Abstract
MicroRNAs are short regulatory RNAs that posttranscriptionally modulate gene expression and thus play crucial roles in controlling cancer-onset, growth, and progression processes. miR107, a highly conserved microRNA that maps to intron 5 of the PANK1 gene, contributes to the regulation of normal and tumor biological processes. Studies have reported that miR107 has oncogenic or tumor-suppressor functions in different human tumors. The pleiotropic functions of miR107 in various cancers are achieved via its targeting different genes that are involved in tumor proliferation, invasiveness, metastasis, angiogenesis, and chemotherapy-response pathways. The carcinogenicity or cancer-suppressor effects of miR107 occur in a tissue- and cell-specific manner, and the expression level of miR107 can be affected by various factors, including epigenetic and genetic factors, treatment exposure, and daily diet. A comprehensive analysis of the current literature suggests that miR107 functions as a central element in the regulation of cancer networks and can be used as a potential diagnostic and prognostic biomarker and drug target for therapeutic intervention.
Background
MicroRNAs (miRNAs) are a family of small (20–25 base pair nucleotides long) noncoding RNAs, most of which play important regulatory roles in regulating normal development and physiology.Citation1,Citation2 miRNAs cause these biological effects through sequence-specific binding of a seed sequence to the 3′-end of the untranslated regions (UTRs) of a target mRNA, which causes either mRNA degradation or inhibition of translation and further leads to posttranscriptional downregulation of target-protein expression.Citation3 Thousands of miRNAs have been identified in humans, and it is thought that expression of two-thirds of all genes are regulated by miRNAs.Citation4 Deregulated expression levels of many miRNAs are associated with carcinogenesis, and have been proposed to be novel prognostic and predictive biomarkers.Citation5–Citation7
Tumor cells are distinguished from normal cells by several peculiar biological traits, such as sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, angiogenesis, and activating invasion and metastasis.Citation8 The alternation of miRNA expression has a crucial role in cancer, as miRNAs can act either as oncogenes or tumor suppressors in the early disease stages, and they can also influence the progression of invasion and metastasis, as well as predict the clinical outcomes of antitumor therapies.Citation9,Citation10
This review focuses on the pleiotropic functions of miR107 in cancers. miR107 is a highly conserved miRNA that maps to intron 5 of the PANK1 gene. miR107 belongs to the miR15/107 group, whose members regulate genes involved in cell division, metabolism, stress response, and angiogenesis in vertebrate species. All miRNAs in this group share the sequence AGCAGC, starting at either the first or the second nucleotide from the 5′-end of the mature miRNA sequence.Citation11 miR107 is ubiquitously expressed in a range of tissues, with relatively high abundance in the brain. Altered expression of miR107 has been reported in metabolism diseases, such as adipogenesis and diabetes, as well as in neurological diseases and cancers.Citation12
The biological functions of miR107 vary significantly in physiological and pathological processes of various cancers, probably as a result of being targeted to different pathways or genes.Citation13,Citation14 Both clinical and non- or preclinical studies have shown that miR107 is deregulated in several human tumors and its expression level significantly associated with disease staging, metastasis, and treatment outcomes.Citation15,Citation16 We present a systematic review of the complex roles of miR107 in tumor pathogenesis, progression, and prognosis, and of the factors that regulate miR107-expression levels.
Carcinogenic effects of miR107 in various types of cancer
The association between miR107-expression level and carcinogenic effects has been widely researched in various human cancers. However, the functions of miR107 in carcinogenesis are controversial and highly cell-type-dependent, as it acts as either a tumor suppressor or an oncogene by presenting different expression levels in various human tumors. The opposing results found between and within studies are not an uncommon phenomenon in the field of miR107 biology, and these inconsistencies may be due to variations in its mechanism of action.Citation17 To help decipher miR107’s function in specific cancer, lists detail of miR107’s function by tumor type.
Table 1 miR107 function by cancer type
miR107 functions as an oncogenic miRNA
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is the most common type of live cancer and the third leading cause of cancer-related death in the world. Previous studies have shown that miRNAs play a fundamental role in HCC, thereby opening a novel avenue for investigating the molecular mechanisms of HCC pathogenesis. The function of miR107 in HCC continues to be researched, as it has been found to have increased expression in HCC tumors compared to paired non-HCC tissue in cancer patients.Citation18,Citation19 Serum miR107 levels are higher in HCC patients compared with healthy controls, and a logit prediction model has shown that the serum levels of miR107, miR92a-3p, miR3126-5p, and α-fetoprotein can serve as sensitive, specific, and noninvasive biomarkers for the diagnosis of HCC, especially in patients at early stages or with low α-fetoprotein levels.Citation20 Moreover, high miR107 expression has been significantly correlated with poor outcome (tumor differentiation and tumor vascular invasion) in HCC by repressing the expression of HMGCS2.Citation21
Pituitary adenomas
Pituitary adenomas are common benign neoplasms that often grow invasively, but very rarely progress to true carcinomas. miR107 expression is significantly upregulated in both sporadic growth hormone-secreting pituitary adenomas and in nonfunctioning pituitary adenomas when compared with normal pituitaries.Citation22 miR107 acts as an oncogenic miRNA in pituitary adenomas, negatively regulating the expression of the pituitary tumor-suppressor gene AIP.
Prostate cancer
Prostate cancer is the most commonly diagnosed male malignancy and the second leading cause of male cancer-related death. A study investigating changes in circulating miRNA levels associated with prostate cancer found that miR107 had higher concentrations in the urine of men with prostate cancer compared with controls.Citation23 There is also evidence that the growth factor granulin is dysregulated via miR107 in prostate cancer, which may provide a potential common therapeutic target.Citation24
Colorectal cancer
Colorectal cancer (CRC) is the fourth most common cancer in the world, with a high mortality rate. Researchers first found that miR107 is a prometastatic miRNA in CRC, in which the expression level of miR107 was positively correlated with the metastatic potential of different CRC cell lines.Citation13 Elevated levels of miR107 have been observed in stage III compared with stage II tumors, and higher expression of miR107 is associated with lymph node metastasis and distant metastasis.Citation13 miR107 exerts its prometastatic function by negatively regulating two metastasis suppressors: DAPK and KLF4. The expression level of miR107 has also been associated with both progression-free survival and overall survival (OS), and overexpression of miR107 is significantly associated with improved objective response to chemotherapy in CRC patients.Citation25 A recent study showed that miR107 expression was aberrantly increased in human CRC tumor tissue and cell lines when compared with colonic control tissues and colon epithelial cells.Citation26
miR107 as a tumor suppressor
Glioma
Glioma is the most common primary malignant tumor in the central nervous system, and is associated with poor prognosis and rapid mortality. Perhaps the strongest evidence of miR107 acting as a tumor suppressor has been found in glioma studies. miR107 is ubiquitously expressed in a range of tissue, with relatively high abundance in the brain. The expression level of miR107 has been shown to be significantly downregulated in human glioma tissue and cell lines when compared with normal brain tissue.Citation15 Low levels of miR107 expression are also significantly associated with advanced pathological features and poor prognosis of human gliomas, such as larger tumor size, lower Karnofsky performance score, and shorter OS and progression-free survival.Citation16
The antitumorigenic roles of miR107 in glioma include the inhibition of cell apoptosis, migration, and invasion. Upregulation of miR107 inhibits the proliferation of gliomas by repressing the expression of SALL4 and activating the FADD–caspase 8–caspase 3/7 cell-apoptosis pathway.Citation15 miR107 exerts its anti-invasion effects through the NOTCH2-signaling pathway.Citation14,Citation27 Overexpression of miR107 inhibits the proliferation of gliomas by arresting the cell cycle at the G0–G1 phase, and also downregulates the expression of CDK6 and NOTCH.Citation28
Non-small-cell lung cancer
Lung cancer (LC) is one of the most common and deadliest cancers in the world, and non-small-cell LC (NSCLC) accounts for 80%–85% of all LC. Two studies have shown that miR107 expression is reduced in lung tumors and LC cell lines compared to normal lungs.Citation29,Citation30 Furthermore, low miR107 expression has been found to be significantly correlated with higher clinicopathological staging stage, regional lymph-node involvement, and tumor differentiation, as well as poor prognosis.Citation29 Recently, it was shown that miR107 suppresses LC-cell proliferation, inhibits metastasis, impedes the cell cycle, and promotes apoptosis by directly targeting EGFR.Citation31
Hematological malignancies
There is limited information on miR107 in hematological cancers. miR107 was initially reported to be upregulated in acute promyelocytic leukemia (APL) cells treated with all-trans retinoic acid (ATRA) compared with untreated cells.Citation32 Further study showed that miR107 was downregulated in APL blasts compared with normal promyelocytes differentiated in vitro from CD34+ progenitors, and its expression level was upregulated after cells had been treated with ATRA.Citation33 Chronic lymphocytic leukemia (CLL) is the most common leukemic disorder, and is characterized by an accumulation of mature B cells in the blood, bone marrow, and secondary lymphoid organs. A recent study assessed global miRNA expression between purified B cells from treatment-naïve CLL patients and healthy controls, and found that miR107 was downregulated in CLL patients.Citation34 Evidence has shown that miR107 acts in CLL by targeting a calcium-channel protein (Cacna2d1) to promote erythroid differentiation.Citation35
Bladder cancer
Bladder cancer is the most prevalent malignant tumor of the urinary system, ranks ninth in cancer incidence around the world, and is the 13th most common cause of death by cancer. Expression of miR107 is decreased in bladder carcinomas in situ when compared with normal bladders from UPK II SV40 mice.Citation36 CircRNAs, a type of noncoding RNA, have been shown to exert major functions in gene regulation. A recent study showed that mechanisms underlying circRNAs in cancer-related pathway might involve miRNA sponge effects via interactions with miRNAs.Citation37 Further results on circRNA in bladder carcinoma found that circTCF25 was significantly more highly expressed in carcinoma tissue compared with control tissue.Citation38 An additional study on the circRNA–miRNA–mRNA axis in bladder cancer showed that overexpression of circTCF25 downregulated miR107, increased CDK6 expression, and promoted proliferation and migration in vitro and in vivo.Citation38
Renal clear-cell carcinoma
Renal cell carcinoma (RCC), which accounts for approximately 85% of all kidney cancers, is the most common type of kidney cancer. Clear-cell RCC occupies the major proportion of RCC cases.Citation39 miR107 plays a tumor-suppressor role in clear-cell RCC by inhibiting cell proliferation and invasiveness.Citation39
Cervical cancer
Cervical cancer is the second most prevalent type of cancer among women worldwide and a complex disease involving numerous oncogenes or the abnormal expression of tumor suppressors. miR107 contributes to the development of cervical cancer by directly targeting CCR5, which acts as a tumor oncogene in cervical cancer.Citation40
Controversy
Head and neck/oral cancer
Head and neck/oral cancer (HNOC) is the sixth most common cancer worldwide, accounting for 4% of cancers in men and 2% of cancers in women.Citation41 miR107 was first found to be highly expressed in nine HNOC cell lines.Citation41,Citation42 However, subsequent studies showed that miR107 is downregulated in oral squamous cell carcinoma (SCC) cell lines and tongue SCC.Citation43–Citation45 Molecular research has found that downregulation of miR107, which targets the PRKCE gene, is a pathogenetic event in HNSCC, and miR107 may thus be a potential anticancer therapeutic for this patient population. Another recent study unraveled the tumor-suppressor role of miR107 in esophageal carcinogenesis, by targeting CDC42.Citation46 HNOC can divide into a severe subtype, and the roles of miR-107 were poorly studied in some kinds of HNOC.Citation47 Based on these in vitro studies, we concluded that further clinical and in vivo studies should be conducted to identify roles of miR107 in HNOC.
Pancreatic cancer
Pancreatic cancer (PCa) is a devastating disease with a poor 5-year survival rate, and local recurrence and systematic metastasis are the major reasons for treatment failure. A study analyzing global miRNA expression in 12 nontumor pancreas and 44 pancreas primary tumors, including 12 insulinomas, 28 nonfunctioning endocrine tumors, and four acinar carcinomas, showed that miR107 is overexpressed in pancreatic endocrine tumors versus normal pancreatic tissue.Citation48 Data from this study suggest that alteration in miR107 expression is related to endocrine and acinar neoplastic transformation, but its expression cannot be used as a biomarker of PCa, due to the very small sample.
In contrast to this result, there is evidence suggesting that miR107 is a tumor-suppressive factor in PCa. BxPC3LN, a lymphatic PCa cell line that exhibits highly metastatic stem-cell-like properties, expresses lower levels of miR107 than the parental BxPC3 cell line.Citation49 Another recent study found that plasma miR107 levels were significantly downregulated in PCa patients compared with healthy volunteers.Citation50 Moreover, low plasma miR107 levels were significantly associated with advanced T and N stage, liver metastasis, and shown to be an independent factor predicting poor prognosis in PCa patients.Citation50 miR107 plasma levels were increased in PCa patients who had undergone curative pancreatectomy compared with preoperative expression levels.Citation50
Gastric cancer
Gastric cancer (GC) is the fourth most common malignancy and the second leading cause of cancer-related death across the world.Citation51 Gastric adenocarcinoma accounts for over 90% of GC cases. miR107 is commonly upregulated in GC and can promote GC-cell migration, invasion, and metastasis, both in vitro and in vivo.Citation52 The first study on miR107 in GC investigated miR107 expression levels in tissues from 50 cases of GC and matched normal tissue by quantitative reverse-transcription polymerase chain reaction.Citation53 The authors discovered that miR107 is frequently upregulated in GC and its overexpression significantly associated with GC metastasis.Citation53 A subsequent study conducted with a larger sample showed that miR107 expression was significantly higher in tumor tissue, and showed significant associations with tumor invasion, lymph-node metastasis, and stage.Citation54 In addition, OS and disease-free survival of patients with high miR107 expression were significantly worse than those of patients with low miR107 expression.Citation54 The overexpression of miR107 in GC has also been evidenced in other studies.Citation55–Citation58 Furthermore, circulating miR107-expression levels are also higher in diffuse-type GC in a mouse model.Citation59
In contrast to previous findings, one study reported that miR107 expression was significantly decreased in GC (determined by comparing miRNA-expression profiles between 80 gastric tumor tissues and matched adjacent nonneoplastic tissues), and the reexpression of miR107 in GC cells significantly decreased proliferation by targeting CDK6.Citation60 In contrast to the mouse model, plasma miR107 levels are not altered in the patients with diffuse-type GC.Citation60
Because of the complex results, it is critical to expand sample sizes and affirm the function of miR107 in the pathogenesis of GC. Also, several questions remain. Dose the expression level of miR107 alter with cancer stage? Why does miR107 expression correlate with response to GC-treatment outcome? Finally, how can miR107 have opposing effects in the same cancer in two different studies? As these questions are answered, researchers will gain a better understanding of the precise function of miR107 in GC.
Breast cancer
Breast cancer is a common highly heterogeneous malignancy and one of the main gynecological cancers worldwide. In breast cancer, hormone-receptor status is one of the most important predictive factors. In one study, miR107 expression was decreased in 30 breast cancer specimens compared with adjacent normal breast tissue,Citation61 and miR107 can inhibit the proliferation and migration of MDA-MB231 cells in a dose- and time-dependent manner. There is also evidence that miR107 is markedly downregulated in both breast cancer cell lines and breast tumors.Citation62
These results are different from previous studies, in which miR107 was shown to be upregulated in breast tumors and cells and elevated miR107 levels associated with more advanced tumor status, increased lymph-node metastasis, and increased metastasis in distant organs.Citation63–Citation65 The complex behavior of miR107 presented in breast cancer are possibly the result of high tumor heterogeneity and hormone responsiveness. Since the expression of miR107 can be downregulated by estrogen treatment, endogenous estrogen levels may significantly influence miR107 levels.Citation66 As breast cancer can be classified into different subgroups based on the state of ER, PR, and HER2, differences in the carcinogenicity of miR107 may also be caused by the statuses of ER, PR, and HER2 in different breast cancer patient groups.
miR107 functions and downstream targets in cancer
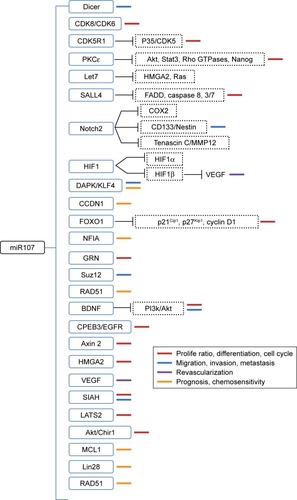
Understanding the functions of miRNAs will provide broad prospects for understanding and overcoming tumors. Altered miR107-expression levels in several cancers have led numerous researchers to investigate its tumor-specific functions and identify its targets in various steps of tumor formation and development. The pleiotropic roles and direct targets of miR107 are further summarized in and discussed in detail herein.
Proliferation, cell cycle, and apoptosis
Altered levels of miR107 have been demonstrated to be associated with proliferation, differentiation, and cell-cycle progression in cancer cells. However, the existing data are controversial. miR107 is effective in suppressing the tumorigenicity of HNSCC in vitro and in vivo by inhibiting proliferation, blocking DNA replication and colony formation, and inhibiting cell invasion. It acts by downregulating the expression of PRKCE, which plays critical roles in the signal-transduction pathways involved in proliferation, differentiation, apoptosis, and migration.Citation67 Evidence has also shown that miR107 can suppress cell proliferation in two LC cell lines and induces G1 cell-cycle arrest by downregulating CCNE1 and CDK6.Citation30 Upregulation of miR107 suppresses glioma cell growth through direct targeting of SALL4, CDK6, and NOTCH, leading to the activation of the FADD–caspase 8–caspase-3/7 apoptosis-signaling pathway.Citation15,Citation28 The antitumor effects of miR107 in inhibiting proliferation and inducing cell-cycle arrest in HNOC, LC, and glioma need further verification.
Data from Song et al suggested that miR107 promotes the proliferation of GC cells by posttranscriptionally targeting CDK8, a key protein in the regulation of cell cycle and cell growth, and thus promoting the development and progression of GC.Citation56 In addition, it also promotes cell proliferation by targeting the transcription factor FOXO1, repressing expression of the cell-cycle inhibitors p21Cip1 and p27Kip1 and increasing the expression of cell-cycle regulator cyclin D1.Citation57 Furthermore, miR107 regulates the expression of GRN, a mitogen and growth factor, in multiple human cancers, implying that a therapeutic strategy of restoring the expression of the miR-15/107 gene group may decrease the malignant potential of tumors by attenuating GRN expression.Citation24,Citation68 Li et al found that miR107 expression was decreased in breast cancer, while overexpression of miR107 suppressed MDA-MB231 breast cell proliferation by targeting CDK8, leading cells to arrest at the G0–G1 phase.Citation61
Revascularization
Tumor vascularization, partly driven by hypoxia, plays a critical role in the progression of solid tumors. HIF1 is significant in hypoxic signaling in tumors. miR107 can decrease hypoxia signaling and inhibit the differentiation of endothelial progenitor cells by targeting HIF1B.Citation69 Overexpression of miR107 in HCT116 colon cancer cells suppresses angiogenesis, tumor growth, and tumor VEGF expression in mice. These in vitro results support the hypothesis that miR107 can act as a tumor suppressor by inhibiting tumor vascularization in tumor-cell lines and tissue.
Migration, invasion, and metastasis
Migration is one of the main reasons for failure of tumor therapy and neoplasm recurrence. High miR107 expression can increase the tumorigenic and metastatic potential of breast cancer by inhibiting LET7 and DICER,Citation64,Citation65 and the expression level of miR107 is significantly elevated in triple-negative metastatic breast cancer patients compared with disease-free patients.Citation63 However, there is also evidence that overexpression of miR107 suppresses breast cancer-cell migration by targeting CDK8.Citation61 miR107 has been shown to function as a prometastatic factor in CRC by negatively regulating the expression of two metastasis suppressors: DAPK and KLF4.Citation13
miR107 overexpression is not only an important factor in the pathological process of GC but also related to increased tumor invasion, metastasis, prognosis, and tumor stage.Citation53,Citation54 In addition, circulating miR107 was significantly increased in a mouse model of early- and late-stage diffuse-type GC.Citation59 miR107 functions as a positive metastatic regulator for GC by targeting DICER.Citation53,Citation54 In contrast, Feng et al found that miR107 was decreased in gastric tumor tissues and could inhibit the invasion of GC cells by targeting CDK6.Citation60
High miR107 expression probably serves a metastasis-inhibiting role in PCa.Citation49,Citation50 Additional studies have shown that the inhibitory effects on the migration and invasion exerted by miR107 in glioma and cervical cancer are due to its involvement in the modulation of NOTCH2–TNC–MMP12 and NOTCH2–COX2 signaling through direct targeting of NOTCH2Citation14,Citation27 and through activation of the ATR–CHK1 pathway,Citation70 respectively.
Cancer therapy resistance mediators
Vast evidence has shown that the expression level of miR107 is associated with chemotherapeutic sensitivity in different cancers, as shown in . For example, low miR107 expression significantly correlated with poor prognosis for NSCLC patients, and mimics transfected with miR107 significantly increased cisplatin resistance by targeting the CDK8 protein in NSCLC cells.Citation29,Citation73 miR107 expression can mediate the sensitivity of PARP inhibitors by targeting genes or factors in the DNA-damage-response pathway, such as RAD51.Citation74
Table 2 Pleiotropic effects of miR107 expression on sensitivity to cancer therapy
In the previous section, we summarized that miR107 has oncogenic and tumor-suppressive effects by suppressing both tumor-suppressive mRNAs and oncogenic mRNAs in different cancer types. Due to the opposite roles of miR107 in the pathogeneses of difference cancer types, it also has various influences on treatment outcomes. Most studies on miRNA-expression levels in cancer have viewed positive correlations between miRNA-expression levels and increased survival as evidence that the miRNA is tumor-suppressive. However, this kind of interpretation can be misleading sometimes. It is critical first to validate the association between miR107-expression levels and clinical treatment outcomes if further studies aim to explore the roles of miR107 in the treatment outcome of cancers. Authors should also further illuminate molecular mechanisms via in vitro and animal studies. We are still far from using miR107 as a potential biomarker for personalized treatment strategies and a potential therapeutic target.
Factors influencing miR107 expression in cancer cell lines
The roles of miRNAs in human disease and treatment response have been widely studied. However, which factors influence the expression and stability of miRNAs have barely been considered. In this section, we summarize various factors that can influence miR107 expression, including genetic and epigenetic factors, treatment exposure, and multiple dietary factors.
Epigenetic and genetic factors
Epigenetic factors
Epigenetic mechanisms, including promoter methylation and histone modification, are critical in the regulation of gene expression. Previous studies have underscored the role of epigenetic regulation of noncoding miRNAs in human cancer, typically through the methylation of CpG islands within miRNA primary transcript-promoter regions.Citation75–Citation77 Recently, Lee et al reported that miR107 was a candidate miRNA that may undergo transcriptional silencing through the methylation of a conserved CpG island in its promoter sequence in pancreatic carcinomas.Citation78 The primary transcript of miR107 (located at chromosome 10q23.31) is coexpressed from an intronic segment of PANK1, a coding gene on chromosome 10. We used two commonly used DNA-methylation prediction programs (MethPrimer and CpG Island Searcher) to predict CpG-island distribution 1,000 base pairs upstream from the 5′UTR, and confirmed that a CpG island exists immediately upstream (-442 to -338) of the PANK1-transcription starting site. Interestingly, a recent study showed that the promoter CpG islands of mir107 were not methylated in NSCLC,Citation79 suggesting that epigenetic silencing of miR107 by DNA methylation is cell-specific and any epigenetic alteration of miR107 in other tumors should not be ignored in further studies.
circRNAs are a class of single-stranded closed RNA molecules and produced from precursor mRNA back-splicing of thousands of genes in eukaryotes.Citation80 circRNAs function as an miRNA- or RNA-binding protein sponge and regulate splicing or transcription. circRNAs are involved in cancer pathogenesis.Citation81 Recent research demonstrated that circTCF25 has a miRNA sponge effect that sequesters miR107 and finally increases CDK6 expression and promotes proliferation and migration in bladder carcinoma.Citation38
lncRNAs are transcripts with no protein-coding function that are longer than 200 nucleotides. lncRNAs are emerging as new factors in the cancer paradigm, demonstrating potential roles in both oncogenic and tumor-suppressive pathways.Citation82 lncRNAs can regulate the expression of miRNA by various mechanisms. miR107-expression levels are regulated by lncRNAs. As an lncRNA, HULC is correlated with the development of HCC. HULC sequesters miR107 and promotes tumor angiogenesis in liver cancer through miR107–E2F1–SPHK1 signaling in liver cancer.Citation83 NEAT1 plays an oncogenic role in human laryngeal SCC and regulates CDK6 expression of laryngeal SCC cells, mediated by miR107.Citation84 c-Myc is an inducible gene that is regulated by specific growth signals in a cell-cycle-dependent manner. c-Myc upregulates the expression and strengthens the activity of lncRNA H19, and H19 promotes cell-cycle progression of NSCLC cells by downregulating miR107 and upregulating CDK6 expression.Citation85
Genomic and transcription factors
The tumor suppressor p53 is a transcription factor that responds to stress and maintains cellular control by deregulating stress responses and thereby maintaining cell and tissue integrity. Mutations in P53 or other disruptions in the p53 pathway are associated with tumor growth and angiogenesis.Citation86,Citation87 P53 can activate the transcription of a group of miRNAs, which in turn suppresses the transcription of genes that regulate apoptosis, DNA repair, and cell-cycle progression.Citation88,Citation89
Computer analysis reveals a potential p53-binding site (1,811 base pairs upstream) of the PANK1/miR107-transcription starting site, and several studies have demonstrated that miR107 belongs to the group of miRNAs regulated by p53.Citation89 Chen et al demonstrated that p53 inhibits the proliferation of glioma cells by targeting miR107, and wild-type p53 protein binding to the promoter region of miR107, leading to increased miR107 expression relative to that of glioma cells expressing mutated P53.Citation28 Furthermore, Toll-like receptors modulate the expression of multiple miRNAs. TLR4 can downregulate miR107 expression through the NFKB pathway, and it can further influence the innate immune system by increasing macrophage adhesion via CDK6.Citation90
Treatment exposure
Surgery
Circulating miRNAs have been identified as potentially convenient biomarkers for many diseases, as they can be easily measured without invasive biopsies. miRNAs in circulation are protected from RNase degradation by binding to protein complexes. It has been hypothesized that circulating miRNAs enter the circulation by passive leakage from apoptotic or necrotic cells or by active secretion of microvesicle-free miRNA or miRNA containing microvesicles,Citation91 and many circulating miRNAs have been used to determine the diagnosis and prognosis of human cancer.Citation92,Citation93 Changes in circulating miRNA levels between pre- and postsurgery samples may be used as biomarkers for cancer diagnosis.Citation67–Citation69 However, it has not yet been explained why miRNA levels change between before and after surgery.
Kodahl et al investigated changes in the circulating miRNA profiles of 24 postmenopausal women with ER-positive early-stage breast cancer before surgery and 3 weeks after tumor resection, and found that the postoperative patients had significant higher miR107 levels compared with preoperative samples.Citation91 On the other hand, Cookson et al showed the opposite result, in which circulating miR107 levels were decreased after surgery in a group of 10 breast cancer patients assessed before and after resection.Citation92–Citation94 To explain this difference, we propose several possible hypotheses. First, if surgery can influence miRNA expression, the time at which samples are taken after surgery should be considered carefully. Second, as miR107 levels in breast cancer are associated with tumor status, increased lymph-node metastasis, and increased metastasis in distant organs, the histopathological stage of the cancer before surgery should be taken into account. Third, since estrogen levels can influence miR107 expression, pre- and post-surgery ER status should also be considered carefully.
Drug exposure
miRNAs are not only associated with disease pathogenesis but also with treatment response to many drugs. Interestingly, disease and drug exposure can inversely influence miRNA expression.Citation95–Citation99 As shown by studies on miR107 and drug treatment, miR107-expression level is not only associated with treatment response but can also be regulated by treatment with various drugs.
Evidence has shown that miR107 is upregulated during ATRA and arsenic trioxide treatment in APL cells.Citation32,Citation100,Citation101 Zhou et al showed that the effects of taxol in attenuating migration and invasion are due to the upregulation of miR107 expression in cervical cancer cells, in which miR107 plays an important role in regulating the expression of MCL1.Citation102 In addition to chemotherapy, radiotherapy can also significantly influence miR107 expression. miR107 is overexpressed in mouse renal cortical tissue after administration of 177Lu-octreotate, which is a radiopharmaceutical used for treatment of neuroendocrine cancers.Citation103 However, a recent study found that long-term and excessive use of 900 MHz radiofrequency radiation downregulated rno-miR107 expression in Wistar albino adult male rat brains.Citation104
There is also evidence that chronic morphine treatment can significantly upregulate miR107 and miR103 levels, leading to downregulation of downstream target genes in vivo and in vitro.Citation105 Soluble β-amyloid peptides have specific repression effects on miR107 expression on neuronal.Citation106 Since miR107 plays a critical role in many cancers, we propose the hypothesis that the antineoplastic effects and treatment resistance of some antitumor drugs are partly due to the exceptional expression of miR107 under treatment exposure.
Lipopolysaccharide (LPS) is recognized as the most potent microbial mediator presaging the threat of invasion of Gram-negative bacteria. Blood miR107 expression levels are upregulated in mice exposed to LPS.Citation107 Another study showed that LPS downregulated miR107 expression level in an MyD88- and p65-dependent manner, as well as PANK1α and PPARα, and then upregulated the expression of CDK6.Citation90
Dietary factors
In addition to drug factors, dietary factors (such as fats, carbohydrates, proteins, vitamins, minerals, and trace elements) may also influence cellular processes by regulating endogenous miRNA expression.Citation108 Davidson et al showed that exposure to fish oil, in which the main effective constituent is a chemoprotective n3-polyunsaturated fatty acid, can selectively upregulate the expression of miR107 in rat colons.Citation109 Studies have further demonstrated that dietary lipids modulate the expression of miR107Citation110 and plant-derived polyphenols can regulate the expression of miR103/107 and prevent diet-induced fatty liver disease in hyperlipidemic mice.Citation111 There is also evidence that miR107 expression can be altered by chronic ethanol feeding.Citation112
Conclusion
We can conclude that miR107 has pleiotropic functions in cancer by controlling the expression of genes involved in several cancer cell-signaling networks. miR107 is involved in tumor proliferation, cell-cycle progression, apoptosis, invasiveness, metastasis, angiogenesis, and chemotherapy response.
As miR107 levels are potential biomarkers for the diagnosis and prognosis of multiple cancers,Citation13,Citation16,Citation29 the diagnostic/prognostic value of circulating miR107 should be explored further in various tumors. Almost all clinical studies on the association between miR107 deregulation and cancer onset compared the expression levels between tumor tissue and corresponding healthy tissue. The results of a study by Boominathan supported the view that circulating miR107 can be used as a novel diagnostic biomarker for breast cancer, as patients with newly diagnosed ER-positive breast cancer had higher circulating miR107 levels than healthy controls.Citation89 Whether circulating miR107 can be used as a novel cancer diagnostic and prognostic biomarker requires further investigation in various cancers.
miR107 can significantly influence the treatment outcomes of various cancers. Conversely, exposure to therapy can also affect the expression of miR107 through unknown mechanisms, a similar phenomenon as observed for other miRNAs.Citation90,Citation91 Based on the complex association between miR107 expression and drug exposure, the relationship between miR107-expression levels, cancer susceptibility, and therapy sensitivity must be considered in future studies. Finally, and most importantly, as miRNA-based in vivo therapy attempts have already been successful,Citation113,Citation114 the potential use of miR107 as a therapeutic target merits careful investigation.
Acknowledgments
This research was supported by grants from the National Key Research and Development Program (2016YFC0905000 and 2016YFC0905001), National High Technology Research and Development Program of China 863 Project (2012AA02A518), National Natural Scientific Foundation of China (81522048, 81573511, 81273595, and 81403017), NSFC (81373477), Innovation-Driven Project of Central South University (2016CX024), and Central South University Innovation Foundation for Postgraduates (2015zzts117).
Disclosure
The authors report no conflicts of interest in this work.
References
- ShuklaGCSinghJBarikSMicroRNAs: processing, maturation, target recognition and regulatory functionsMol Cell Pharmacol201133839222468167
- BartelDPMicroRNAs: target recognition and regulatory functionsCell2009136221523319167326
- ChenKRajewskyNThe evolution of gene regulation by transcription factors and microRNAsNat Rev Genet2007829310317230196
- FriedmanRCFarhKKBurgeCBBartelDPMost mammalian mRNAs are conserved targets of microRNAsGenome Res20091919210518955434
- BlenkironCGoldsteinLDThorneNPMicroRNA expression profiling of human breast cancer identifies new markers of tumor subtypeGenome Biol2007810R21417922911
- Rodriguez-MontesJASanchezPMRole of micro-RNA in colorectal cancer screeningCir Esp2014921065465825088411
- GuoZZhaoCWangZMicroRNAs as ideal biomarkers for the diagnosis of lung cancerTumour Biol20143510103951040725053595
- HanahanDWeinbergRAHallmarks of cancer: the next generationCell2011144564667421376230
- HuangJLyuHWangJLiuBMicroRNA regulation and therapeutic targeting of survivin in cancerAm J Cancer Res201551203125628918
- PennaEOrsoFTavernaDmiR-214 as a key hub that controls cancer networks: small player, multiple functionsJ Invest Dermatol2015135496096925501033
- FinnertyJRWangWXHébertSSWilfredBRMaoGNelsonPTThe miR-15/107 group of microRNA genes: evolutionary biology, cellular functions, and roles in human diseasesJ Mol Biol2010402349150920678503
- FoleyNHO’NeillLAmiR-107: a Toll-like receptor-regulated miRNA dysregulated in obesity and type II diabetesJ Leukoc Biol201292352152722645244
- ChenHYLinYMChungHCmiR-103/107 promote metastasis of colorectal cancer by targeting the metastasis suppressors DAPK and KLF4Cancer Res201272143631364122593189
- ChenLChenXRZhangRMicroRNA-107 inhibits glioma cell migration and invasion by modulating Notch2 expressionJ Neurooncol20131121596623299462
- HeJZhangWZhouQLow-expression of microRNA-107 inhibits cell apoptosis in glioma by upregulation of SALL4Int J Biochem Cell Biol20134591962197323811124
- JiYWeiYWangJAoQGongKZuoHDecreased expression of microRNA-107 predicts poorer prognosis in gliomaTumour Biol20153664461446625596705
- SvoronosAAEngelmanDMSlackFJOncomiR or tumor suppressor? The duplicity of microRNAs in cancerCancer Res201676133666367027325641
- WangYChenFZhaoMmiR-107 suppresses proliferation of hepatoma cells through targeting HMGA2 mRNA 3′UTRBiochem Biophys Res Commun2016480345546027773820
- ZhangJJWangCYHuaLYaoKHChenJTHuJHmiR-107 promotes hepatocellular carcinoma cell proliferation by targeting Axin2Int J Clin Exp Pathol2015855168517426191213
- ZhangYLiTQiuYSerum microRNA panel for early diagnosis of the onset of hepatocellular carcinomaMedicine (Baltimore)2017962e564228079796
- SuSGYangMZhangMFmiR-107-mediated decrease of HMGCS2 indicates poor outcomes and promotes cell migration in hepatocellular carcinomaInt J Biochem Cell Biol201791Pt A535928867541
- TrivellinGButzHDelhoveJMicroRNA miR-107 is overexpressed in pituitary adenomas and inhibits the expression of aryl hydrocarbon receptor-interacting protein in vitroAm J Physiol Endocrinol Metab20123036E708E71922811466
- BryantRJPawlowskiTCattoJWChanges in circulating microRNA levels associated with prostate cancerBr J Cancer2012106476877422240788
- WangWXKyprianouNWangXNelsonPTDysregulation of the mitogen granulin in human cancer through the miR-15/107 microRNA gene groupCancer Res201070229137914220884628
- Molina-PineloSCarneroARiveraFmiR-107 and miR-99a-3p predict chemotherapy response in patients with advanced colorectal cancerBMC Cancer20141465625197016
- LiuFLiuSAiFmiR-107 promotes proliferation and inhibits apoptosis of colon cancer cells by targeting prostate apoptosis response-4 (Par4)Oncol Res201725696797427938501
- ChenLChenXRChenFFMicroRNA-107 inhibits U87 glioma stem cells growth and invasionCell Mol Neurobiol201333565165723572380
- ChenLZhangRLiPP53-induced microRNA-107 inhibits proliferation of glioma cells and down-regulates the expression of CDK6 and Notch-2Neurosci Lett201353432733223220650
- ZhongKZChenWWHuXYJiangALZhaoJClinicopathological and prognostic significance of microRNA-107 in human non small cell lung cancerInt J Clin Exp Pathol2014774545455125120851
- TakahashiYForrestARMaenoEHashimotoTDaubCOYasudaJmiR-107 and miR-185 can induce cell cycle arrest in human non small cell lung cancer cell linesPLoS One200948e667719688090
- WangPLiuXShaoYMicroRNA-107-5p suppresses non-small cell lung cancer by directly targeting oncogene epidermal growth factor receptorOncotarget2017834570125702328915650
- GarzonRPichiorriFPalumboTMicroRNA gene expression during retinoic acid-induced differentiation of human acute promyelocytic leukemiaOncogene200726284148415717260024
- CarecciaSMainardiSPelosiAA restricted signature of miRNAs distinguishes APL blasts from normal promyelocytesOncogene200928454034404019749800
- PallaschCPPatzMParkYJmiRNA deregulation by epigenetic silencing disrupts suppression of the oncogene PLAG1 in chronic lymphocytic leukemiaBlood2009114153255326419692702
- RuanJLiuXXiongXmiR107 promotes the erythroid differentiation of leukemia cells via the downregulation of Cacna2d1Mol Med Rep20151121334133925373460
- de la PeñaFAKanasakiKKanasakiMTangiralaNMaedaGKalluriRLoss of p53 and acquisition of angiogenic microRNA profile are insufficient to facilitate progression of bladder urothelial carcinoma in situ to invasive carcinomaJ Biol Chem201128623207782078721388952
- ChenLLYangLRegulation of circRNA biogenesisRNA Biol201512438138825746834
- ZhongZLvMChenJScreening differential circular RNA expression profiles reveals the regulatory role of circTCF25-miR-103a-3p/miR-107-CDK6 pathway in bladder carcinomaSci Rep201663091927484176
- SongNMaXLiHMicroRNA-107 functions as a candidate tumor suppressor gene in renal clear cell carcinoma involving multiple genesUrol Oncol2015335201205
- CheLFShaoSFWangLXDownregulation of CCR5 inhibits the proliferation and invasion of cervical cancer cells and is regulated by microRNA-107Exp Ther Med201611250350926893637
- LiuXChenZYuJXiaJZhouXMicroRNA profiling and head and neck cancerComp Funct Genomics200983751419753298
- TranNMcleanTZhangXMicroRNA expression profiles in head and neck cancer cell linesBiochem Biophys Res Commun20073581121717475218
- KozakiKImotoIMogiSOmuraKInazawaJExploration of tumor-suppressive microRNAs silenced by DNA hypermethylation in oral cancerCancer Res20086872094210518381414
- WongTSLiuXBHoACYuenAPNgRWWeiWIIdentification of pyruvate kinase type M2 as potential oncoprotein in squamous cell carcinoma of tongue through microRNA profilingInt J Cancer2008123225125718464261
- WongTSLiuXBWongBYNgRWYuenAPWeiWIMature miR-184 as potential oncogenic microRNA of squamous cell carcinoma of tongueClin Cancer Res20081492588259218451220
- SharmaPSainiNSharmaRmiR-107 functions as a tumor suppressor in human esophageal squamous cell carcinoma and targets Cdc42Oncol Rep20173753116312728393193
- ChuYZhuHLvLZhouYHuoJmiRNAs in oesophageal squamous cancerNeth J Med2013712697523462054
- RoldoCMissiagliaEHaganJPMicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behaviorJ Clin Oncol200624294677468416966691
- LuoGLongJCuiXHighly lymphatic metastatic pancreatic cancer cells possess stem cell-like propertiesInt J Oncol201342397998423338123
- ImamuraTKomatsuSIchikawaDDepleted tumor suppressor miR-107 in plasma relates to tumor progression and is a novel therapeutic target in pancreatic cancerSci Rep201771570828720759
- MaYYTaoHQMicroribonucleic acids and gastric cancerCancer Sci2012103462062522168593
- ShresthaSHsuSDHuangWYA systematic review of microRNA expression profiling studies in human gastric cancerCancer Med20143487888824902858
- LiXZhangYShiYMicroRNA-107, an oncogene microRNA that regulates tumour invasion and metastasis by targeting DICER1 in gastric cancerJ Cell Mol Med20111591887189521029372
- InoueTIinumaHOgawaEInabaTFukushimaRClinicopathological and prognostic significance of microRNA-107 and its relationship to DICER1 mRNA expression in gastric cancerOncol Rep20122761759176422407237
- LiXZhangYZhangHmiRNA-223 promotes gastric cancer invasion and metastasis by targeting tumor suppressor EPB41L3Mol Cancer Res20119782483321628394
- SongYQMaXHMaGLMicroRNA-107 promotes proliferation of gastric cancer cells by targeting cyclin dependent kinase 8Diagn Pathol2014916425163571
- LiFLiuBGaoYUpregulation of microRNA-107 induces proliferation in human gastric cancer cells by targeting the transcription factor FOXO1FEBS Lett2014588453854424374340
- ZhangMWangXLiWCuiYmiR-107 and miR-25 simultaneously target LATS2 and regulate proliferation and invasion of gastric adenocarcinoma (GAC) cellsBiochem Biophys Res Commun2015460380681225824045
- RotkruaPShimadaSMogushiKAkiyamaYTanakaHYuasaYCirculating microRNAs as biomarkers for early detection of diffuse-type gastric cancer using a mouse modelBr J Cancer2013108493294023385731
- FengLXieYZhangHWuYmiR-107 targets cyclin-dependent kinase 6 expression, induces cell cycle G1 arrest and inhibits invasion in gastric cancer cellsMed Oncol201229285686321264532
- LiXYLuoQFWeiCKLiDFLiJFangLmiRNA-107 inhibits proliferation and migration by targeting CDK8 in breast cancerInt J Clin Exp Med201471324024482686
- GaoBHaoSTianWMicroRNA-107 is downregulated and having tumor suppressive effect in breast cancer by negatively regulating BDNFJ Gene Med20171912e2932
- ShenSSunQLiangZA prognostic model of triple-negative breast cancer based on miR-27b-3p and node statusPLoS One201496e10066424945253
- MartelloGRosatoAFerrariFA microRNA targeting dicer for metastasis controlCell201014171195120720603000
- ChenPSSuJLChaSTmiR-107 promotes tumor progression by targeting the let-7 microRNA in mice and humansJ Clin Invest201112193442345521841313
- CicatielloLMutarelliMGroberOMEstrogen receptor α controls a gene network in luminal-like breast cancer cells comprising multiple transcription factors and microRNAsAm J Pathol201017652113213020348243
- DattaJSmithALangJCMicroRNA-107 functions as a candidate tumor-suppressor gene in head and neck squamous cell carcinoma by downregulation of protein kinase CεOncogene201231364045405322158047
- WangWXWilfredBRMadathilSKmiR-107 regulates granulin/progranulin with implications for traumatic brain injury and neurode-generative diseaseAm J Pathol2010177133434520489155
- YamakuchiMLottermanCDBaoCP53-induced microRNA-107 inhibits HIF-1 and tumor angiogenesisProc Natl Acad Sci U S A2010107146334633920308559
- ZhouCLiGZhouJHanNLiuZYinJmiR-107 activates ATR/Chk1 pathway and suppress cervical cancer invasion by targeting MCL1PLoS One2014911e11186025386925
- BhiseNSChauhanLShinMMicroRNA-mRNA pairs associated with outcome in AML: from in vitro cell-based studies to AML patientsFront Pharmacol2015632426858643
- AkcakayaPCaramutaSAhlenJMicroRNA expression signatures of gastrointestinal stromal tumours: associations with imatinib resistance and patient outcomeBr J Cancer2014111112091210225349971
- ZhangZZhangLYinZYmiR-107 regulates cisplatin chemosensitivity of A549 non small cell lung cancer cell line by targeting cyclin dependent kinase 8Int J Clin Exp Pathol20147107236724125400821
- HuangJWWangYDhillonKKSystematic screen identifies miRNAs that target RAD51 and RAD51D to enhance chemosensitivityMol Cancer Res201311121564157324088786
- HanLWitmerPDCaseyEValleDSukumarSDNA methylation regulates microRNA expressionCancer Biol Ther2007681284128817660710
- LujambioARoperoSBallestarEGenetic unmasking of an epigenetically silenced microRNA in human cancer cellsCancer Res20076741424142917308079
- LehmannUHasemeierBChristgenMEpigenetic inactivation of microRNA gene hsa-mir-9-1 in human breast cancerJ Pathol20082141172417948228
- LeeKHLottermanCKarikariCEpigenetic silencing of microRNA miR-107 regulates cyclin-dependent kinase 6 expression in pancreatic cancerPancreatology20099329330119407485
- RykovSVKhodyrevDSProninaIVKazubskayaTPLoginovVIBragaEANovel miRNA genes methylated in lung tumorsGenetika2013497896901 Russian24450160
- ChenLLThe biogenesis and emerging roles of circular RNAsNat Rev Mol Cell Biol201617420521126908011
- QuSLiuZYangXThe emerging functions and roles of circular RNAs in cancerCancer Lett201741430130929174799
- HuXSoodAKDangCVZhangLThe role of long noncoding RNAs in cancer: the dark matter mattersCurr Opin Genet Dev20174881529054012
- LuZXiaoZLiuFLong non-coding RNA HULC promotes tumor angiogenesis in liver cancer by up-regulating sphingosine kinase 1 (SPHK1)Oncotarget20167124125426540633
- WangPWuTZhouHLong noncoding RNA NEAT1 promotes laryngeal squamous cell cancer through regulating miR-107/CDK6 pathwayJ Exp Clin Cancer Res2016352226822763
- CuiJMoJLuoMc-Myc-activated long non-coding RNA H19 downregulates miR-107 and promotes cell cycle progression of non-small cell lung cancerInt J Clin Exp Pathol2015810124001240926722426
- YeudallWAP53 mutation in the genesis of metastasisSubcell Biochem20148510511725201191
- LiXLJonesMFSubramanianMLalAMutant p53 exerts oncogenic effects through microRNAs and their target gene networksFEBS Lett2014588162610261524726728
- HermekingHP53 enters the microRNA worldCancer Cell200712541441817996645
- BoominathanLThe tumor suppressors p53, p63, and p73 are regulators of microRNA processing complexPLoS One201055e1061520485546
- HennessyEJSheedyFJSantamariaDBarbacidMO’NeillLAToll-like receptor-4 (TLR4) down-regulates microRNA-107, increasing macrophage adhesion via cyclin-dependent kinase 6J Biol Chem201128629255312553921628465
- KodahlARZeuthenPBinderHKnoopASDitzelHJAlterations in circulating miRNA levels following early-stage estrogen receptor-positive breast cancer resection in post-menopausal womenPLoS One201497e10195025004125
- HeneghanHMMillerNLoweryAJSweeneyKJNewellJKerinMJCirculating microRNAs as novel minimally invasive biomarkers for breast cancerAnn Surg2010251349950520134314
- ChengHZhangLCogdellDECirculating plasma miR-141 is a novel biomarker for metastatic colon cancer and predicts poor prognosisPLoS One201163e1774521445232
- CooksonVJBentleyMAHoganBVCirculating microRNA profiles reflect the presence of breast tumours but not the profiles of microRNAs within the tumoursCell Oncol (Dordr)201235430130822821209
- LewisANijhuisAMehtaSIntestinal fibrosis in Crohn’s disease: role of microRNAs as fibrogenic modulators, serum biomarkers, and therapeutic targetsInflamm Bowel Dis20152151141115025636122
- TaipaleenmakiHBrowneGAkechJTargeting of Runx2 by miRNA-135 and miRNA-203 impairs progression of breast cancer and metastatic bone diseaseCancer Res20157571433144425634212
- SzaboGSatishchandranAMicroRNAs in alcoholic liver diseaseSemin Liver Dis2015351364225632933
- ZhangYGengLTalmonGWangJMicroRNA-520 g confers drug resistance by regulating p21 expression in colorectal cancerJ Biol Chem2015290106215622525616665
- MacdonaghLGraySGFinnSPCuffeSO’ByrneKJBarrMPThe emerging role of microRNAs in resistance to lung cancer treatmentsCancer Treat Rev201541216016925592062
- GarzonRPichiorriFPalumboTMicroRNA gene expression during retinoic acid-induced differentiation of human acute promyelocytic leukemiaOncogene200726284148415717260024
- WuYLiXFYangJHLiaoXYChenYZMicroRNAs expression profile in acute promyelocytic leukemia cell differentiation induced by all-trans retinoic acid and arsenic trioxideZhonghua Xue Ye Xue Za Zhi2012337546551 Chinese22967415
- ZhouCLiGZhouJHanNLiuZYinJmiR-107 activates ATR/Chk1 pathway and suppress cervical cancer invasion by targeting MCL1PLoS One2014911e11186025386925
- SchülerEParrisTZHelouKForssell-AronssonEDistinct microRNA expression profiles in mouse renal cortical tissue after 177Lu-octreotate administrationPLoS One2014911e11264525386939
- DasdagSAkdagMZErdalMELong term and excessive use of 900 MHz radiofrequency radiation alter microRNA expression in brainInt J Radiat Biol201591430631125529971
- LuZGXuJXuMMPasternakGWPanYXMorphine regulates expression of μ-opioid receptor MOR-1A, an intron-retention carboxyl terminal splice variant of the μ-opioid receptor (OPRM1) gene via miR-103/miR-107Mol Pharmacol201485236838024302561
- LiJJDoliosGWangRLiaoFFSoluble beta-amyloid peptides, but not insoluble fibrils, have specific effect on neuronal microRNA expressionPLoS One201493e9077024595404
- HsiehCHRauCSJengJCWhole blood-derived microRNA signatures in mice exposed to lipopolysaccharidesJ Biomed Sci2012196922849760
- WagnerAEPiegholdtSFerraroMPallaufKRimbachGFood derived microRNAsFood Funct20156371471825644027
- DavidsonLAWangNShahMSLuptonJRIvanovIChapkinRSn-3 Polyunsaturated fatty acids modulate carcinogen-directed non-coding microRNA signatures in rat colonCarcinogenesis200930122077208419825969
- Daimiel-RuizLKlett-MingoMKonstantinidouVDietary lipids modulate the expression of miR-107, an miRNA that regulates the circadian systemMol Nutr Food Res201559355256525522185
- JovenJEspinelERullAPlant-derived polyphenols regulate expression of miRNA paralogs miR-103/107 and miR-122 and prevent diet-induced fatty liver disease in hyperlipidemic miceBiochim Biophys Acta20121820789489922503922
- DippoldRPVadigepalliRGonyeGEPatraBHoekJBChronic ethanol feeding alters miRNA expression dynamics during liver regenerationAlcohol Clin Exp Res201337Suppl 1E59E6922823254
- TominagaNYoshiokaYOchiyaTA novel platform for cancer therapy using extracellular vesiclesAdv Drug Deliv Rev201595505526482189
- LiFMahatoRImiRNAs as targets for cancer treatment: therapeutics design and deliveryAdv Drug Deliv Rev201581vvi25500272