Abstract
Epidermal growth factor receptor-tyrosine kinase inhibitors have improved progression-free survival and overall survival in non-small-cell lung cancer (NSCLC) patients with sensitive mutations. However, response of uncommon mutation to epidermal growth factor receptor-tyrosine kinase inhibitors is still unclear. S768I is one of the uncommon mutations. A female patient with advanced NSCLC with a single S768I mutation achieved effectiveness from afatinib after showing no response to gefitinib. The patient had progression-free survival after taking afatinib for 6 months, and her follow-up is continuing. It suggests that afatinib may be a more effective treatment for NSCLC patients with a single S768I mutation, compared to first-generation tyrosine kinase inhibitors.
Introduction
Lung cancer is the leading cause of cancer deaths according to GLOBOCAN estimates and has caused stressful burden on the society.Citation1 Cancer Statistics in China revealed similar phenomenon.Citation2 In recent years, epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) have improved the progression-free survival (PFS) in non-small-cell lung cancer (NSCLC) patients with sensitive mutations, compared to traditional chemotherapyCitation3–Citation5 (9.2–13.1 vs 4.6–6.3 months). The sensitive mutations include deletions in exon 19 or L858R mutations in exon 21, which account for 85% of all epidermal growth factor receptor (EGFR) mutations and are associated with sensitivity to EGFR-TKIs.Citation6,Citation7 Different generations of EGFR-TKIs showed no significant differences in sensitive mutations. Meanwhile, the incidence rates of uncommon mutation such as G719X, S768I, L861Q, and exon 20 insertion mutations are 4%–13%,Citation8 and their response effects to EGFR-TKIs remain unclear.Citation8–Citation10
Exon 20 p.S768I mutation is one of the uncommon mutations. Previous reports contradict on which generation has a better effect on S768I mutation.Citation8–Citation10 LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6 studiesCitation11 showed that afatinib (Boehringer Ingelheim Pharmaceutical Co., Ingelheim, Germany) revealed benefits among eight patients who carried S768I mutation. However, only one case had the single S768I mutation. Here, we report a case of afatinib’s response in an advanced NSCLC female patient who failed treatment with gefitinib (AstraZeneca plc, London, UK).
Case report
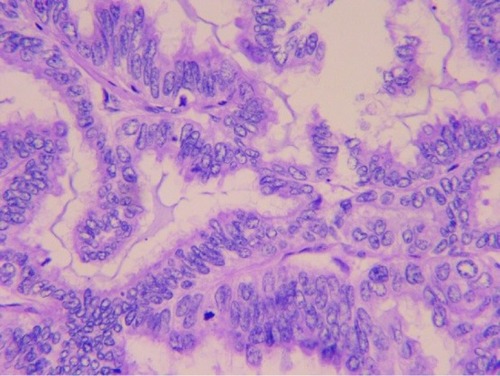
In March 2013, a 52-year-old Chinese female with no smoking history had sudden coughs with bloody sputum and chest pain. Positron emission tomography-computed tomography (CT) taken in Peking Union Medical College Hospital showed a mass in the left upper lung sized 39×49 mm2 and the standard uptake value was 5.8. Meanwhile, several masses sized 8 mm in the mediastinum were observed without increased radioactive uptake. On April 16, 2013, upper left lung resection and mediastinal lymph node dissection were performed. Finally, the patient was diagnosed with lung adenocarcinoma on the left upper lobe with stage IIa (pT2aN1M0), as shown in . Molecular pathology suggested EGFR exon 20 p.S768I mutation (2303G>T). Also, in her family, her father and one uncle died of lung cancer and another uncle died of kidney cancer.
From June to October 9, 2013, the patient was treated with adjuvant chemotherapy in another hospital (pemetrexed plus cisplatin, but the dose was unknown) for four therapy circles. Chest CT scan showed no recurrence. However, on October 10, 2014, a regular chest CT scan showed a new mass with a diameter of 10 mm in the left upper lung and several new masses in the right lung with a maximum diameter of 4 mm, that is, metastasis in mediastinal 4R, 4L, six regions, and left pleural effusion.
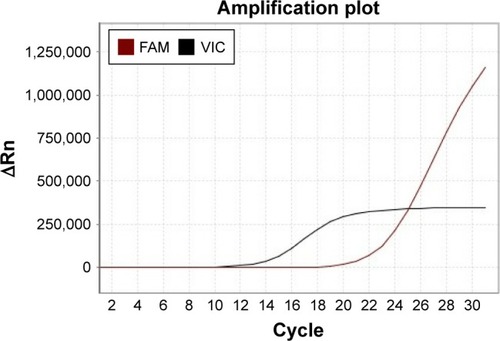
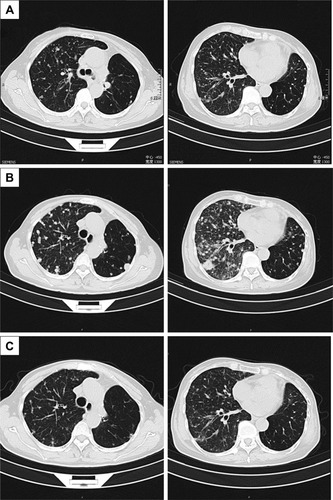
From October 2014 to December 2016, the patient accepted four-line therapies with chemotherapies and bevacizumab. Among the treatments, the fourth line treatment maintained 15 cycles and the patient benefited the longest PFS lasting for 14 months (). On December 30, 2016, a circulating tumor DNA liquid biopsy by the Amplification Refractory Mutation System was performed. And the result was the same as the surgical specimen two years ago (). Because afatinib was not available in China at that time, we recommended the first-generation EGFR-TKI gefitinib (250 mg/day) with bevacizumab. One month later, the chest CT scan revealed that the metastases increased widely in both lungs, indicating that gefitinib was of primary resistance (). On March 3, 2017, the patient started taking afatinib (40 mg/day) with bevacizumab. The chest CT scan revealed the metastases shrank obviously after 1 month (). After 3 months, the patient had two-grade diarrhea and one-grade rash on the back neck. The efficacy evaluation was partial response. Now, the patient has PFS for 6 month ().
Table 1 Detailed medications and treatment
Figure 3 Thoracic computed tomography (CT) before taking gefitinib (A); after taking gefitinib, metastases increased widely in both lungs (B); after taking afatinib for one month, the metastases shrank obviously (C).
The patient has signed written informed consent for publishing the case details and any accompanying images.
Discussion
The patient was diagnosed with left lung adenocarcinoma without sensitive EGFR mutation. The initial stage was IIa, and the margin was negative. According to the National Comprehensive Cancer Network (NCCN) Guideline,Citation12 chemotherapy was recommended as the adjuvant therapy. After four cycles of therapy and 16-month disease-free survival, locoregional recurrence and distant metastases occurred. Chemotherapy or bevacizumab combined with chemotherapy was recommended according to the NCCN Guideline.Citation12
According to the NCCN Guideline, bevacizumab, a humanized monoclonal antibody against vascular endothelial growth factor, can be used as the first-line therapy for lung adenocarcinoma until the disease progressed.Citation12 Many classic clinical studiesCitation13–Citation16 also proved its efficacy in NSCLC, compared with traditional chemotherapy. The classic ECOG4599Citation16 and BEYONDCitation13 studies have proved that bevacizumab with carboplatin/paclitaxel was well tolerated and resulted in a clinically meaningful treatment benefit in Chinese patients with advanced nonsquamous NSCLC. shows the survival benefits from chemotherapy or bevacizumab.
When the disease progressed, the tissue samples could not be obtained due to the poor physical strength of the patient, so we performed circulating tumor DNA liquid biopsy, which was the feasible sample for EGFR mutation analysis.Citation17,Citation18 EGFR exon 20 S768I mutation was detected just like before. Hellmann et alCitation19 reported a case whose mass spectrometry genotyping revealed EGFR S768I mutation among surgical specimens in 2001, 2003, and 2013, respectively. Besides, erlotinib had resulted in partial radiographic response until T790M mutation occurred.
To the best of our knowledge, this is the first case wherein a patient with S768I mutation received first- and second-generation EGFR-TKIs successively, and the response was completely different. The S768I mutation is a rare subset of EGFR mutants located in exon 20 (incidence 1%–2%).Citation10,Citation20 Common mutations including exon 19 deletions and L858R in exon 21 have shown sensitivity to EGFR-TKIs, no matter which generations they are.Citation6,Citation7 However, the effects of uncommon EGFR mutations such as S768I mutation remain largely unknown. Although the analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6Citation11 proved that eight patients with S768I mutation all showed partial remission with afatinib, the limitation is obvious since the sample size was small and only one patient had single S768I mutation. In addition, Kuiper et alCitation21 and Beau-Faller et alCitation22 discovered that the disease control rate (DCR) was better with EGFR-TKIs for complex mutations than for a single mutation. Although some cases could be explained because they carried attractive mutations such as 19 deletions or L858R in exon 21 simultaneously, most conditions remain unclear.
The female patient carried single S768I mutation according to two molecular pathologic tests. The effects of first- and second-generation EGFR-TKIs were completely different. To evaluate the effect of the single S768I mutation on the response to TKIs, we reviewed the previous cases and studies (). shows that all the patients accepting afatinib showed partial remission or stable disease. The DCR is 100%. By contrast, the DCR of patients who accepted the first-generation TKIs such as erlotinib, gefitinib, and icotinib was 46.2%. Our case showed similar result. In addition to clinical researches, some experimental studies confirmed the value of different TKIs on S768I mutation. Tanizaki et alCitation23 examined the sensitivity of Ba/F3 cells expressing EGFR (L858R) or EGFR (S768I) to EGFR-TKIs by calculating the median inhibitory concentration (IC50) values and a ratio relative to those for cells expressing EGFR (L858R). The result was inspiring because the IC50 values of afatinib were minimal. Regarding the IC50 ratios, the second-generation drugs’ ratios were much smaller than those of the first- and the third-generation drugs. Kancha et alCitation24 and Banno et alCitation25 carried out similar experiments and drew similar conclusions. Therefore, S768I mutation is more sensitive to the second-generation TKI (afatinib) than the first-generation TKIs (erlotinib, gefitinib, and icotinib).
Table 2 Summary of reported clinical response to TKIs in patients harboring the single EGFR S768I mutation
Up to now, 6-month PFS was achieved from afatinib combined with bevacizumab. The NEJ002 studyCitation26 found that the median survival time of patients treated with gefitinib, platinum, and pemetrexed or docetaxel was around 3 years. Previous studies proved the benefits of EGFR-TKIs combined with anti-vascular endothelial growth factor therapy, such as bevacizumab and apatinib.Citation27–Citation29 Furthermore, the European Society for Medical OncologyCitation30 recommends bevacizumab combined with erlotinib as the first-line therapy for metastatic NSCLC. Comprehensive treatment could bring benefits to advanced NSCLC patients.
T790M mutation accounted for half of the known mechanisms of resistance.Citation31 Patients with single S768I mutation also acquired secondary T790M mutation.Citation19,Citation32 Meanwhile, another clinical caseCitation33 reported that patients with L858R mutation showed resistance after TKI therapy and secondary S768I mutation occurred in repeated molecular pathology. May be the occurrence of secondary S768I mutation could be the potential resistance mechanism.
Conclusion
Our case indicates that the second-generation TKI (afatinib) could be better than the first-generation TKI (gefitinib). Afatinib may be an effective treatment for NSCLC patients with single S768I mutation. Comprehensive treatment could bring benefits to advanced NSCLC patients. However, further clinical data are required for patients with advanced NSCLC harboring a single S768I mutation in order to provide more powerful evidences.
Author contributions
HD, HC, YP, and YQ were responsible for collection and assembly of the patient’s data. HD, YP, QL, JZ, WS, CS, and CL performed data analysis and literature searching. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This case report was supported by China–Japan Friendship Hospital. We would like to thank Resident Huang Chen (Pathology Department, China–Japan Friendship Hospital, Beijing, China) for pathologic reading. Our thanks also go to Professor Qing Wu (English Department, School of Humanities, Beijing University of Chinese Medicine, Beijing, China) and Liang Liang (a postgraduate studying Translation Theory and Practice at Renmin University of China) for improving language quality. We also express our gratitude to the patient and her husband. HD and YP are co-first authors.
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- ZhouCWuYLChenGErlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 studyLancet Oncol201112873574221783417
- RosellRCarcerenyEGervaisRErlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trialLancet Oncol201213323924622285168
- MitsudomiTMoritaSYatabeYWest Japan Oncology GroupGefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trialLancet Oncol201011212112820022809
- LynchTJBellDWSordellaRActivating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinibN Engl J Med2004350212129213915118073
- PaezJGJannePALeeJCEGFR mutations in lung cancer: correlation with clinical response to gefitinib therapyScience200430456761497150015118125
- WuTHsiueEHLeeJHLinCCYangJCNew data on clinical decisions in NSCLC patients with uncommon EGFR mutationsExpert Rev Respir Med2016111515527927060
- ImprotaGPettinatoAGieriSEpidermal growth factor receptor exon 20 p.S768I mutation in non-small cell lung carcinoma: a case report combined with a review of the literature and investigation of clinical significanceOncol Lett201511139339826870223
- KobayashiYMitsudomiTNot all epidermal growth factor receptor mutations in lung cancer are created equal: perspectives for individualized treatment strategyCancer Sci201610791179118627323238
- YangJCSequistLVGeaterSLClinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6Lancet Oncol201516783083826051236
- EttingerDSWoodDEAkerleyWNCCN guidelines insights: non-small cell lung cancer, version 4.2016J Natl Compr Canc Netw201614325526426957612
- ZhouCWuYChenGBEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancerJ Clin Oncol201533192197220426014294
- ReckMvon PawelJZatloukalPOverall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL)Ann Oncol20102191804180920150572
- ReckMvon PawelJZatloukalPPhase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAilJ Clin Oncol20092781227123419188680
- SandlerAYiJDahlbergSTreatment outcomes by tumor histology in Eastern Cooperative Group Study E4599 of bevacizumab with paclitaxel/carboplatin for advanced non-small cell lung cancerJ Thorac Oncol2010591416142320686429
- ReckMHagiwaraKHanBctDNA determination of EGFR mutation status in European and Japanese patients with advanced NSCLC: the ASSESS StudyJ Thorac Oncol201611101682168927468938
- SchwaederleMCPatelSPHusainHUtility of genomic assessment of blood-derived circulating tumor DNA (ctDNA) in patients with advanced lung adenocarcinomaClin Cancer Res201723175101511128539465
- HellmannMDRevaBYuHClinical and in vivo evidence that EGFR S768I mutant lung adenocarcinomas are sensitive to erlotinibJ Thorac Oncol2014910e73e7425521405
- ArcilaMENafaKChaftJEEGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristicsMol Cancer Ther201312222022923371856
- KuiperJLHashemiSMSThunnissenENon-classic EGFR mutations in a cohort of Dutch EGFR-mutated NSCLC patients and outcomes following EGFR-TKI treatmentBr J Cancer2016115121504151227875527
- Beau-FallerMPrimNRuppertAMRare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10117 patients: a multicentre observational study by the French ERMETIC-IFCT networkAnn Oncol201425112613124285021
- TanizakiJBannoETogashiYCase report: durable response to afatinib in a patient with lung cancer harboring two uncommon mutations of EGFR and a KRAS mutationLung Cancer2016101111527794398
- KanchaRKvon BubnoffNPeschelCDuysterJFunctional analysis of epidermal growth factor receptor (EGFR) mutations and potential implications for EGFR targeted therapyClin Cancer Res200915246046719147750
- BannoETogashiYNakamuraYSensitivities to various epidermal growth factor receptor-tyrosine kinase inhibitors of uncommon epidermal growth factor receptor mutations L861Q and S768I: what is the optimal epidermal growth factor receptor-tyrosine kinase inhibitor?Cancer Sci201610781134114027240419
- InoueAKobayashiKMaemondoMNorth-East Japan Study GroupUpdated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002)Ann Oncol2013241545922967997
- SetoTKatoTNishioMErlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 studyLancet Oncol201415111236124425175099
- OtsukaKHataAEGFR-TKI rechallenge with bevacizumab in EGFR-mutant non-small cell lung cancerCancer Chemother Pharmacol201576483584126349474
- PengYCuiHLiuZApatinib to combat EGFR-TKI resistance in an advanced non-small cell lung cancer patient with unknown EGFR status: a case reportOnco Targets Ther2017102289229528490886
- NovelloSBarlesiFCalifanoRMetastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-upAnn Oncol201627Suppl 5v1v2727664245
- SequistLVWaltmanBADias-SantagataDGenotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitorsSci Transl Med201137575ra26
- RussoAFranchinaTRicciardiGRRAdamoVRapid acquisition of T790M mutation after treatment with afatinib in an NSCLC patient harboring EGFR Exon 20 S768I mutationJ Thorac Oncol2017121e6e827988103
- LongoLMengoliMCBertoliniFBettelliSManfrediniSRossiGSynchronous occurrence of squamous-cell carcinoma “transformation” and EGFR exon 20 S768I mutation as a novel mechanism of resistance in EGFR-mutated lung adenocarcinomaLung Cancer2017103242628024692
- MasagoKFujitaSIrisaKGood clinical response to gefitinib in a non-small cell lung cancer patient harboring a rare somatic epidermal growth factor gene point mutation; Codon 768 AGC > ATC in Exon 20 (S768I)Jpn J Clin Oncol201040111105110920522446
- Lund-IversenMKleinbergLFjellbirkelandLHellandABrustugunOTClinicopathological characteristics of 11 NSCLC patients with EGFR-exon 20 mutationsJ Thorac Oncol2012791471147322895145
- WeberBHagerHSorensenBSEGFR mutation frequency and effectiveness of erlotinib: a prospective observational study in Danish patients with non-small cell lung cancerLung Cancer201483222423024388704
- PallanLTanierePKohPRare EGFR exon 20 S768I mutation predicts resistance to targeted therapy: a report of two casesJ Thorac Oncol2014910e75
- ChiuCHYangCTShihJYEpidermal growth factor receptor tyrosine kinase inhibitor treatment response in advanced lung adeno-carcinomas with G719X/L861Q/S768I mutationsJ Thorac Oncol201510579379925668120
- HeigenerDFSchumannCSebastianMAfatinib Compassionate Use Consortium (ACUC)Afatinib in non-small cell lung cancer harboring uncommon EGFR mutations pretreated with reversible EGFR inhibitorsOncologist201520101167117426354527
- YangXChenHZhangHEffectiveness of tyrosine kinase inhibitors on uncommon epidermal growth factor receptor mutations in non-small cell lung cancerZhongguo Fei Ai Za Zhi2015188493499 Chinese26302346
- LeventakosKKippBRRumillaKMWintersJLYiESMansfieldASS768I mutation in EGFR in patients with lung cancerJ Thorac Oncol201611101798180127211795
- KlughammerBBruggerWCappuzzoFExamining treatment outcomes with erlotinib in patients with advanced non-small cell lung cancer whose tumors harbor uncommon EGFR mutationsJ Thorac Oncol201611454555526773740
- ChengGSongZChenDClinical efficacy of first-generation EGFR-TKIs in patients with advanced non-small-cell lung cancer harboring EGFR exon 20 mutationsOnco Targets Ther201694181418627468240
- KobayashiHWakudaKTakahashiTEffectiveness of afatinib in lung cancer with paralytic ileus due to peritoneal carcinomatosisRespirol Case Rep201646e0019728031832
- ZhangYWangZHaoXClinical characteristics and response to tyrosine kinase inhibitors of patients with non-small cell lung cancer harboring uncommon epidermal growth factor receptor mutationsChin J Cancer Res2017291182428373750
- ZhuXBaiQLuYResponse to tyrosine kinase inhibitors in lung adenocarcinoma with the rare epidermal growth factor receptor mutation S768I: a retrospective analysis and literature reviewTarget Oncol2017121818827538584