind to MHC class II molecules.Citation46
TIM-3
Expression of the type I transmembrane protein TIM-3 was shown in many immune cell types, including Th1, Th17, NK, and NKT cells as well as Tregs; on APCs, TIM-3 is co-expressed with PD-1.Citation50 TIM-3 binds to galectin-9, which causes apoptosis of CD4+ and CD8+ cells through the calcium–calpain–caspase-1 pathway.Citation51,Citation52 Galectin-9 is expressed on the surface of many cancer cell types, whereas the expression of TIM-3 was observed in tumor-infiltrating T cells in mice. TIM-3 directly inhibits Th1-mediated autoimmunity, and it indirectly promotes immunosuppression by inducing expansion of myeloid-derived suppressor cells (MDSCs), through an unknown mechanism.Citation22,Citation53 Blocking TIM-3 increases the production of IFNγ by lymphocytes, but it is unclear as to what forms the molecular basis of this action.Citation54 In patients with gastric, colorectal, liver, and pancreatic cancers, TIM-3 tumor expression correlated with tumor invasion, reduced survival, and metastasis; thus, TIM-3 can be implicated in carcinogenesis.Citation55
B- and T-lymphocyte attenuator (BTLA)
BTLA is a glycoprotein containing an immunoglobulin domain, and it is expressed on T cells, resting B cells, macrophages, DCs, and NK cells.Citation56 BTLA downregulates the activity of lymphocytes after binding to its ligand – the herpesvirus entry mediator (HVEM) molecule. HVEM belongs to the TNF receptor superfamily, whereas BTLA and CD160 are members of the immunoglobulin superfamily.Citation57 The functions and structures of these co-stimulatory molecules are related to positive and negative co-stimulatory pathways.Citation57,Citation58 Binding of BTLA to HVEM inhibits the proliferation of CD8+ T cells, production of proinflammatory cytokines, and formation of memory T cells; at the same time, it promotes peripheral tolerance.Citation59 Studies in the HVEM−/− knockout mouse have shown, however, that immunosuppressive function is preserved in this animal model.Citation60
Novel immune-checkpoint molecules
Novel immune-checkpoint molecules that could be future targets for cancer treatments are being investigated. They include, for example, HHLA2, TMIGD2, B7x, B7 homologue 3 (B7-H3), T-cell immunoglobulin and ITIM domain (TIGIT), CD96, 2B4, and adenosine A2a receptor (A2aR).Citation22,Citation61 Moreover, blockade of the V-domain Ig Suppressor of T cell Activation (VISTA) protein is a promising add-on therapy to PD-1 inhibitors because it inhibits T-cell activation via different pathways than does PD-1.Citation62 In addition to immune-checkpoint inhibition, enhancement of immune-stimulatory pathways (OX40, GITR, and CD40) is considered in cancer treatment. Future studies will show which new molecule will be used to treat cancer along the currently approved CTLA-4 and PHD-1 inhibitors. In our opinion, because of an advanced program of clinical trials, LAG-3 will be the third approved target for immune-checkpoint inhibition.Citation46
Blockade of T-cell dysfunction as a new method of cancer immunotherapy
TCR-mediated antigen recognition is the most important signal for T-cell activation. In addition, there are co-stimulatory and co-inhibitory molecules on the surface of effector T cells that take part in the immune response against tumor cells. Ligands for these molecules are found on the surface of APCs and tumor cells ().Citation63 The interaction between specific cell-surface molecules and their ligands directs lymphocyte response. PD-L1 and PD-L2, both located on the surface of APCs and tumor cells, inhibit lymphocyte activity by binding to PD-1 on the lymphocyte surface.Citation64 The interaction between CD28 on the T-cell surface and CD80 (B7/B7.1) and CD86 (B70/B7-2) on the surface of APCs or tumor cells is crucial for the activation of effector T cells. However, when CD80 and CD86 bind to the cytotoxic T-cell antigen 4 (CTLA-4) instead of CD28, lymphocyte anergy and apoptosis occur.Citation65 In the tumor microenvironment, wherein signals that inhibit effector T cells predominate, tumor cells may avoid immune response.Citation66 Because mAbs against the molecules implicated in T-cell dysfunction may boost the immune response in the tumor microenvironment, these antibodies have a therapeutic potential in certain cancers. Currently, mAbs against the PD-1/PD-L1 pathway, CTLA-4, lymphocyte activation gene 3 (LAG-3), T-cell immunoglobulin and mucin domain 3 (TIM-3), and BTLA are either an approved treatment or are undergoing phase III clinical trials in patients with different cancers; some of these treatments are investigated in preclinical studies.Citation63 presents a list of completed clinical trials of immune-checkpoint inhibitors in patients with cancer.Citation67
Table 2 Completed clinical trials of molecules used to overcome T-cell exhaustion in patients with cancer
Blockade of PD-1 and its ligands
PD-1 and PD-L1 are expressed both on tumor cells and on tumor-specific immune cells.Citation22,Citation23,Citation29 In humans, PD-L1 is expressed by different tumors, and it is a negative prognostic factor in some of them. In cervical cancer, however, PD-L1 expression was associated with a longer overall survival.Citation68 The expression of PD-L1 on tumor cells may be associated with a decreased number of tumor-infiltrating lymphocytes.Citation69 PD-L2 is expressed by colorectal cancer, non-small-cell lung cancer, head and neck squamous cell carcinoma, hepatocellular carcinoma, cervical cancer, and some B-cell leukemias.Citation70 Moreover, PD-1 and its ligands are expressed by immune cells in the tumor microenvironment. In breast cancer, Hodgkin’s lymphoma, and head and neck cancer, PD-1 expression by tumor-infiltrating lymphocytes correlated with tumor size and a lower overall survival.Citation71 Further, increased PD-1 expression was observed on DCs in the tumor microenvironment, which reduced the DC-mediated activation of T cells.Citation72 PD-L1 expression on tumor cells is induced mainly by IFNγ, which is produced by tumor-infiltrating lymphocytes.Citation73 Thus, tumor cells can protect themselves from lymphocytes by expressing PD-L1, which inhibits lymphocyte activation and considerably reduces their efficacy.Citation74 Tumor-associated DCs expressing PD-L1 produce suppressive IL-10.Citation75 In animal studies, the PD-1:PD-L1 interaction enables Treg-mediated suppression of CD8+ T cells in the tumor microenvironment.Citation76 Blockade of the PD-1:PD-L1/2 pathway may increase the therapeutic effectiveness in patients with cancer by reducing the exhaustion of effector T cells.
In 2002, the therapeutic effects of anti-PD-1 antibodies were first observed in mice with PD-L1-positive tumors.Citation77 These and other preclinical findings encouraged phase I clinical trials in patients with cancer.Citation66 In the first clinical trials of an anti-PD-1 mAb (MDX-1106, nivolumab), an objective response was observed in multiple cancer types, including melanoma, non-small-cell lung cancer, and renal cell cancer.Citation78 The drug-related toxicity was acceptable, and the anticancer effect was long term.Citation56 Nivolumab proved effective not only in immunogenic tumors like melanoma and renal cell cancer, but also in non-small-cell lung cancer (considered insensitive to immunotherapy), hepatocellular cancer, metastatic colorectal cancer, squamous cell carcinoma of the head and neck, and urothelial carcinoma.Citation56,Citation79,Citation80 In addition, nivolumab was investigated in patients with hematological malignancies (Hodgkin’s lymphoma) because PD-1 ligands are expressed in these cancers.Citation81 Currently, nivolumab and nivolumab-combined therapies are approved by the US Food and Drug Administration (FDA) for the treatment of melanoma, lung cancer, advanced and metastatic renal cell carcinoma, Hodgkin’s lymphoma, head and neck cancers, urothelial carcinoma, colorectal cancer, and hepatocellular carcinoma ().Citation82
In phase I clinical trials, treatment with pembrolizumab – an anti-PD-1 mAb – was associated with a favorable objective response and a high survival rate in patients with advanced melanoma.Citation83 Further studies showed that pembrolizumab was effective in patients with advanced urothelial carcinoma, gastric cancer, non-small-cell lung cancer, and squamous cell carcinoma of the head and neck.Citation56 Similarly to nivolumab, the effects of pembrolizumab are durable, and the frequency of third- or fourth-level adverse effects related to drug administration is relatively low.Citation84 In a phase III clinical trial, in patients with advanced melanoma, pembrolizumab was associated with a higher survival rate and a higher percentage of objective responses as compared with ipilimumab – an anti-CTLA-4 mAb.Citation85 Currently, pembrolizumab is approved for the treatment of advanced melanoma, advanced or metastatic non-small-cell lung cancer, recurrent or metastatic head and neck squamous cell carcinoma, Hodgkin’s lymphoma, advanced or metastatic urothelial carcinoma, and recurrent locally advanced or metastatic gastric or gastroesophageal junction cancers.Citation82 Treatment with another anti-PD-1 mAb – pidilizumab – showed an encouraging effect in patients with diffuse large B-cell lymphoma (DLBCL) after autologous hematopoietic stem-cell transplantation and with relapsed follicular lymphoma.Citation86,Citation87
The effects of anti-PD-L1 mAbs can differ from those of the anti-PD-1 mAbs because PD-L1 and PD-1 have different ligands.Citation66 BMS-936559 – an anti-PD-L1 mAb – caused an objective and durable response in patients with melanoma, lung cancer, kidney cancer, and ovarian cancer.Citation88 MPDL3280A (atezolizumab), another anti-PD-L1 mAb, proved effective in patients with metastatic urinary bladder cancer.Citation89 Based on favorable outcomes of clinical trials, the FDA approved atezolizumab for the treatment of urothelial carcinoma, certain types of metastatic lung cancer, and bladder cancer.Citation82 In 2017, the FDA approved avelumab – another anti-PD-L1 mAb – for the treatment of metastatic Merkel cell carcinoma and urothelial carcinoma.Citation82 The effectiveness and safety of avelumab are currently under investigation in ovarian cancer, non-small-cell lung cancer, gastric cancer, and mesothelioma.Citation90 Darvulumab, an anti-PD-L1 mAb, was recently approved for the treatment of patients with advanced bladder cancer and for unresectable stage III non-small-cell lung cancer.Citation82 Currently, the effectiveness of mAbs against the PD-1:PD-L1/2 pathway is being investigated in patients with nearly 250 different neoplastic disorders.Citation67 Although several anti-PD-1 and ant-PD-L1 mAbs are approved, they have similar efficacy and toxicity profiles.Citation91,Citation92
Blockade of CTLA-4
At the end of the 20th century, studies showed that removing signals that blocked co-stimulation led to a stronger anticancer response. In mice with immunogenic colorectal cancer, treatment with anti-CTLA-4 mAbs before the transfer of tumor cells prevented disease development, mostly due to the activation of CD8+ T cells. Moreover, anti-CTLA-4 mAbs caused cancer regression in mice with developed tumors, including weakly immunogenic tumors. This treatment led to the formation of immunological memory against tumor cells.Citation93,Citation94 Such encouraging preclinical findings prompted clinical trials with anti-CTLA-4 mAbs in patients with various neoplastic diseases.Citation95 The effectiveness of ipilimumab, an anti-CTLA-4 mAb, was investigated both as a standalone treatment and in combination with other treatments (IL-2, melanoma vaccine, and chemotherapy).Citation96,Citation97 In 2011, ipilimumab was approved by the FDA for the treatment of patients with advanced melanoma. Subsequently, in 2015, ipilimumab was approved as an adjuvant treatment in patients with melanoma after surgery and, in 2017, as a treatment for children with advanced melanoma.Citation82,Citation99 Ipilimumab is a promising treatment for relapsed and refractory B-cell non-Hodgkin lymphomas, metastatic renal cell carcinoma, small-cell and non-small-cell lung cancer, prostate cancer, urothelial carcinoma, and ovarian cancer.Citation100 In phase I and phase II clinical trials in patients with metastatic melanoma, tremelimumab (ticilimumab), also an anti-CTLA-4 antibody, was associated with a durable tumor regression.Citation101 Subsequently, the effect of tremelimumab was shown in patients with advanced gastric and esophageal adenocarcinoma, colorectal carcinoma, non-small-cell lung cancer, and malignant mesothelioma.Citation63 Treatment with anti-CTLA-4 mAbs such as ipilimumab and tremelimumab is associated with significant immune-related adverse effects.Citation84 A high incidence of immune-related adverse events of anti-CTLA-4 treatments is likely due to the depletion of Treg cells and a systemic activation of autoimmune T cells in the lymphoid tissue.Citation56 Currently, approximately 300 clinical trials are investigating the effectiveness of ipilimumab and ipilimumab-combined therapies, and 100 clinical trials are investigating the effectiveness of tremelimumab and tremelimumab-combined therapies.Citation67
Blockade of LAG-3
In ovarian and prostate cancers, LAG-3 is expressed by CD8+ tumor-specific T cells that co-express PD-1, which suggests that LAG-3 might be implicated in the formation of an immunosuppressive tumor microenvironment.Citation102 In preclinical trials, LAG-3 blockade with mAbs was investigated as a standalone therapy and combined with anti-PD-1 and anti-CTLA-4 mAbs.Citation103 In mice, the blockade of either PD-1 or LAG-3 did not effectively inhibit tumor development after transfer of cancer cells, but a dual blockade was more effective and was associated with higher percentages of T CD8+/IFNγ+ and CD4+ T cells.Citation104 In another study, dual PD-1 and LAG-3 blockade caused a considerable tumor regression in all treated mice.Citation105 A triple blockade of PD-1, CTLA-4, and LAG-3 significantly increased the effectiveness of cytotoxic T lymphocytes injected to mice with leukemia.Citation106 However, targeting of multiple T-cell inhibitory molecules might be associated with an increased incidence of autoimmune adverse events.Citation107 To date, two approaches to inhibit LAG-3 signaling have been developed: a LAG-3-Ig fusion protein and anti-LAG-3 mAbs (IMP321, LAG525, IMP701, TSR-033, REGN3767, and BMS-986016).Citation22,Citation108 Inhibition of LAG-3 may be effective not only due to the enhancement of Th1 responses, but also due to the stimulation of DC maturation, in which IL-12 is implicated.Citation22 The effectiveness of IMP321 was shown in phase I clinical trials in patients with breast cancer, renal cell carcinoma, and pancreatic cancer.Citation109–Citation111 Currently, approximately 20 clinical trials are investigating anti-LAG-3 mAbs as a standalone treatment or combined with other therapies; bispecific proteins binding to PD-1 and LAG-3 are investigated in different metastatic cancers, small-cell lung cancer, gastrointestinal cancers, virus-associated tumors, hematologic neoplasms, brain tumors, and melanoma.Citation67
Blockade of TIM-3
TIM-3-blocking mAbs enhance T-cell proliferation and increase cytokine production, which explains their antitumor activity.Citation22 Tim-3+ tumor-infiltrating Tregs can greatly inhibit the proliferation of naïve T cells.Citation112 In mice, anti-TIM-3 mAbs trigger an anticancer response, which is dependent mostly on CD8+ T cells that secrete IFNγ and on CD4+ T cells. Although a substantial proportion of tumor-infiltrating CD4+TIM-3+ cells co-express Foxp3, the role of TIM-3 in Treg signaling remains unknown.Citation113 In a mouse model of hepatitis B, TIM-3 blockade was associated with increased production of IFNγ by CD8+ cells.Citation114 Anti-TIM-3 antibodies slowed tumor growth in mice, which was associated with a decreased percentage of exhausted TIM-3+ lymphocytes.Citation115 A more potent anticancer response was observed when anti-TIM-3 mAbs were given in combination with anti-PD-1 or anti-CTLA-4 mAbs, when compared with the individual effects of these antibodies.Citation116 The presence of TIM-3+ T cells correlates with disease severity and poor prognosis in patients with non–small-cell lung carcinoma and follicular lymphoma.Citation22,Citation117 In contrast, expression of galectin-9 – the main TIM-3 ligand – is associated with a favorable outcome in many solid tumors, which suggests that galectin-9 may have other effects in cancer than those associated with TIM-3 signaling.Citation118 Currently, anti-TIM-3 mAbs (MBG453, Sym023, TSR-022, and LY3321367) are being investigated in phase I and II clinical trials in patients with advanced malignancies, including leukemia; these treatments will be investigated in patients with solid tumors and lymphomas from June 2018 (six clinical trials).Citation22,Citation67
Blockade of BTLA
Tumor cells change the BTLA/HVEM signaling by either promoting the development of dysfunctional T cells with persistent BTLA expression (cells susceptible to inactivation) or by expressing HVEM – for example, in melanoma.Citation22,Citation119 In patients with advanced melanoma, BTLA is expressed by tumor-specific CD8+ T cells; moreover, an in vitro BTLA blockade of melanoma-specific CD8+ T cells increased their proliferation and secretion of IL-2, IFNγ, and TNFα; these effects were even greater with a triple blockade (anti-BTLA, anti-PD-1, and anti-TIM-3).Citation59 Both BTLA and HVEM are expressed by tumor cells and T-follicular helper cells in patients with chronic lymphocytic leukemia. These findings might direct the development of future immunotherapies.Citation120 BTLA and HVEM are investigated as treatment targets in preclinical studies.Citation56
The place of immune-checkpoint inhibitors in cancer treatment
Although several immune-checkpoint inhibitors are now available in clinical practice, the place of cancer immunotherapy is unclear. It is not often evident which patients will benefit from immune treatments more than from standard therapies. The choice between the immune and standard cancer treatments is even more difficult because the immune treatments are associated with a new class of adverse effects.
Based on the available data, a significant proportion of patients do not respond to treatment with immune-checkpoint inhibitors. Among patients with advanced melanoma, less than 20% respond to ipilimumab, approximately one third respond to pembrolizumab, and less than a half respond to nivolumab.Citation121–Citation123 When anti-CTLA-4 (ipilimumab) and anti-PD-1 (nivolumab) treatments are given in combination, the response rate increases to approximately 60%.Citation124 The same treatment combination is associated with a response rate of approximately 40%–50% among patients with lung cancer.Citation125 These observations suggest non-redundant effects of CTLA-4 and PD-1 blockade.Citation123 For instance, T cells expressing CTLA-4 are found predominantly in secondary lymphoid organs, whereas PD-1 expression is characteristic for T cells in the tumor microenvironment.Citation126 Moreover, CTLA-4 targets mostly recently primed cells, and PD-1 targets primarily effector T cells.Citation126 Furthermore, CTLA-4 and PD-1 have different intracellular signaling pathways ().
It is important to establish predictive factors for selecting patients who will most likely benefit from immunotherapy. In general, it is believed that patients with tumors that are well infiltrated by immune cells (hot tumors) respond to immunotherapy better than patients with tumors that display scarce immune infiltration (cold tumors). Infiltration of tumors by immune cells depends both on tumor immunogenicity and on host immune function. For example, immunogenic tumors – that is, those characterized by a high mutational load and, thus, a high neoantigen load – respond well to immune-checkpoint inhibitors.Citation126 Similarly, high counts of circulating immune cells with proliferative potential (CD8+ Ki67+), relative to tumor burden, are associated with a favorable response to immune-checkpoint inhibition.Citation127 Moreover, expression of immune-checkpoint ligands by tumors seems important, because immune-checkpoint inhibitors are thought to act by competing with its ligands. Indeed, high concentrations of PD-L1 are associated with a favorable clinical response to PD-1 blockade.Citation128,Citation129 In line with this observation, in patients with urothelial cancer who have a low PD-L1 status, the effects of PD-1 blockade are worse than those of chemotherapy.Citation130 Other predictors of clinical response to immune-checkpoint inhibitors are being investigated. For example, a recent study showed that patients with melanoma who responded to anti-PD-1 immunotherapy had different gut microbiome than did non-responders.Citation131
Immune-checkpoint inhibitors are associated with a new class of immune-mediated adverse events. In general, immune-related adverse effects occur more commonly with anti-CTLA-4 blockade (~50%) than with anti-PD-1 blockade (~25%).Citation132 Moreover, the frequency of immune-related adverse effects is higher with a combination of CTLA-4 and PD-1 blockade. However, patients with advanced cancer seem to better tolerate PD-1/PD-L1 blockade than chemotherapy.Citation133 The immune-related adverse effects are due to immune overactivation, and they include skin changes, diarrhea related to colitis, hepatotoxicity, pneumonitis, and different endocrinopathies such as autoimmune thyroid disease (hypothyroidism and hyperthyroidism), hypophysitis, adrenal insufficiency, and type 1 diabetes mellitus. These adverse effects are usually managed with glucocorticoids, which, in turn, may cause infections such as tuberculosis.Citation134 The adverse effects of immune-checkpoint inhibitors are often severe and lead to treatment discontinuation. For example, approximately half the patients who received adjuvant ipilimumab after surgery for melanoma discontinued treatment due to adverse effects.Citation135 Thus, the adverse effects of immune-checkpoint inhibitors should be weighed against their expected benefit, particularly when considering combined CTLA-4 and PD-1 blockade. Although this combined treatment is more effective than its individual components, it is associated with the highest risk of immune-related adverse effects.
Conclusion
In most advanced cancers, chemotherapy is possibly approaching or has already reached the greatest possible therapeutic effect. The different methods used to overcome T-cell dysfunction have proved effective in some cancers, and this approach might replace chemotherapy in the future. Treatments aimed at boosting immune function have several advantages over other treatments, such as a relatively short treatment period (several weeks). Moreover, they do not need to be specifically prepared for each individual patient (like DC vaccines). Some of these treatments are already approved on the basis of encouraging outcomes of clinical trials. Cancer immunotherapy has lower toxicity compared with chemotherapy; in some cancers, it may achieve a long-term disease control. Currently, finding reliable response factors to immunotherapy is crucial to properly select the best treatment for each patient. Treatment with mAbs that boost immune function, particularly a simultaneous use of antibodies that target different mechanisms of T-cell exhaustion, in combination with other treatments, shows the greatest promise for patients with cancer, including those with cancer resistant to standard therapies.
Acknowledgments
This work was supported by the Polish National Science Center (grant no UMO-2016/21/B/NZ6/02279) and the Medical University of Lublin (grant no DS460). The sponsors had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- GoralSThe three-signal hypothesis of lymphocyte activation/targets for immunosuppressionDial Transplant20114011416
- QiXJiaBZhaoXYuDAdvances in T-cell checkpoint immunotherapy for head and neck squamous cell carcinomaOncoTargets Ther20171057455754
- PaukenKEWherryEJOvercoming T cell exhaustion in infection and cancerTrends Immunol201536426527625797516
- ThommenDSSchumacherTNT Cell Dysfunction in CancerCancer Cell201833454756229634943
- WherryEJKurachiMMolecular and cellular insights into T cell exhaustionNat Rev Immunol201515848649926205583
- MuellerSNAhmedRHigh antigen levels are the cause of T cell exhaustion during chronic viral infectionProc Natl Acad Sci U S A2009106218623862819433785
- WłasiukPPutowskiMGiannopoulosKPD1/PD1L pathway, HLA-G and T regulatory cells as new markers of immunosuppression in cancersPostępy Hig Med Dośw (online)2016701044105827708209
- BalkhiMYMaQAhmadSJunghansRPT cell exhaustion and Interleukin 2 downregulationCytokine201571233934725516298
- ChłopekMKowalikAGóźdźSKoziakKThe role of interleukin 15 in neoplasiaPostepy Hig Med Dosw (online)201771051928181907
- GrenierJMYeungSTKhannaKMCombination Immunotherapy: Taking Cancer Vaccines to the Next LevelFront Immunol2018961029623082
- CruszSMBalkwillFRInflammation and cancer: advances and new agentsNat Rev Clin Oncol2015121058459626122183
- KumarRYuFZhenYHPD-1 blockade restores impaired function of ex vivo expanded CD8+ T cells and enhances apoptosis in mismatch repair deficient EpCAM+PD-L1+OncoTargets Ther20171034533465
- HaSJWestEEArakiKSmithKAAhmedRManipulating both the inhibitory and stimulatory immune system towards the success of therapeutic vaccination against chronic viral infectionsImmunol Rev2008223131733318613845
- CrespoJSunHWellingTHTianZZouWT cell anergy, exhaustion, senescence, and stemness in the tumor microenvironmentCurr Opin Immunol201325221422123298609
- EleftheriadisTAntoniadiGLiakopoulosVKortsarisAT-Cell Zeta Chain Expression, Phosphorylation and Degradation and their Role in T-Cell Signal Transduction and Immune Response Regulation in Health And DiseaseCurr Signal Transduct Ther200612191208
- TorelliGFPaoliniRTatarelliCDefective expression of the T-cell receptor-CD3 zeta chain in T-cell acute lymphoblastic leukaemiaBr J Haematol2003120220120812542476
- CornwellWDRogersTJUncoupling of T cell receptor zeta chain function during the induction of anergy by the superantigen, staphylococcal enterotoxin AToxins2010271704171722069657
- IshidaYAgataYShibaharaKHonjoTInduced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell deathEmbo J19921111388738951396582
- OkazakiTMaedaANishimuraHKurosakiTHonjoTPD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosineProc Natl Acad Sci U S A20019824138661387111698646
- PatsoukisNSariDBoussiotisVAPD-1 inhibits T cell proliferation by upregulating p27 and p15 and suppressing Cdc25ACell Cycle201211234305430923032366
- KruegerJRuddCETwo Strings in One Bow: PD-1 Negatively Regulates via Co-receptor CD28 on T CellsImmunity201746452953128423334
- Marin-AcevedoJADholariaBSoyanoAEKnutsonKLChumsriSLouYNext generation of immune checkpoint therapy in cancer: new developments and challengesJ Hematol Oncol20181113929544515
- ChemnitzJMParryRVNicholsKEJuneCHRileyJLSHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activationJ Immunol2004173294595415240681
- LichteneggerFSRotheMSchnorfeilFMTargeting LAG-3 and PD-1 to Enhance T Cell Activation by Antigen-Presenting CellsFront Immunol2018938529535740
- WongRMSmithKATamVLTLR-9 signaling and TCR stimulation co-regulate CD8+ T cell-associated PD-1 expressionImmunol Lett20091271606719751765
- HeimLFriedrichJEngelhardtMNFATc1 promotes anti-tumoral effector functions and memory CD8+ T cell differentiation during non-small cell lung cancer developmentCancer Res201878133619363329691251
- FreemanGJLongAJIwaiYEngagement of the Pd-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte ActivationJ Exp Med200019271027103411015443
- GianchecchiEDelfinoDVFierabracciARecent insights into the role of the PD-1/PD-L1 pathway in immunological tolerance and autoimmunityAutoimmun Rev201312111091110023792703
- LiechtensteinTDufaitIBricogneCPD-L1/PD-1 Co-Stimulation, a Brake for T cell Activation and a T cell Differentiation SignalJ Clin Cell Immunol2012S12
- KeirMELatchmanYEFreemanGJSharpeAHProgrammed death-1 (PD-1):PD-ligand 1 interactions inhibit TCR-mediated positive selection of thymocytesJ Immunol2005175117372737916301644
- PaukenKEJenkinsMKAzumaMFifeBTPD-1, but Not PD-L1, Expressed by Islet-Reactive CD4+ T Cells Suppresses Infiltration of the Pancreas During Type 1 DiabetesDiabetes20136282859286923545706
- PiantaSMagattiMVertuaEAmniotic mesenchymal cells from pre-eclamptic placentae maintain immunomodulatory features as healthy controlsJ Cell Mol Med201620115716926515425
- HiroseTTanakaYTanakaAPD-L1/PD-L2-expressing B-1 cells inhibit alloreactive T cells in micePLoS One2017126e017876528570665
- NieXChenWZhuYB7-DC (PD-L2) costimulation of CD4+ T-helper 1 response via RGMbCell Mol Immunol Epub20175828782755
- ZhongXTumangJRGaoWBaiCRothsteinTLPD-L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for V(H)11/V(H)12 and phosphatidylcholine bindingEur J Immunol20073792405241017683117
- SaundersPAHendrycksVRLidinskyWAWoodsMLPD-L2:PD-1 involvement in T cell proliferation, cytokine production, and integrin-mediated adhesionEur J Immunol200535123561356916278812
- Ramos-HernándezNRamonHEBealAMLarocheADeklevaEAOliverPMNdfip1 enforces a requirement for CD28 costimulation by limiting IL-2 productionJ Immunol201319141536154623851689
- FranciscoLMSalinasVHBrownKEPD-L1 regulates the development, maintenance, and function of induced regulatory T cellsJ Exp Med2009206133015302920008522
- SakaguchiSWingKOnishiYPrieto-MartinPYamaguchiTRegulatory T cells: how do they suppress immune responses?Int Immunol200921101105111119737784
- TaiXvan LaethemFPobezinskyLBasis of CTLA-4 function in regulatory and conventional CD4+ T cellsBlood2012119225155516322403258
- KlepschVHermann-KleiterNBaierGBeyond CTLA-4 and PD-1: Orphan nuclear receptor NR2F6 as T cell signaling switch and emerging target in cancer immunotherapyImmunol Lett2016178313626992368
- CarrenoBMBennettFChauTACTLA-4 (CD152) can inhibit T cell activation by two different mechanisms depending on its level of cell surface expressionJ Immunol200016531352135610903737
- CiszakLFrydeckaIWolowiecDSzteblichAKosmaczewskaACTLA-4 affects expression of key cell cycle regulators of G0/G1 phase in neoplastic lymphocytes from patients with chronic lymphocytic leukaemiaClin Exp Med201616331733226003188
- ZhangZMYangXMZhangCAntitumor effects and mechanisms of dendritic cells stimulated by sCD40L on ovarian cancer cells in vitroOncoTargets Ther20136503515
- DyckLMillsKHGImmune checkpoints and their inhibition in cancer and infectious diseasesEur J Immunol201747576577928393361
- AndrewsLPMarciscanoAEDrakeCGVignaliDAALAG3 (CD223) as a cancer immunotherapy targetImmunol Rev20172761809628258692
- GrossoJFKelleherCCHarrisTJLAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systemsJ Clin Invest2007117113383339217932562
- HuangCTWorkmanCJFliesDRole of LAG-3 in Regulatory T CellsImmunity200421450351315485628
- GrossoJFGoldbergMVGetnetDFunctionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cellsJ Immunol2009182116659666919454660
- LajkoAMeggyesMPolgarBSzeredayLThe immunological effect of Galectin-9/TIM-3 pathway after low dose Mifepristone treatment in mice at 14.5 day of pregnancyPLoS One2018133e019487029566059
- NgiowSFTengMWLSmythMJProspects for TIM3-Targeted Antitumor ImmunotherapyCancer Res201171216567657122009533
- KashioYNakamuraKAbedinMJGalectin-9 induces apoptosis through the calcium-calpain-caspase-1 pathwayJ Immunol200317073631363612646627
- ZhuCAndersonACSchubartAThe Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunityNat Immunol20056121245125216286920
- KoohiniZHossein-NatajHMobiniMHosseinian-AmiriARafieiAAsgarian-OmranHAnalysis of PD-1 and Tim-3 expression on CD4+ T cells of patients with rheumatoid arthritis; negative association with DAS28Clin Rheumatol20183782063207129626269
- PengP-JiLiYSunSOn the significance of Tim-3 expression in pancreatic cancerSaudi J Biol Sci20172481754175729551917
- TorphyRSchulickRZhuYNewly Emerging Immune Checkpoints: Promises for Future Cancer TherapyInt J Mol Sci201718122642
- WangZWangKYangHAssociations between HVEM/LIGHT/BTLA/CD160 polymorphisms and the occurrence of antibody-mediate rejection in renal transplant recipientsOncotarget201785910007910009429245962
- PilatNSayeghMHWekerleTCostimulatory pathways in transplantationSemin Immunol201123429330321616680
- FourcadeJSunZPaglianoOCD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1Cancer Res201272488789622205715
- PaseroCOliveDInterfering with coinhibitory molecules: BTLA/HVEM as new targets to enhance anti-tumor immunityImmunol Lett20131511–2717523439006
- JanakiramMShahUALiuWZhaoASchoenbergMPZangXThe third group of the B7-CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7-H3Immunol Rev20172761263928258693
- LiuJYuanYChenWImmune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responsesProc Natl Acad Sci U S A2015112216682668725964334
- CatakovicKKlieserENeureiterDGeisbergerRT cell exhaustion: from pathophysiological basics to tumor immunotherapyCell Commun Signal2017151128073373
- ShaoYZhuWDaJBisdemethoxycurcumin in combination with alpha-PD-L1 antibody boosts immune response against bladder cancerOncoTargets Ther20171026752683
- LiuQHuPDengGSoluble cytotoxic T-lymphocyte antigen 4: a favorable predictor in malignant tumors after therapyOncoTargets Ther20171021472154
- SwatlerJKozłowskaEImmune checkpoint-targeted cancer immunotherapiesPostępy Hig Med Dośw (online)201670254226864062
- Clinical Trials Available from: https://clinicaltrials.gov/ct2/results?cond=&term=immune+checkpoint+inhibitor&cntry=&state=&city=&dist=Accessed July 28, 2018
- KarimRJordanovaESPiersmaSJTumor-Expressed B7-H1 and B7-DC in Relation to PD-1+ T-Cell Infiltration and Survival of Patients with Cervical CarcinomaClin Cancer Res200915206341634719825956
- LarbcharoensubNMahapromKJiarpinitnunCCharacterization of PD-L1 and PD-1 Expression and CD8 Tumor-infiltrating Lymphocyte in Epstein-Barr Virus-associated Nasopharyngeal CarcinomaAm J Clin Oncol Epub201841826270441
- YearleyJHGibsonCYuNPD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in CancerClin Cancer Res201723123158316728619999
- MuenstSSoysalSDGaoFObermannECOertliDGillandersWEThe presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancerBreast Cancer Res Treat2013139366767623756627
- ChenCLPanQZWengDSSafety and activity of PD-1 blockade-activated DC-CIK cells in patients with advanced solid tumorsOncoimmunology201874e141772129632736
- KodumudiKNSiegelJWeberAMScottESarnaikAAPilon-ThomasSImmune Checkpoint Blockade to Improve Tumor Infiltrating Lymphocytes for Adoptive Cell TherapyPLoS One2016114e015305327050669
- TaubeJMAndersRAYoungGDColocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escapeSci Transl Med20124127ra37
- LamichhanePKaryampudiLShreederBIL10 Release upon PD-1 Blockade Sustains Immunosuppression in Ovarian CancerCancer Res201777236667667828993412
- DongLZhengXWangKWangGZouHPD-1/PD-L1 pathway participates in gastric surgery-induced imbalance of Th17/Treg cells in miceJ Trauma Acute Care Surg201885354955929554041
- IwaiYIshidaMTanakaYOkazakiTHonjoTMinatoNInvolvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockadeProc Natl Acad Sci U S A20029919122931229712218188
- BrahmerJRDrakeCGWollnerIPhase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlatesJ Clin Oncol201028193167317520516446
- TopalianSLHodiFSBrahmerJRSafety, Activity, and Immune Correlates of Anti–PD-1 Antibody in CancerN Engl J Med Overseas Ed20123662624432454
- OvermanMJMcDermottRLeachJLNivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 studyLancet Oncol20171891182119128734759
- AnsellSMLesokhinAMBorrelloIPD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin’s LymphomaN Engl J Med Overseas Ed20153724311319
- Resources for Information on Approved Drugs Available from: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/default.htmAccessed July 28, 2018
- RobertCRibasAWolchokJDAnti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trialThe Lancet2014384994811091117
- SosaALopez CadenaESimon OliveCKarachaliouNRosellRClinical assessment of immune-related adverse eventsTher Adv Med Oncol201810175883591876462
- RobertCSchachterJLongGVPembrolizumab versus Ipilimumab in Advanced MelanomaN Engl J Med Overseas Ed20153722625212532
- ArmandPNaglerAWellerEADisabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trialJ Clin Oncol201331334199420624127452
- WestinJRChuFZhangMSafety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trialLancet Oncol2014151697724332512
- BrahmerJRTykodiSSChowLQMSafety and Activity of Anti–PD-L1 Antibody in Patients with Advanced CancerN Engl J Med Overseas Ed20123662624552465
- InmanBALongoTARamalingamSHarrisonMRAtezolizumab: A PD-L1-Blocking Antibody for Bladder CancerClin Cancer Res20172381886189027903674
- KellyKInfanteJRTaylorMHSafety profile of avelumab in patients with advanced solid tumors: A pooled analysis of data from the phase 1 JAVELIN solid tumor and phase 2 JAVELIN Merkel 200 clinical trialsCancer201812492010201729469949
- PillaiRNBeheraMOwonikokoTKComparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: A systematic analysis of the literatureCancer2018124227127728960263
- PrasadVKaestnerVNivolumab and pembrolizumab: Monoclonal antibodies against programmed cell death-1 (PD-1) that are interchangeableSemin Oncol201744213213528923211
- LeachDRKrummelMFAllisonJPEnhancement of Antitumor Immunity by CTLA-4 BlockadeScience19962715256173417368596936
- van ElsasAHurwitzAAAllisonJPCombination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentationJ Exp Med1999190335536610430624
- MakerAVPhanGQAttiaPTumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II studyAnn Surg Oncol200512121005101616283570
- WolchokJDHodiFSWeberJSDevelopment of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanomaAnn N Y Acad Sci20131291111323772560
- O’DaySJMaioMChiarion-SileniVEfficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase {II} studyAnn Oncol20102181712171720147741
- PostowMAYuanJKitanoSLesokhinAMWolchokJDMarkers for anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) therapy in melanomaMethods Mol Biol20141102839524258975
- DrénoBIpilimumab more and more discussed: Urgent need for predictive markers of responseJ Eur Acad Dermatology Venereol2018326849
- WangRFWangHYImmune targets and neoantigens for cancer immunotherapy and precision medicineCell Res2017271113728025978
- RibasAClinical development of the anti-CTLA-4 antibody tremelimumabSemin Oncol201037545045421074059
- CappucciniFPollockEStribblingSHillAVSRedchenkoI5T4 oncofoetal glycoprotein: an old target for a novel prostate cancer immunotherapyOncotarget2017829474744748928537896
- HellmannMDFriedmanCFWolchokJDCombinatorial cancer immunotherapiesAdv Immunol201613025127726923003
- WooSRTurnisMEGoldbergMVImmune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escapeCancer Res201272491792722186141
- FoySPSenninoBdela CruzTPoxvirus-Based Active Immunotherapy with PD-1 and LAG-3 Dual Immune Checkpoint Inhibition Overcomes Compensatory Immune Regulation, Yielding Complete Tumor Regression in MicePLoS One2016112e015008426910562
- Berrien-ElliottMMJacksonSRMeyerJMDurable adoptive immunotherapy for leukemia produced by manipulation of multiple regulatory pathways of CD8+ T-cell toleranceCancer Res201373260561623188506
- PardollDMThe blockade of immune checkpoints in cancer immunotherapyNat Rev Cancer201212425226422437870
- SharmaPAllisonJPImmune checkpoint targeting in cancer therapy: toward combination strategies with curative potentialCell2015161220521425860605
- BrignoneCEscudierBGrygarCMarcuMTriebelFA phase I pharmacokinetic and biological correlative study of IMP321, a novel MHC class II agonist, in patients with advanced renal cell carcinomaClin Cancer Res200915196225623119755389
- BrignoneCGutierrezMMeftiFFirst-line chemoimmunotherapy in metastatic breast carcinoma: combination of paclitaxel and IMP321 (LAG-3Ig) enhances immune responses and antitumor activityJ Transl Med2010817120653948
- Wang-GillamAPlambeck-SuessSGoedegebuurePA phase I study of IMP321 and gemcitabine as the front-line therapy in patients with advanced pancreatic adenocarcinomaInvest New Drugs201331370771322864469
- LiuZMcMichaelELShayanGNovel effector phenotype of Tim-3 regulatory T cells leads to enhanced suppressive function in head and neck cancer patientsClin Cancer Res201824184529453829712685
- DuWYangMTurnerATIM-3 as a Target for Cancer Immunotherapy and Mechanisms of ActionInt J Mol Sci2017183645
- JuYHouNZhangXNBlockade of Tim-3 pathway ameliorates interferon-gamma production from hepatic CD8+ T cells in a mouse model of hepatitis B virus infectionCell Mol Immunol200961354319254478
- NgiowSFvon ScheidtBAkibaHYagitaHTengMWSmythMJAnti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumorsCancer Res201171103540355121430066
- Perez-GraciaJLLabianoSRodriguez-RuizMESanmamedMFMeleroIOrchestrating immune check-point blockade for cancer immunotherapy in combinationsCurr Opin Immunol201427899724485523
- AndersonACJollerNKuchrooVKLag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune RegulationImmunity2016445989100427192565
- ZhouXSunLJingDGalectin-9 Expression Predicts Favorable Clinical Outcome in Solid Tumors: A Systematic Review and Meta-AnalysisFront Physiol2018945229765332
- PaulosCMJuneCHPutting the brakes on BTLA in T cell-mediated cancer immunotherapyJ Clin Invest20101201768020038807
- M’HidiHThibultMLChetailleBHigh expression of the inhibitory receptor BTLA in T-follicular helper cells and in B-cell small lymphocytic lymphoma/chronic lymphocytic leukemiaAm J Clin Pathol2009132458959619762537
- FellnerCIpilimumabFCIpilimumab (yervoy) prolongs survival in advanced melanoma: serious side effects and a hefty price tag may limit its useP T201237950353023066344
- RibasAHamidODaudAAssociation of Pembrolizumab With Tumor Response and Survival Among Patients With Advanced MelanomaJAMA201631515160027092830
- LarkinJChiarion-SileniVGonzalezRCombined Nivolumab and Ipilimumab or Monotherapy in Untreated MelanomaN Engl J Med20153731233426027431
- LongGVAtkinsonVCebonJSStandard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): an open-label, phase 1b trialLancet Oncol20171891202121028729151
- HellmannMDRizviNAGoldmanJWNivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort studyLancet Oncol2017181314127932067
- ZappasodiRMerghoubTWolchokJDEmerging Concepts for Immune Checkpoint Blockade-Based Combination TherapiesCancer Cell201833458159829634946
- HuangACPostowMAOrlowskiRJT-cell invigoration to tumour burden ratio associated with anti-PD-1 responseNature20175457652606528397821
- HerbstRSSoriaJCKowanetzMPredictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patientsNature2014515752856356725428504
- GaronEBRizviNAHuiRPembrolizumab for the treatment of non-small-cell lung cancerN Engl J Med2015372212018202825891174
- Safety Alerts for Human Medical ProductsKeytruda (pembrolizumab) or Tecentriq (atezolizumab): FDA Alerts Health Care Professionals and Investigators: FDA Statement – Decreased Survival in Some Patients in Clinical Trials Associated with Monotherapy Available from: https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm608253.htmAccessed July 28, 2018
- GopalakrishnanVSpencerCNNeziLGut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patientsScience201835963719710329097493
- El OstaBHuFSadekRChintalapallyRTangSCNot all immune-checkpoint inhibitors are created equal: Meta-analysis and systematic review of immune-related adverse events in cancer trialsCrit Rev Oncol Hematol201711911229065979
- NishijimaTFShacharSSNyropKAMussHBSafety and Toler-ability of PD-1/PD-L1 Inhibitors Compared with Chemotherapy in Patients with Advanced Cancer: A Meta-AnalysisOncologist201722447047928275115
- PicchiHMateusCChouaidCInfectious complications associated with the use of immune checkpoint inhibitors in oncology: reactivation of tuberculosis after anti PD-1 treatmentClin Microbiol Infect201824321621829269089
- EggermontAMChiarion-SileniVGrobJJAdjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trialLancet Oncol201516552253025840693
