Abstract
Background and objective
The aim of this study was to evaluate the expression of cytomembranic programmed death-ligand 1 (PD-L1) and its clinical significance in locoregionally advanced nasopharyngeal carcinoma (NPC).
Patients and methods
Formalin-fixed, paraffin-embedded tissue biopsies from 85 patients with histological diagnosis of locoregionally advanced NPC treated with radical intensity-modulated radiotherapy and concurrent cisplatin-based chemotherapy were studied. By using immunohistochemistry staining, expressions of cytomembranic PD-L1 on tumor cells were detected.
Results
After a median follow-up duration of 65.8 months, 7 (8.2%), 5 (5.9%), and 5 (5.9%) patients suffered from local failure, regional failure, and distant metastases, respectively. The 5-year local failure-free survival, regional failure-free survival, distant failure-free survival, and overall survival (OS) rates were 90.9%, 94.8%, 94.0%, and 92.2%, respectively. Our results revealed that a high expression of cytomembranic PD-L1 was correlated with shorter OS (5y-OS: 82.5% vs 97.6%, P=0.022). In the multivariate analysis, only the cytomembranic PD-L1 was an independent prognostic factor for OS (hazard ratio: 6.176, 95% confidence interval, 1.166–32.710, P=0.032).
Conclusion
Cytomembranic PD-L1 expression levels correlated with OS in locoregionally advanced NPC. Agreement between different methods is needed for further application of PD-L1 biomarker assays in NPC.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Nasopharyngeal carcinoma (NPC) is an endemic malignancy in southern China, with a peak annual incidence approaching 30 per 100,000 persons.Citation1 More than 70% of patients with newly diagnosed NPC are classified as having locoregionally advanced disease.Citation2 With the advent of concurrent chemoradiotherapy, intensity-modulated radiotherapy (IMRT), and imaging techniques, locoregional control has substantially improved and distant metastasis is now the main source of treatment failure for NPC.Citation1
Programmed death-ligand 1 (PD-L1) is an immune checkpoint which regulates Type 1 T helper immune responses and mediates cancer immune evasion.Citation3 Recently, a Phase Ib trial of metastatic NPC suggested encouraging results after treatment with a programmed death-1 (PD-1) inhibitor.Citation4 However, to date, only a few studies of NPC on PD-L1 are available, and the prognostic role of PD-L1 has not yet been fully evaluated.Citation5–Citation11 Thus, the aim of this study is to evaluate the expression of cytomembranic PD-L1 and its clinical significance in locoregionally advanced NPC.
Patients and methods
Patients and samples
This study was approved by the independent ethics committee, Zhejiang Cancer Hospital. Consent was waived and patient records were deidentified and anonymized prior to analysis.
For this study, 116 NPC patients were consecutively sampled by 1 medical care group from March 2010 to May 2012. Patients were selected based on the following criteria: 1) histologically proven locoregionally advanced NPC with available biopsy specimens; 2) Karnofsky score ≥70; 3) receiving radical IMRT and concurrent cisplatin-based chemotherapy at initial diagnosis;Citation12 4) no previous malignancy or other concomitant malignant disease; and 5) PD-L1 staining was detectable in the tumor cells. Therefore, there were 85 patients who qualified for this study. All patients underwent disease staging using the American Joint Committee on Cancer (AJCC) 2010 staging system. The clinical characteristics are listed in .
Table 1 Patient characteristics
Immunohistochemistry (IHC) staining
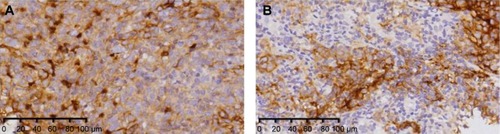
Immunohistochemical staining of 5 μm sections from formalin-fixed paraffin-embedded nasopharyngeal biopsies specimens was performed in the Department of Pathology of our hospital with the antibody 1:200 anti-PD-L1 (E1L3N; Cell Signaling Technology, Danvers, MA, USA) using the standard protocol for routine diagnostic specimens. Hematoxylin and eosin sections were also reviewed for the presence of tumors. The immunoreactivity of PD-L1 was scored semi-quantitatively as follows: the percentage of tumor cells with cytomembranic positivity (0, ≤5%; 1, 6 to ≤25%; 2, 26 to ≤50%; 3, 51 to ≤75%; 4, >75%) was added with the intensity of staining (0, negative; 1, weak; 2, moderate; 3, strong), resulting in a score of 0–7. Patients with a score of 1 or 2 were considered low expression.
Statistical analysis
The Statistical Package for Social Sciences, version 17.0 (SPSS, Chicago, IL, USA), software was used for statistical analysis. The local failure-free survival (LFFS), regional failure-free survival (RFFS), distant failure-free survival (DFFS), and overall survival (OS) were estimated by use of the Kaplan–Meier method. LFFS, RFFS, DFFS, and OS were measured from Day 1 of treatment to the date of the event. Log-rank test was used in univariate analysis. χ2 and Fisher’s exact tests were used to compare the differences between the cytomembrane high group and the cytomembrane low group. Multivariate analysis was performed using the Cox proportional hazards model. All statistical tests were two sided, and P<0.05 was considered to be statistically significant.
Results
General information
Among the 85 patients enrolled, including 63 males and 22 females, the median age was 47 years (range from 18 to 67 years). The median body mass index (BMI) was 22.8 kg/m2 (range, 16.7–30.1 kg/m2). All tumors were classified as having nonkeratinizing phenotype. Thirty patients (35.3%) had Stage T4 disease, and 8 patients (9.4%) had Stage N3 disease. After a median follow-up duration of 65.8 months, 7 (8.2%) patients died and 7 (8.2%), 5 (5.9%), and 5 (5.9%) patients suffered from local failure, regional failure, and distant metastasis, respectively. The 5-year LFFS, RFFS, DFFS, and OS rates were 90.9%, 94.8%, 94.0%, and 92.2%, respectively.
Clinicopathologic correlations
PD-L1 staining was detectable in 85 patients (73.3%, 85 of 116 patients) and was mainly located at the membrane or in the cytoplasm region (or both) in the tumor cells. The membrane-mainly tumors accounted for 9.4% of patients (8/85), cytoplasm-mainly tumors accounted for 65.9% of patients (56/85), and similar expression on both the membrane and cytoplasm accounted for 24.7% of patients (21/85). Cytomembranic PD-L1 staining was classified as high expression in 29 patients. Representative cytomembranic staining of PD-L1 in NPC is shown in . In this study, high expression of cytomembranic PD-L1 staining was not significantly correlated with the clinicopathological parameters of age, sex, BMI, or clinical stage at diagnosis. Detailed data are summarized in .
Figure 1 Immunohistochemical staining for PD-L1 in patients with locoregionally advanced NPC.
Abbreviations: NPC, nasopharyngeal carcinoma; PD-L1, programmed death-ligand 1.
Prognostic values related with PD-L1
The values of various potential prognostic factors including age, sex, BMI, overall stage, and PD-L1 on predicting LFFS, RFFS, DFFS, and OS were evaluated. The outcomes of univariate analysis are shown in . Our results revealed that a high expression of cytomembranic PD-L1 was correlated with worse OS (5y-OS: 82.5% vs 97.6%, P=0.022, ). In the multivariate analysis, only PD-L1 was suggested to be an independent prognostic factor for OS (hazard ratio: 6.176, 95% confidence interval, 1.166–32.710, P=0.032).
Figure 2 Kaplan–Meier curves stratified by the patterns of immunohistochemical staining for cytomembranic PD-L1 (low vs high).
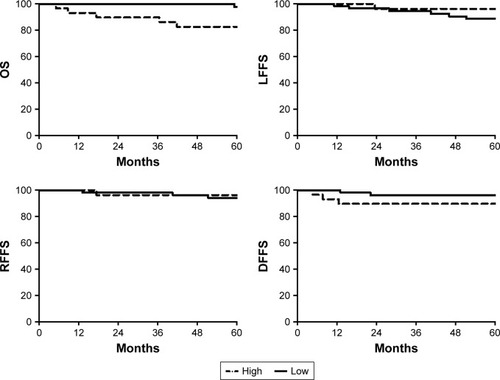
Table 2 Impact of prognostic factors on treatment results by univariate analysis
Discussion
In our study, cytomembranic PD-L1 was overexpressed in 73.3% of locoregionally advanced NPC patients, and 25.0% of patients had tumors with high expression of cytomembranic PD-L1. Our results revealed that locoregionally advanced NPC patients with high cytomembranic PD-L1 expression had a significantly reduced survival outcome, which was similar to the results of previous studies.Citation5–Citation7 However, Lee et alCitation8 reported that there was a longer survival rate in nonmetastatic NPC patients treated by IMRT with high PD-L1 expression; Chan et alCitation10 reported that OS and progression-free survival did not correlate with baseline PD-L1 expression in 161 patients with NPC receiving standard-of-care treatment. These differences could have multifactorial causes, such as of the difference in PD-L1 monoclonal antibodies, staining protocols, detection systems, and different scoring algorithms used.
Hsu et alCitation9 demonstrated that the expression rate of PD-1 in intratumoral CD8 cells significantly correlated with a poorer prognosis of OS, disease-free survival, and LFFS of 46 NPC patients. However, in a larger cohort (total n=161), the impact of PD-L1 on tumor-infiltrating immune cells was not obvious.Citation10 Biomarker expression in lymphoid or other immune effector cells is a special challenge for pathologists.Citation13 Inter- and intraobserver bias for tumor cells is higher than for tumor-infiltrating lymphocytes,Citation14 and the pathologist cannot always recognize whether the existing lymphocyte population is inflammation or oncogene driven.Citation14,Citation15 Furthermore, reproducible PD-L1 IHC scoring of tumor cells seems feasible, whereas scoring of immune cells did not yield reproducible results.Citation16 In our study, the impact of PD-L1 on tumor-infiltrating immune cells was not analyzed.
The scoring system of IHC for PD-L1 has yet to be defined.Citation8 However, several groups have evaluated the degree of agreement on PD-L1 among different methods. The extent of concordance among three validated, commercially available PD-L1 IHC assays (Dako 22C3, Ventana SP263, and Dako 28-8) was compared in 493 non-small-cell lung cancer samples. The three assays indicated similar patterns of tumor membrane staining, with high correlation between percentage PD-L1 staining. Between assays at multiple expression cut-offs, including 1%, 10%, 25%, and 50% tumor membrane staining, an overall percentage agreement of >90% was achieved.Citation17 In the present study, the expression of cytomembranic PD-L1 in locoregionally advanced NPC was evaluated. Agreement between different methods is needed for application of PD-L1 biomarker assays in NPC.
There are several limitations in the current study, including the inclusion of patients with cytomembranic PD-L1 staining who completed treatment only, the retrospective nature of the study design, and the limited number of patients with Stage T4 or N3 disease, and these could affect the outcomes. Nevertheless, our report is noteworthy because this is the first study to evaluate cytomembranic PD-L1 expression in locoregionally advanced NPC.
Conclusion
Cytomembranic PD-L1 expression levels correlated with OS in locoregionally advanced NPC. Agreement between different methods is needed for application of PD-L1 biomarker assays in NPC.
Acknowledgments
We thank Dr Feng Jiang, Dr Qifeng Jin, Dr Ting Jin, Dr Shuang Huang, Dr Yuanyuan Chen, Dr Yongfeng Piao, Dr Yonghong Hua, and Dr Xinglai Feng for collecting the data. The present study was supported by Zhejiang Province Medical and Health Science and Technology Project (2017182785).
Disclosure
The authors report no conflicts of interest in this work.
References
- LeeAWMaBBNgWTManagement of nasopharyngeal carcinoma: current practice and future perspectiveJ Clin Oncol2015333356336426351355
- MaoYPXieFYLiuLZRe-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imagingInt J Radiat Oncol Biol Phys2009731326133419153016
- ChenDSIrvingBAHodiFSMolecular pathways: next-generation immunotherapy–inhibiting programmed death-ligand 1 and programmed death-1Clin Cancer Res2012186580658723087408
- HsuCLeeSHEjadiSAntitumor activity and safety of pembrolizumab in patients with PD-L1-positive nasopharyngeal carcinoma: interim results from a phase 1b studyAnn Oncol201526Suppl 9ix94
- ZhangJFangWQinTCo-expression of PD-1 and PD-L1 predicts poor outcome in nasopharyngeal carcinomaMed Oncol20153238625702326
- FangWZhangJHongSEBV-driven LMP1 and IFN-γ up-regulate PD-L1 in nasopharyngeal carcinoma: implications for onco-targeted therapyOncotarget2014523121891220225361008
- ZhouYShiDMiaoJPD-L1 predicts poor prognosis for nasopharyngeal carcinoma irrespective of PD-1 and EBV-DNA loadSci Rep201774362728256540
- LeeVHLoAWLeungCYCorrelation of PD-L1 expression of tumor cells with survival outcomes after radical intensity-modulated radiation therapy for non-metastatic nasopharyngeal carcinomaPLoS One2016116e015796927341634
- HsuMCHsiaoJRChangKCIncrease of programmed death-1- expressing intratumoral CD8 T cells predicts a poor prognosis for nasopharyngeal carcinomaMod Pathol201023101393140320657553
- ChanOSKowanetzMNgWTCharacterization of PD-L1 expression and immune cell infiltration in nasopharyngeal cancerOral Oncol201767526028351581
- ZhouYMiaoJWuHPD-1 and PD-L1 expression in 132 recurrent nasopharyngeal carcinoma: the correlation with anemia and outcomesOncotarget2017831512105122328881642
- JinTQinWFJiangFInterim analysis of a prospective randomized non-inferiority trial of cisplatin and fluorouracil induction chemotherapy with or without docetaxel in nasopharyngeal carcinomaOncotarget Epub2016728
- HutarewGPD-L1 testing, fit for routine evaluation? From a pathologist’s point of viewMemo20169420120628058063
- TengMWNgiowSFRibasAClassifying cancers based on T-cell infiltration and PD-L1Cancer Res201575112139214525977340
- FehrenbacherLSpiraABallingerMAtezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, openlabel, phase 2 randomised controlled trialLancet20163871837184626970723
- ScheelAHDietelMHeukampLCHarmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomasMod Pathol201629101165117227389313
- RatcliffeMJSharpeAMidhaAAgreement between programmed cell death ligand-1 diagnostic assays across multiple protein expression cut-offs in non-small cell lung cancerClin Cancer Res201723CCR-1116CCR-2375
