Abstract
Purpose
Previous studies investigating the association between interleukin-17A (IL-17A) G197A polymorphism and gastric cancer risk have provided inconsistent results. We, therefore, conducted this meta-analysis to clarify the association between IL-17A G197A polymorphism and gastric cancer risk.
Methods
We searched PubMed, Excerpta Medica Database, and CNKI databases to identify relevant studies up to June 10, 2017. A total of 16 case-control studies including 6,624 cases and 7,631 controls were identified.
Results
Overall, significant associations between IL-17A G197A polymorphism and gastric cancer risk were observed (A vs G: OR =1.24, 95% CI =1.14–1.36; AA vs GG: OR =1.63, 95% CI =1.35–1.96; GA vs GG: OR =1.12, 95% CI =1.01–1.25; AA+GA vs GG: OR =1.23, 95% CI =1.11–1.35; AA vs GA+GG: OR =1.54, 95% CI =1.27–1.87). Similar associations were also observed in Asian population (A vs G: OR =1.25, 95% CI =1.15–1.37; AA vs GG: OR =1.62, 95% CI =1.33–1.97; GA vs GG: OR =1.16, 95% CI =1.07–1.25; AA+GA vs GG: OR =1.24, 95% CI =1.15–1.33; AA vs GA+GG: OR =1.51, 95% CI =1.23–1.85), in Caucasian population (AA vs GA+GG: OR =2.19, 95% CI =1.40–3.44), and in the hospital-based controls’ subgroup (A vs G: OR =1.30, 95% CI =1.17–1.45; AA vs GG: OR =1.81, 95% CI =1.46–2.25; AA+GA vs GG: OR =1.27, 95% CI =1.12–1.43; AA vs GA+GG: OR =1.71, 95% CI =1.34–2.18).
Conclusions
The current meta-analysis suggests that IL-17A G197A polymorphism might enhance gastric cancer risk.
Introduction
Interleukin-17 (IL-17) is a relatively newly described family of pro-inflammatory cytokines that consists of six family members (IL-17A–F).Citation1 IL-17 is produced by CD4+ memory T cells, and it is involved in both innate and adaptive immune responses.Citation2,Citation3 It has been reported that IL-17A, a pro-inflammatory cytokine, is associated with the pathogenesis of chronic inflammatory diseases, autoimmune diseases,Citation4,Citation5 and cancer progression.Citation6,Citation7
There are many studies that focus on the relationship between IL-17A G197A polymorphism and gastric cancer.Citation8–Citation23 These studies are all based on experimental results, but their results are always inconsistent. Since 2015, only one meta-analysis has been conducted, and 11 case-control studies were included in this meta-analysis.Citation24 Today, more than five studies that assessed the association between IL-17A G197A polymorphism and the risk of gastric cancer have been published. Therefore, we performed an updated meta-analysis to further determine an accurate relationship between IL-17A G197A polymorphism and gastric cancer susceptibility.
Materials and methods
Publication search
We conducted a publication search in PubMed, Excerpta Medica Database, and CNKI databases (up to June 10, 2017) using the following search strategy: “interleukin-17A or interleukin 17A or IL-17A or IL17A”, “polymorphism”, and “gastric cancer”. No language restrictions were applied. Studies had to meet the following criteria: 1) case-control studies; 2) diagnoses of all patients with malignant tumors were confirmed by pathological or histological examination; 3) the study assessed the association between gastric cancer risk and the IL-17A G197A polymorphism. The following exclusion criteria were used: 1) unpublished studies or abstracts; 2) duplicate publications; and 3) insufficient data were reported.
Data extraction
For each study, the following characteristics were extracted: first author, year of publication, ethnicity, sample size (total cases and controls), source of controls, genotype distributions in cases and controls, and P-value of Hardy–Weinberg equilibrium (HWE).Citation25 Disagreements were resolved by discussion.
Statistical analysis
Odds ratios (ORs) with corresponding 95% CIs were calculated to clarify the strength of the association between IL-17A G197A polymorphism and gastric cancer risk. Five genetic models were assessed: homozygote model (AA vs GG), heterozygote model (GA vs GG), recessive model (AA vs GA+GG), dominant model (AA+GA vs GG), and allele model (A vs G). Subgroup analyses were conducted according to ethnicity and source of controls.
Heterogeneity was calculated by using both χ2-based Q-statistic and I2-statistic.Citation26 If P≥0.1 and I2<50%, the fixed-effects model (Mantel–Haenszel method) was chosen.Citation27 Otherwise, the random effects model (Der Simonian–Laird method) was used.Citation28 Moreover, sensitivity analysis was performed to assess the stability of the results. Publication bias was assessed with funnel plots and Egger’s test.Citation29 All of the statistical tests were carried out with STATA version 12.0 (Stata corporation, College Station, TX, USA). P<0.05 was considered significant, and all P-values were two sided.
Results
Characteristics of eligible studies
A flow diagram illustrating the study selection process is shown in . Through literature search and selection, a total of 16 publicationsCitation8–Citation23 including 6,624 cases and 7,631 controls were included in the meta-analysis. shows the main characteristics of the included studies.
Table 1 Characteristics of studies included in the meta-analysis
Meta-analysis
Overall, the IL-17A G197A polymorphism was associated with an increased gastric cancer risk in all genetic models (A vs G: OR =1.24, 95% CI =1.14–1.36, ; AA vs GG: OR =1.63, 95% CI =1.35–1.96, ; GA vs GG: OR =1.12, 95% CI =1.01–1.25, ; AA+GA vs GG: OR =1.23, 95% CI =1.11–1.35, ; AA vs GA+GG: OR =1.54, 95% CI =1.27–1.87, ). The HWE of each study was taken into consideration. After eliminating studies whose distribution of genotype in controls deviated from HWE, the outcome remained statistically significant. These results are shown in .
Table 2 Meta-analysis of the IL-17A polymorphism and gastric cancer risk
Figure 2 Forest plot of the association between IL-17A G197A polymorphism and gastric cancer risk in the allele model (A vs G) among the overall populations.
Abbreviations: IL, interleukin; OR, odds ratio.
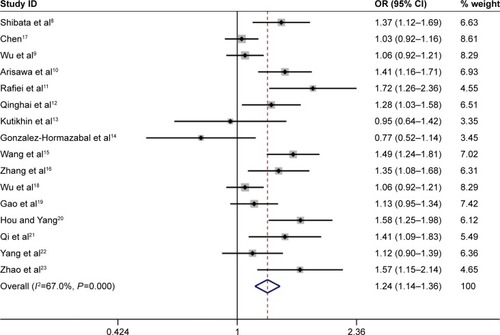
Figure 3 Forest plot of the association between IL-17A G197A polymorphism and gastric cancer risk in the homozygote model (AA vs GG) among the overall populations.
Abbreviations: IL, interleukin; OR, odds ratio.
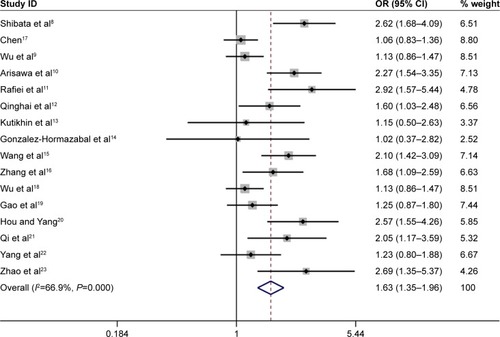
Figure 4 Forest plot of the association between IL-17A G197A polymorphism and gastric cancer risk in the heterozygote model (GA vs GG) among the overall populations.
Abbreviations: IL, interleukin; OR, odds ratio.
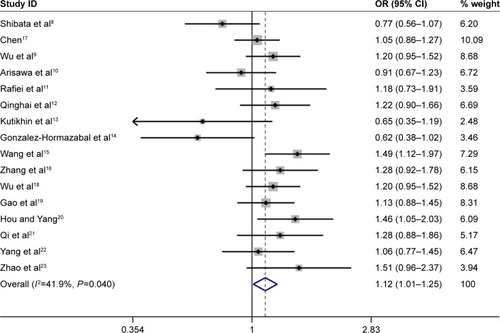
Figure 5 Forest plot of the association between IL-17A G197A polymorphism and gastric cancer risk in the dominant model (AA+GA vs GG) among the overall populations.
Abbreviations: IL, interleukin; OR, odds ratio.
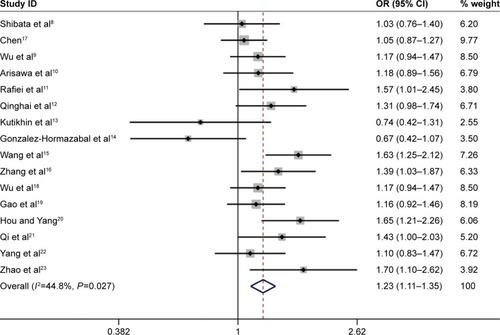
Figure 6 Forest plot of the association between IL-17A G197A polymorphism and gastric cancer risk in the recessive model (AA vs GA+GG) among the overall populations.
Abbreviations: IL, interleukin; OR, odds ratio.
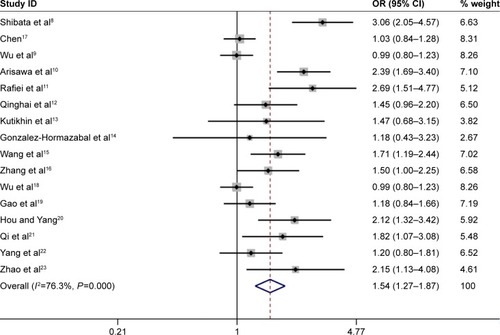
When subgroup analysis was carried out based on ethnicity, significant associations were found in all five genetic models in Asian population (A vs G: OR =1.25, 95% CI =1.15–1.37; AA vs GG: OR =1.62, 95% CI =1.33–1.97; GA vs GG: OR =1.16, 95% CI =1.07–1.25; AA+GA vs GG: OR =1.24, 95% CI =1.15–1.33; AA vs GA+GG: OR =1.51, 95% CI =1.23–1.85), and statistically significant associations were found in the following genetic model in Caucasian population (AA vs GA+GG: OR =2.19, 95% CI =1.40–3.44).
When results were stratified by source of controls, IL-17A G197A polymorphism was associated with a significantly increased gastric cancer risk in the hospital-based controls’ subgroup (A vs G: OR =1.30, 95% CI =1.17–1.45; AA vs GG: OR =1.81, 95% CI =1.46–2.25; AA+GA vs GG: OR =1.27, 95% CI =1.12–1.43; AA vs GA+GG: OR =1.71, 95% CI =1.34–2.18). However, no associations were observed in population-based controls’ subgroup in all five comparison models. All comparisons are listed in .
Sensitivity analysis and publication bias
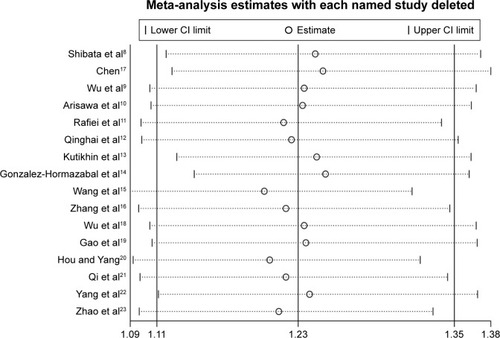
Sensitivity analyses showed that omitting an individual study from all the analyses did not affect the pooled ORs significantly and no substantial change was detected, indicating that the overall results of the present study are stable ().
Figure 7 Sensitivity analysis about IL-17A G197A polymorphism and gastric cancer risk in the dominant model (AA+GA vs GG).
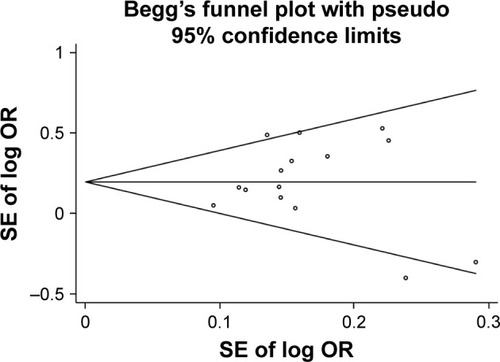
Begg’s funnel plot was used to assess the publication bias of included literature. The shapes of the funnel plots did not show any evidence of obvious asymmetry, indicating the absence of publication bias ().
Figure 8 Begg’s funnel plots to examine publication bias between IL-17A G197A polymorphism and gastric cancer risk in the dominant model (AA+GA vs GG).
Abbreviations: SE, standard error; OR, Odds ratio.
Discussion
Genetic and environmental factors, life style, and Helicobacter pylori infections have been considered as playing essential roles in the development of gastric cancer,Citation30,Citation31 but the precise etiology of the disease remains inconsistent.
IL-17 is a critical inflammatory cytokine that plays an important role in chronic inflammation, autoimmune diseases, and cancer.Citation32 The IL-17A G197A is located in the 5′ region near the IL-17A gene, and it may regulate the gene transcription.Citation33 A previous study has conflicting results about the association between IL-17A G197A polymorphism and gastric cancer risk, which may be because of relatively small sample size and different genetic background.Citation8 Meta-analysis is a powerful method to evaluate gene–disease associations, by collecting all available published studies to obtain more precise results.Citation34
With the development of molecular epidemiology, numerous studies explored the effects of IL-17A G197A polymorphism on gastric cancer susceptibility. In 2014, Yu et alCitation35 carried out a meta-analysis and revealed that the IL-17A G197A polymorphism was associated with a significantly increased gastric cancer risk. In their work, they identified only six case-control studies evaluating the association between the IL-17A G197A polymorphism and gastric cancer risk. In 2015, Li et alCitation24 conducted a meta-analysis to assess the association between IL-17A G197A polymorphism and gastric cancer susceptibility with 11 case-control studies and revealed that IL-17A G197A polymorphism was associated with gastric cancer risk. Therefore, we collected all available published literature and performed an updated meta-analysis of 16 independent case-control studies containing 6,624 cases and 7,631 controls. In the meta-analysis, significant associations between IL-17A G197A polymorphism and gastric cancer risk were observed in all five genetic models. The HWE of each study was taken into consideration. After eliminating studies whose distribution of genotype in controls deviated from HWE, the outcome remained statistically significant. Similar associations were also observed in Asian population (A vs G: OR =1.25, 95% CI =1.15–1.37; AA vs GG: OR =1.62, 95% CI =1.33–1.97; GA vs GG: OR =1.16, 95% CI =1.07–1.25; AA+GA vs GG: OR =1.24, 95% CI =1.15–1.33; AA vs GA+GG: OR =1.51, 95% CI =1.23–1.85), in Caucasian population (AA vs GA+GG: OR =2.19, 95% CI =1.40–3.44), and in the hospital-based controls’ subgroup (A vs G: OR =1.30, 95% CI =1.17–1.45; AA vs GG: OR =1.81, 95% CI =1.46–2.25; AA+GA vs GG: OR =1.27, 95% CI =1.12–1.43; AA vs GA+GG: OR =1.71, 95% CI =1.34–2.18).
Several limitations need to be addressed. First, due to heterogeneity, the results of our meta-analysis should be interpreted. Second, the overall outcomes were based on unadjusted ORs. Lacking the information on detailed individual data limited our more precise analysis on adjusted estimates by other factors like age and sex. This limitation may cause serious confounding bias. Third, meta-analysis is a type of retrospective study, and recall and selection bias may be present.
In conclusion, our meta-analysis revealed that IL-17A G197A polymorphism may increase gastric cancer risk. However, larger studies are still required to assess the interaction of IL-17A G197A polymorphism with gastric cancer risk.
Disclosure
The authors report no conflicts of interest in this work.
References
- KawaguchiMAdachiMOdaNKokubuFHuangSKIL-17 cytokine familyJ Allergy Clin Immunol2004114612651273 quiz 127415577820
- KornTBettelliEOukkaMKuchrooVKIL-17 and Th17 CellsAnnu Rev Immunol20092748551719132915
- MoseleyTAHaudenschildDRRoseLReddiAHInterleukin-17 family and IL-17 receptorsCytokine Growth Factor Rev200314215517412651226
- OuyangWKollsJKZhengYThe biological functions of T helper 17 cell effector cytokines in inflammationImmunity200828445446718400188
- SteinmanLA brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damageNat Med200713213914517290272
- YangBKangHFungAZhaoHWangTMaDThe role of interleukin 17 in tumour proliferation, angiogenesis, and metastasisMediators Inflamm2014201462375925110397
- ZouWRestifoNPT(H)17 cells in tumour immunity and immunotherapyNat Rev Immunol201010424825620336152
- ShibataTTaharaTHirataIArisawaTGenetic polymorphism of interleukin-17A and -17F genes in gastric carcinogenesisHum Immunol200970754755119414056
- WuXZengZChenBAssociation between polymorphisms in interleukin-17A and interleukin-17F genes and risks of gastric cancerInt J Cancer20101271869219904747
- ArisawaTTaharaTShiroedaHGenetic polymorphisms of IL17A and pri-microRNA-938, targeting IL17A 3′-UTR, influence susceptibility to gastric cancerHum Immunol201273774775222537748
- RafieiAHosseiniVJanbabaiGPolymorphism in the interleukin-17A promoter contributes to gastric cancerWorld J Gastroenterol201319345693569924039363
- QinghaiZYanyingWYunfangCXukuiZXiaoqiaoZEffect of interleukin-17A and interleukin-17F gene polymorphisms on the risk of gastric cancer in a Chinese populationGene2014537232833224315816
- KutikhinAGYuzhalinAEVolkovANZhivotovskiyASBrusinaEBCorrelation between genetic polymorphisms within IL-1B and TLR4 genes and cancer risk in a Russian population: a case-control studyTumour Biol20143554821483024446182
- Gonzalez-HormazabalPMuslehMBustamanteMRole of cytokine gene polymorphisms in gastric cancer risk in ChileAnticancer Res20143473523353024982364
- WangNYangJLuJIL-17 gene polymorphism is associated with susceptibility to gastric cancerTumour Biol20143510100251003025012243
- ZhangXZhengLSunYZhangXAnalysis of the association of interleukin-17 gene polymorphisms with gastric cancer risk and interaction with Helicobacter pylori infection in a Chinese populationTumour Biol20143521575158024218334
- ChenJAssociation Study of Polymorphisms in IL23R and IL17A Genes With the Susceptibility of Gastric Cancer [M.S. thesis]Nanjing, ChinaNanjing Medical University2010
- WuXZengZXuLAssociation of interleukin-17 G197A gene polymorphism and interleukin-17 expression with gastric cancerJ Pract Med2014301246249
- GaoYWXuMXuYLiDZhouSEffect of three common IL-17 single nucleotide polymorphisms on the risk of developing gastric cancerOncol Lett2015931398140225663919
- HouCYangFInterleukin-17A gene polymorphism is associated with susceptibility to gastric cancerInt J Clin Exp Pathol2015867378738426261639
- QiWTGaoJLZhangSSRole of IL-17 gene polymorphisms in the susceptibility to gastric cancerGenet Mol Res2015144133641336926535650
- YangLJGaoWBaiJYCorrelation between Interleukin-17 gene polymorphism and gastric cancer susceptibility in Han Chinese populationEur Rev Med Pharmacol Sci20162071271128227097946
- ZhaoWMShayimuPLiuLFangFHuangXLAssociation between IL-17A and IL-17F gene polymorphisms and risk of gastric cancer in a Chinese populationGenet Mol Res2016153
- LiXFShenMCaiJWAssociation of interleukin-17 gene polymorphisms and Helicobacter pylori infection with gastric cancer susceptibility: a cumulative and comprehensive meta-analysisInt J Clin Exp Med2015810176231763326770352
- HaberMExact significance levels of goodness-of-fit tests for the Hardy-Weinberg equilibriumHum Hered19813131611667262889
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- MantelNHaenszelWStatistical aspects of the analysis of data from retrospective studies of diseaseJ Natl Cancer Inst195922471974813655060
- Der SimonianRLairdNMeta-analysis in clinical trialsControl Clin Trials1986731771883802833
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ19973156296349310563
- DongLMPotterJDWhiteEUlrichCMCardonLRPetersUGenetic susceptibility to cancer: the role of polymorphisms in candidate genesJAMA2008299202423243618505952
- CrewKDNeugutAIEpidemiology of gastric cancerWorld J Gastroenterol200612335436216489633
- SongXQianYIL-17 family cytokines mediated signaling in the pathogenesis of inflammatory diseasesCell Signal201325122335234723917206
- LiuXKLinXGaffenSLCrucial role for nuclear factor of activated T cells in T cell receptor-mediated regulation of human interleukin-17J Biol Chem200427950527625277115459204
- NtaisCPolycarpouAIoannidisJPMeta-analysis of the association of the cathepsin D Ala224Val gene polymorphism with the risk of Alzheimer’s disease: a HuGE gene-disease association reviewAm J Epidemiol2004159652753615003956
- YuHSunSLiuFXuQHMeta-analysis of associations between interleukin-17 gene polymorphisms and risk of gastric cancerAsian Pac J Cancer Prev201415208709871325374195