Abstract
Background
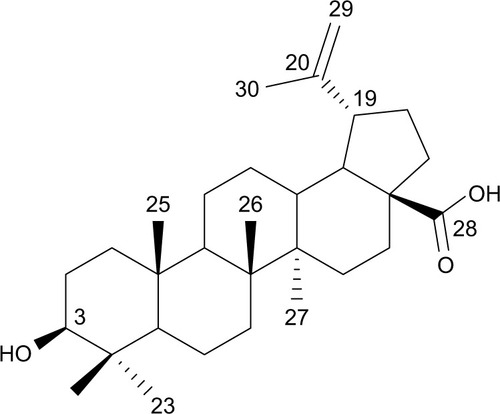
Betulinic acid (BA) is a complex lupane triterpenoid with unique antineoplastic activity. However, its antiproliferative activity is far from satisfaction. In order to improve its anticancer efficacy, betulinic acid was conjugated with a nitric oxide (NO)-releasing moiety to get a novel hybrid, BA-78.
Methods
The antiproliferative activity of BA-78 against 6 cell lines and the ability of releasing nitric oxide were determined. The pro-apoptosis mechanism of BA-78 was investigated as well.
Results
BA-78 exhibited time-dependent release of NO, and it displayed higher antiproliferative potential than BA through increasing apoptosis and inducing cell cycle arrest at G1 phase. Western blotting results showed that BA-78 increased the expression of Bax, Bid, Bad and cytochrome C and reduced the level of anti-apoptosis proteins including Bcl-2 and Bcl-xl.
Conclusion
Our study revealed that novel compound BA-78, possessing betulinic acid and nitric oxide (NO)-releasing moiety, could be developed as an antitumor agent.
Introduction
Natural products have always been the main source of anticancer drugs, especially in the treatment of solid tumors, against which targeted therapies have shown disappointing efficiency.Citation1 Betulinic acid (BA) (), isolated from the root of Pulsatilla chinensis (Bge) has long been used in traditional treatments.Citation2 BA exhibits multiple biological activities, including anti-tumor, anti-HIV and antiangiogenesis,Citation3,Citation4 among which, its unique, safe and remarkable anticancer pharmacological profile is of particular interest.Citation5 It is generally accepted that the apoptotic events induced by BA were associated with apoptosis-related proteins.Citation6 It activates the mitochondrial pathway of apoptosis, which results in the release of cytochrome c (Cyt-c).Citation7 However, the development of BA for cancer therapy is hampered largely by its modest efficacy and broad range of pharmacological activity. Therefore, it is highly desirable to develop novel derivatives of BA to improve its cytotoxic potency while reducing its side effects.
Nitric oxide (NO) is a gas signaling molecule, which plays multiple roles in physiological conditions or under pathologic processes.Citation8 Studies have shown that high level of NO cannot only induce apoptosis of tumor cells, but can also sensitize tumor cells to chemotherapy.Citation9 However, limited by the gas property of NO, NO donors are alternatively used as surrogates for NO in anticancer studies. Diazeniumdiolates (NONOates) are considered as reliable sources of NO,Citation10 as shown in , NONOate prodrugs could form the parent anion under physiological conditions (pH 7.4, 37°C), which further decomposes to release 2 molecules of NO. Recently, abundant NONOate-based hybrids have been developed as anticancer agents, including PABA/NO (2), JS-K (3), 5-Fu/NO (4), and oridonin/NO (5) ().Citation11–Citation14 It was observed that the derivatives releasing higher amount of NO showed superior antiproliferative activity, suggesting that NO was a component of the cytostatic effects of such kind of hybrids.
Figure 2 Mechansim of NO release from diazeniumdiolate and the structures of PABA/NO (2), JS-K (3), 5-Fu/NO (4), and oridonin/NO (5).
Encouraged by these findings, we designed and synthesized a novel hybride (BA-78) containing BA and NONOate moieties to improve the antiproliferative activity of natural BA. Herein, we report the synthesis, in vitro antiproliferative activity and NO release ability of BA-78, and evaluate its possible mechanism of action.
Materials and methods
Materials
Mouse melanoma cells (B16F10), human breast cancer cells (MCF-7), human colon cancer cells (HCT-116), human adenocarcinomic alveolar basal epithelial cells (A549), human hepatocarcinoma (HepG2) and human embryonic lung fibroblast (Helf) were purchased from American Type Culture Collection (Manassas, VA, USA). 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) and propidium iodide (PI) were purchased from Sigma Chemical Co. (St Louis, MO, USA). The Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit was purchased from BD Parmingen (San Diego, CA, USA). Griess reagent was purchased from Beyotime (Jiangsu, China). Primary antibodies against Bax, Bcl-xl, Bak, Bid, Bad, Bcl-2, Cyt-c and β-actin were purchased from Cell Signaling Technology, (Boston, MA, USA) and fluorescent secondary antibodies (goat-anti-rabbit or goat-anti-mouse) were purchased from Life Technologies (Grand Island, NY, USA). All cell culture reagents, including no-essential amino acids and the bicinchoninic acid (BCA) protein assay kit were purchased from Life Technologies.
Chemistry
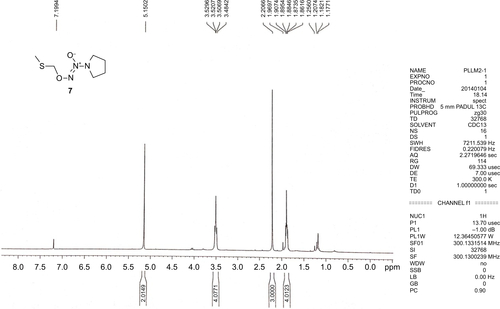
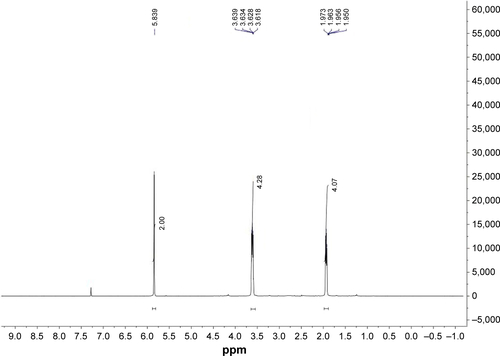
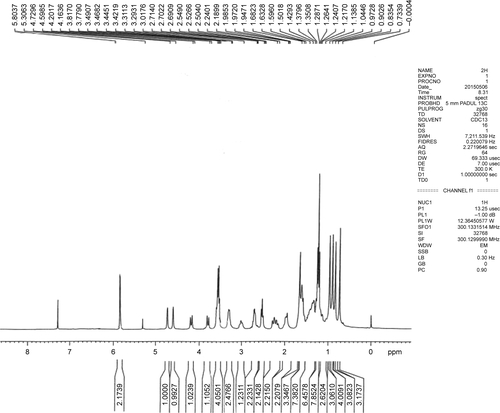
All commercially available solvents and reagents were used without further purification. Flash column chromatography was carried out on 200–300 mesh silica gel. 1H nuclear magnetic resonance (NMR) and 13C NMR spectra were recorded with a Bruker AV-300 spectrometer in the indicated solvents (trimethylchlorosilane [TMS] as internal standard). Mass spectra were obtained using FTMS-2000. The synthetic method and physicochemical date of compounds 6 and 9 have been disclosed in previous reports, the NMR spectrums of compounds 7, 8, and 10 are available in –.Citation15,Citation16
O2-(Methylthiomethyl)-1-(pyrrolidin-1-yl) diazen-1-ium-1,2-diolate (7)
The sodium NONOate 6 (6.88 g, 45.0 mmol) was added to a suspension of sodium carbonate (4.78 g, 45.0 mmol) and dimethylformamide (DMF) (100 mL) at room temperature, and this mixture was stirred for 10 min. Chloromethyl methyl sulfide (3.72 mL, 45.0 mmol) was added dropwise, and the reaction was allowed to proceed at room temperature for 4 h with stirring. Ethyl acetate (200 mL) was added to quench the reaction, the solids were filtered off, the organic phase was washed with water (5×60 mL), and dried with Na2SO4. The solvent was removed in vacuo to give a liquid residue, which was purified by silica gel column chromatography using EtOAc/hexane (1:4, v/v) as the eluent. Compound 7 was obtained as a canary yellow liquid (3.80 g, 45%): 1H NMR (300 MHz, CDCl3): δ (ppm) 5.15 (s, 2 H), 3.53–3.48 (m, 4 H), 2.21 (s, 3 H), 1.91–1.86 (m, 4 H).
O2-Chloromethyl-1-(pyrrolidin-1-yl) diazen-1-ium-1,2-diolate (8)
A solution of compound 7 (273 mg, 1.44 mmol) in dry dichloromethane (30 mL) was cooled to 0°C and sulfuryl chloride (1.44 mL, 1.0 M solution in dichloromethane) was added dropwise, the ice bath was removed and the reaction mixture was stirred at room temperature for another 2 h. The solvent was evaporated to afford compound 8 in nearly quantitative yield, which was used without further purification. 1HNMR (300 MHz, CDCl3): δ (ppm) 5.80 (s, 2 H), 3.63 (m, 4 H), 1.95 (m, 4 H).
BA-78
To a solution of 0.1 g, 9 mmol in anhydrous DMF was added Cs2CO3 (1 eq.) and compound 8 (1.2 eq.). The reaction was stirred at room temperature for 6 h. The reaction mixture was diluted with 20 mL of ethyl acetate, and the organic layer was separated. The aqueous layer was extracted with ethyl acetate (2×10 mL). The combined organic layer was dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The crude material was purified by flash column chromatography (1:1 hexane/ethyl acetate) to afford product 10 (51%) as a white solid. 1H-NMR (CDCl3, 300 MHz): δ 0.73, 0.84, 0.90, 0.97, 1.68 (each 3 H, s), 1.59 (m, 4 H), 1.98 (2 H, m), 2.24 (2 H, m), 3.01 (1 H, m), 3.49 (4 H, t), 3.81, 4.01 (each 1 H, m), 4.59, 4.72 (each 1 H, m), 5.80 (2 H, s); 13C-NMR (CDCl3, 75 MHz): δ 15.9, 16.6, 18.1, 19.3, 20.8, 15.4, 26.2, 29.7, 29.8, 30.6, 31.1, 32.3, 37.0, 38.3, 38.5, 39.2, 40.6, 40.8, 42.1, 42.4, 45.1, 46.9, 49.2, 50.5, 56.3, 67.2, 72.1, 96.5, 109.6, 152.8, 173.4, 179.7; mass spectrometry (MS) electrospray ionization (ESI) m/z [M+H]+ 642.4, high resolution mass spectrometer (HRMS) (ESI) m/z calculated for C37H63N4O6 [M+NH4]+ 659.4742, found 659.4738.
Determination of in vitro antiproliferative activity
The viability of 5 human cancer cell lines (B16F10, MCF-7, HCT-116, A549, and HepG2) treated with BA, BA-78, and Cisplatin, was determined using the MTT assay. Briefly, cells were seeded in 96-microwell plates, incubated at 37°C in a humidified incubator with 5% CO2 for 24 h prior to the experiments. After removing the medium, 100 μL of fresh medium containing the test compounds at 5 concentrations (ie, 10−4, 10−5, 10−6, 10−7, and 10−8 M) were added to microtiter plates and cells were incubated with each compound at 37°C for another 72 h. At the end of the incubation, the medium was discarded, cells were washed with PBS and 100 μL of MTT buffer was added to each well. Cells were incubated with MTT for at least 4 h at 37°C and the viabilities were proportioned to the value of optical densities, which was quantitatively measured by spectrophotometry (Biorad, Nazareth, Belgium) at 570 nm. The dose–response curve (not shown) was created by plotting the percent viability against the log of concentrations of the corresponding compound for each cell line. The molar concentrations that caused 50% cell killing (IC50) were determined.
Detection of NO production
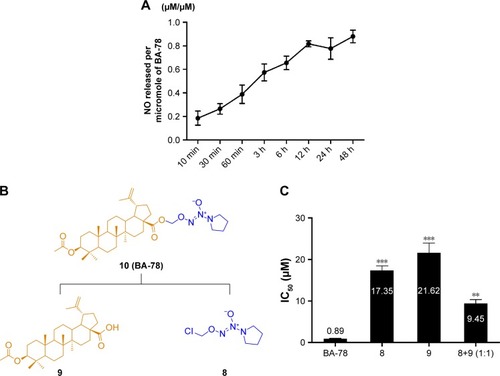
NO was indirectly determined by measuring nitrite content in the culture supernatant using Griess reagent. Briefly, B16F10 cells (2×104 cells/mL) were seeded in a 96-well plate and incubated at 37°C for 24 h followed by incubation with 10 μM of BA-78 for different times (ie, 10, 30, 60 min, 3, 6, 12, 24, and 48 h). For each well, 50 μL of supernatants was collected in a tube, the collection was incubated with 25 μL of sulfanilic acid for 10 min at room temperature in the dark. After the reaction, 25 μL of N-(1-naphthyl) ethylenediamine was added into the tube and the plate was incubated for another 10 min at room temperature in the dark. The absorbance was read at 550 nm by a spectrophotometry (BioTek Instruments, Inc., Waltham, MA, USA). The amount of NO was quantified according to the standard curve prepared under the same condition and expressed as NO released/micromole of BA-87.
Determination of apoptosis by flow cytometry
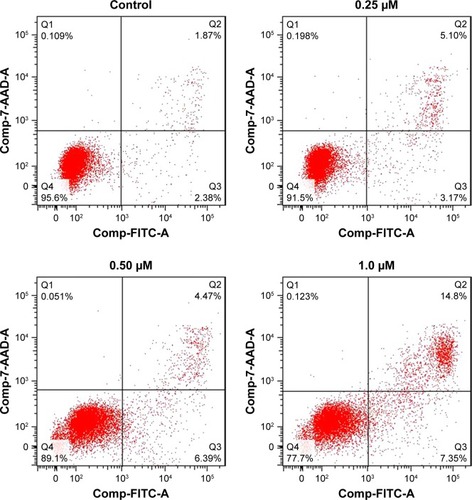
B16F10 cells (5×105 cells/mL) were seeded in 6-well plates and treated with BA-78 at different concentrations (ie, 0, 0.25, 0.5, and 1.0 μM) for 36 h. The cells were then washed twice and digested with trypsin. After centrifugation, the supernatants were removed and cells were resuspended in 500 μL of 1× binding buffer to obtain a concentration of 1×106 cell/mL. Annexin V-FITC 5 μL and PI 10 μL were added and cells were incubated at room temperature for another 15 min in the dark. The stained cells were analyzed by a FC500 cytometer (Beckman Coulter, Brea, CA, USA).
Western blot analysis
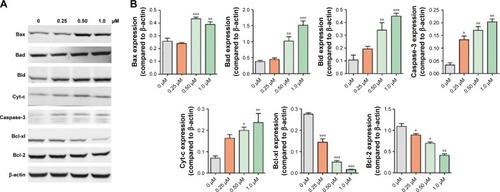
B16F10 cells were incubated with BA-78 at different concentrations (ie, 0, 0.25, 0.5, and 1.0 μM) for 48 h. Cells were washed with cold PBS twice and lysed on ice for 10 min, and lysates were centrifuged at 13,000 g for 15 min. The protein concentration in the supernatant was determined using BCA protein assay. Equal amounts of protein were loaded and separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membrane. Membranes were blocked for 1 h at room temperature with 5% bovine serum albumin and then were incubated with primary antibodies against Bax, Bad, Bid, Bak, Bcl-xl, Bcl-2, cytochrome c, caspase-3 and β-actin (all purchased from Cell Signaling Technology) overnight at 4°C. After the membranes were washed, they were then incubated with fluorescent secondary antibodies for 60 min at room temperature. The membranes were washed and proteins were detected with LI-COR Odyssey Infrared Imaging System.
Cell cycle analysis
5×104 B16F10 cells were seeded into 6-well plates and incubated overnight. After incubation with various concentrations of BA-78 for 48 h, cells were harvested, washed with cold PBS and fixed with 70% ethanol in PBS at −20°C for 12 h. Subsequently, the cells were resuspended in PBS containing 100 μg/mL RNase and 50 μg/mL PI and incubated at 37°C for 30 min. Cell cycle distribution of nuclear DNA was determined by flow cytometry on a FC500 cytometer (Beckman Coulter).
Statistical analysis
Data were expressed as mean ± standard error of the mean. Data with normal distribution were analyzed by t-test or one-way analysis of variance non-parametric analysis followed by the Bonferroni post hoc test. Data with abnormal distribution were analyzed by Kruskal–Wallis test (GraphPad 5.0). P<0.05 was considered statistically significant.
Results
Chemistry
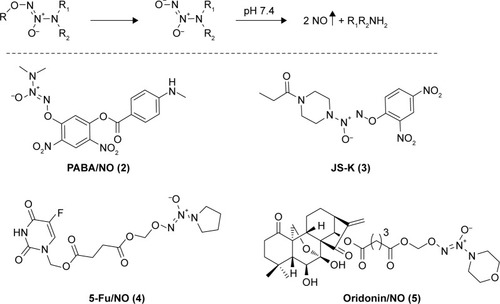
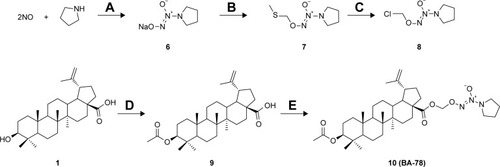
As shown in , the synthesis of NONOates was carried according to the literature in the presence of nanometer-sized TiO2.Citation16 Briefly, reaction of pyrrolidine with NO gas at room temperature under atmospheric pressure afforded O2-sodium-1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolates (6) in 78% yield. The sodium salts were alkylated with chloromethyl methyl sulfide to afford O2-(methylthiomethyl)-1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolates (7), which were subsequently reacted with sulfuryl chloride in dichloromethane to afford the O2-chloromethyl-protected NONOate (8). This preparative approach of NONOates under atmospheric pressure using easily available apparatus promoted the following synthesis of BA-78. Next, acetylated BA (9) was prepared by using acetic anhydride in 92% yield, which subsequently reacted with NONOate (8) to afford the target compound 10 (BA-78) in yield of 51%.
Cytostatic effects of BA-78 against human cancer cells
With BA-78 in hand, we first evaluated its cytotoxicity on cancer cell by MTT method. As shown in , BA-78 displayed preferred inhibitory efficacies than BA against the selected malignant cell lines, with IC50 values ranging from 0.89 to 7.44 μM. The most sensitive cancer cell line was B16F10, against which the IC50 value of BA-78 was 0.89 μM, which were even lower than that of positive control cisplatin. This is in line with reports that BA is more potent against melanoma. In addition, similar experiments were performed with the Helf and human normal liver cells (L02). The results showed that BA-87 had little inhibiting effects on the growth of Helf and L02 cells; the IC50 values on Helf and L02 cells were much higher than that on cancer cells. From the growth response curves (Supplementary materials) of BA-78 on these cells at a concentration of 1 μM, it can be inferred that the growth of Helf and L02 cells were not affected at all, while the growth of tumor cells were affected significantly. These data suggested that BA-78 has selective cancer cell killing properties, and will be safe for cancer treatment.
Table 1 Antiproliferative activities of BA-78 against different cell lines
Nitrogen oxide-releasing efficacy of BA-78
The NO-releasing efficacy has been proven to be an important component for the antiproliferative activity of NO-based hybrids.Citation17 Using Griess assay, we tried to characterize the NO release in cancer cells and identify the correlation between NO production level and cytotoxicity of BA-87. As shown in , ~80% of nitrite/nitrate was detected in the supernatant of B16F10 cell culture subjected with BA-78 after 12 h, suggesting that NO might be a key factor in the cytotoxicity of such hybride. Furthermore, the cytotoxicity of components 8, 9, and their equimolar combination (8+9) were tested against B16F10 cells (). As expected, the IC50 values of components 8, 9, and their combination were significantly higher than that of BA-87 (). Previous results suggested that the antiproliferative activity of BA-78 was attributed to the synergic effects of BA and NO donor moieties.
Figure 3 Time-dependent production of NO in B16F10 cells.
Abbreviations: B16F10, mouse melanoma cells; BA-78, synthetic route for hybride; NO, nitric oxide.
BA-78 increased level of cell apoptosis
High level of NO is toxic and able to induce apoptosis of tumor cell.Citation18 To investigate whether BA-87 can induce cell apoptosis, B16F10 cells were incubated with 0, 0.25, 0.5, and 1.0 μM of BA-78 and Annexin V-FITC/PI double staining was applied to test cell apoptosis at the end of the incubation. Annexin V is a phospholipid-binding protein with strong affinity for phosphatidylserine, which appears on the cell surface as a general indicator of apoptosis. As shown in , BA-78 dose dependently increased the percentage of Annexin V-positive cells (right quadrants, Q2+Q3) ranging from 8.27% (0.25 μM) to 22.15% (1 μM), which were significantly higher than the basement apoptosis rate in control cells (only 4.25%). shows the percentages of early apoptosis cells (Q3) and advanced apoptosis cells (Q2).
Table 2 Flow cytometry analysis of apoptotic B16F10 cells
Figure 4 Effect of BA-78 on the induction of apoptosis in B16F10 cells.
Abbreviations: B16F10, mouse melanoma cells; BA-78, synthetic route for hybride; FITC-A, fluorescein isothiocyanate-annexin.
BA-78 reduced level of anti-apoptosis proteins and increased level of pro-apoptosis proteins
The previous flow cytometry results indicated the pro-apoptosis activity of BA-78. We further evaluated the results by detecting the expression of apoptosis-related proteins. The Bcl-2 family of proteins determines cell fate by regulating the integrity of the mitochondrial outer membrane (MOM). It includes adaptor proteins only containing BH3 domain such as Bim and Bid, which initiate apoptosis. The second group are prosurvival proteins (Bcl-2 and Bcl-xL), which inhibit the activation of a pro-apoptosis group consisting of Bax and Bak. The latter constitute the pore-like structures in the MOM, from which Cyt-c could be released and initiate mitochondrial apoptosis pathway.Citation19 As shown in , the expression of pro-apoptotic proteins increased with the incubation of BA-78, while the expression of antiapoptotic proteins profoundly decreased. Furthermore, the cytosolic expression of Cyt-c, which plays a key role in the caspase-dependent apoptotic pathway, also increased after the treatment of BA-78. These results suggested that apoptotic pathway was involved in BA-78 induced cell death.
Figure 5 WB analysis of apoptosis-related proteins in B16F10 cells treated with BA-78.
Abbreviations: B16F10, mouse melanoma cells; BA-78, synthetic route for hybride; WB, Western blot.
BA-78 induced cell cycle arrest at G1 phase
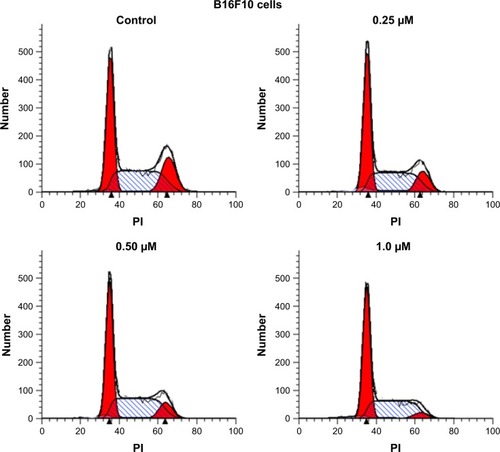
Because mitochondrial dysfunction has a close relationship with events such as apoptosis, necrosis and cell cycle arrest, we further analyzed the effect of BA-78 on cell cycle of B16F10 cells. B16F10 cells were incubated with different concentrations of BA-78 (eg, 0, 0.25, 0.5 and 1.0 μM) for 36 h, and after staining with PI, the cell cycle was analyzed by flow cytometry. As shown in , BA-78 dose dependently increased the number of B16F10 cells in G1 phase, suggesting that BA-78 could induce cell arrest at G1 phase. Accordingly, the number of cells in G2 phase reduced from 18.45% in control group to 4.84% in 1 μM of BA-78 treatment group as displayed in , indicating that BA-87 redirected cells from mitotic phase to quiescent stage. Taken together, these data showed that the anti-proliferation activity of BA-78 against cancer cell was at least partially dependent on cell cycle arrest at G1 phase.
Table 3 The influence of cell cycle progression in B16F10 cells by BA-78 at different concentrations
Figure 6 Cell cycle arrest induced by BA-78.
Abbreviations: B16F10, mouse melanoma cells; BA-78, synthetic route for hybride; PI, propidium iodide.
Scheme 1 Synthetic route for hybride (BA-78).
Abbreviations: DCM, dichloromethane; DMAP, dimethylaminopyridine; DMF, dimethylformamide.
Discussion
BA is a naturally occurring pentacyclic triterpenoid, which has been used as an anticancer agent.Citation20 In recent years, a number of structural modifications of BA have been carried out to improve its efficacy.Citation21 The positions of C-1, C-2, C-3, C-4, C-20 and C-28 are the diversity centers of BA, and some derivatives resulted from structural modifications at these positions exhibited potent anticancer activities.Citation22–Citation24 Rational design of pharmacological agents by connecting NO donors takes account of specific modifications of known molecules with the purpose of optimizing their efficacy and safety.Citation25–Citation28 NO-releasing derivatives of BA have been reported previously, a series of compounds were synthesized by connecting BA with furoxan NO donors, and they exhibited obvious cytotoxicity against human hepatocellular carcinoma cells in vitro.Citation29 In the present study, we successfully prepared a novel NO-releasing derivative of BA (BA-78) using another kind of NO donors, NONOate. As shown in , a number of NONOates-based compounds have been developed as anticancer agents. NONOates release NO spontaneously under physiological conditions (pH 7.4, 37°C) with a range of half-lives from a few seconds to several days, displaying antithrombotic, vasorelaxant, and cytostatic activities.Citation30 The nitrite measured after culture of tumor cells with BA-78 may be generated by at least 2 different modes. These are as follows: 1) oxidation of NO released from the drug by molecular oxygen, and 2) oxidation of NO by tumor cell catalase. Both processes lead to the generation of nitrite. BA showed moderate cytotoxicity on cancer cells used in our study (17.53–38.30 μM), and the connection with NONOate dramatically increased the antiproliferative efficacy of BA-78 (0.89–7.44 μM). We attributed this improvement mainly to the NO-releasing ability of BA-78. However, it should be noted that NO can both promote and inhibit tumor progression and metastasis, this dual nature of NO is greatly dependent on the dose and duration of NO exposure and cellular sensitivity.Citation31
Low concentration of NO can promote invasion and metastases in different tumor models, and high concentration of NO can inhibit tumor growth and metastases.Citation32,Citation33 NO alone shows a high IC50 concerning its cytostatic efficacy, while the reported anticancer candidates with NO-releasing residue have much lower IC50 (from hundreds nanomoles to several micromoles). It has been reported by Heigold et al that NO induced apoptosis selectively in NOX-expressing transformed cells through formation of peroxynitrite in early stages of carcinogenesis.Citation34 Later, it was found that at late stages of tumor progression, membrane-associated catalase protects from apoptosis induced by exogenous NO.Citation35 There is a delicate balance between oxidation of NO/decomposition of peroxynitrite by tumor cell protective catalase and NO-mediated inhibition of catalase.Citation36 Besides, it should be kept in mind that the intracellular NO is not always correlated with those obtained in simple phosphate buffer for the sake of cell permeability and tumor microenvironment. In addition, our study showed that BA-78 exerted much higher inhibitory activity against tumor growth than NO, it seems that other features of BA-87 besides NO release might contribute to its antitumor activity. Many research by us and others have verified these assumptions.
BA was reported to induce apoptosis by damaging the mitochondrial membrane, leading to Cyt-c release into cytosol, which, in turn, regulates the downstream caspase activation.Citation37,Citation38 In the present study, we also found the increase in Cyt-c expression in cytosol. Besides, there is a direct relationship between antiproliferative activity of BA and depression of antiapoptotic Bcl-2 family proteins.Citation39 Bcl-2 family proteins serve as critical regulators of apoptosis.Citation40,Citation41 As shown in , the expression of pro-apoptotic proteins increased with the incubation of BA-78, while the expression of anti-apoptotic proteins significantly decreased. This observation was in line with previous reports. We therefore concluded that BA-78 induced apoptosis through mitochondrial apoptotic pathway.
Mitochondria are the cell’s power plant that must be in a proper functional state in order to produce the energy necessary for basic cellular functions, such as proliferation. This is especially the case in fast-growing tumor cells.Citation42 Moreover, mitochondrial fission participates in cell mitosis, and dysfunction of mitochondrial function can, no doubt, induce arrest of cell cycle. In this study, we accordingly found that BA-78 induced cell cycle arrest in the G1 phase, which might be ascribed to the dysfunction of mitochondrial dynamics.
Conclusion
A novel BA/NONOate hybride nominated as BA-78 was designed and synthesized in this research. BA-78 exhibited excellent antiproliferative ability in melanoma B16F10 cells with an IC50 as low as 0.89 μM. Notably, NO release assay suggested that the potent cytotoxicity of BA-78 was partly attributed to NO produced from NONOate. The anticancer mechanism studies further revealed that the antiproliferative activity of BA-78 was ascribed to the induction of mitochondrial apoptosis and cell cycle arrest in the G1 phase. Taken together, these findings provide a preliminary basis for the rational design and mechanism study of NO-based derivative of BA.
Acknowledgments
This work was supported by grants from the Outstanding Leaders Training Program of Pudong Health Bureau of Shanghai (NO PWR12015-06, NO PWRd2017-11) and by a program of Shanghai Pudong Hospital (NO 201602), Fudan University. Shanghai Key Laboratory of Bioactive Small Molecules, Department of Pharmacology, School of Pharmacy and Institutes of Biomedical Sciences, Fudan University, Shanghai, China. Laiyin Zhang is the first author for this study.
Supplementary materials
Disclosure
The authors report no conflicts of interest in this work.
References
- NewmanDJDeveloping natural product drugs: supply problems and how they have been overcomePharmacol Ther20161621926706239
- ZhouJLiCJYangJZLupane triterpenoids from the stems of Euonymus carnosusJ Nat Prod201477227628424467317
- YeWCJiNNZhaoSXTriterpenoids from Pulsatilla chinensisPhytochemistry19964237998028768325
- YogeeswariPSriramDBetulinic acid and its derivatives: a review on their biological propertiesCurr Med Chem200512665766615790304
- ZhangDMXuHGWangLBetulinic acid and its derivatives as potential antitumor agentsMed Res Rev20153561127115526032847
- ZhangXHuJChenYBetulinic acid and the pharmacological effects of tumor suppression (Review)Mol Med Rep20161454489449527748864
- MullauerFBKesslerJHMedemaJPBetulinic acid, a natural compound with potent anticancer effectsAnticancer Drugs201021321522720075711
- LowensteinCJDinermanJLSnyderSHNitric oxide: a physiologic messengerAnn Intreern Med19941203227237
- VasudevanDThomasDDInsights into the diverse effects of nitric oxide on tumor biologyVitam Horm20149626529825189391
- KeeferLKNitric oxide (NO)- and nitroxyl (HNO)-generating diazeniumdiolates (NONOates): emerging commercial opportunitiesCurr Top Med Chem20055762563616101424
- ShamiPJSaavedraJEBonifantCLAntitumor activity of JS-K [O2-(2,4-dinitrophenyl)1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate] and related O2-aryl diazeniumdiolates in vitro and in vivoJ Med Chem200649144356436616821795
- AndreiDMaciagAEChakrapaniHCitroMLKeeferLKSaavedraJEAryl bis(diazeniumdiolates): potent inducers of S-glutathionylation of cellular proteins and their in vitro antiproliferative activitiesJ Med Chem200851247944795219053760
- CaiTBTangXNagorskiJBrauschweigerPGWangPGSynthesis and cytotoxicity of 5-fluorouracil/diazeniumdiolate conjugatesBioorg Med Chem200311234971497514604659
- XuSWangGLinYNovel anticancer oridonin derivatives possessing a diazen-1-ium-1,2-diolate nitric oxide donor moiety: design, synthesis, biological evaluation and nitric oxide release studiesBioorg Med Chem Lett201626122795280027158140
- Medina-O’DonnellMRivasFReyes-ZuritaFJSemi-synthesis and antiproliferative evaluation of PEGylated pentacyclic triterpenesEur J Med Chem2016118647827128174
- HuangZZhangYFangLNanometresized titanium dioxide-catalyzed reactions of nitric oxide with aliphatic cyclic and aromatic aminesChem Commun (Camb)20091317631765
- LiDHanTTianKNovel nitric oxide-releasing spirolactonetype diterpenoid derivatives with in vitro synergistic anticancer activity as apoptosis inducerBioorg Med Chem Lett201626174191419627491707
- BobbaAAtlanteAMoroLCalissanoPMarraENitric oxide has dual opposite roles during early and late phases of apoptosis in cerebellar granule neuronsApoptosis20071291597161017503222
- KvansakulMHindsMGThe Bcl-2 family: structures, interactions and targets for drug discoveryApoptosis201520213615025398535
- PishaEChaiHLeeISDiscovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosisNat Med1995110104610517489361
- MukherjeeRKumarVSrivastavaSKAgarwalSKBurmanACBetulinic acid derivatives as anticancer agents: structure activity relationshipAnticancer Agents Med Chem20066327127916712455
- KhanIGuruSKRathSKA novel triazole derivative of betulinic acid induces extrinsic and intrinsic apoptosis in human leukemia HL-60 cellsEur J Med Chem201610810411626629862
- SpivakAYKeiserJVargasMSynthesis and activity of new triphenylphosphonium derivatives of betulin and betulinic acid against Schistosoma mansoni in vitro and in vivoBioorg Med Chem201422216297630425245671
- DuttaDChakrabortyBSarkarAChowdhuryCDasPA potent betulinic acid analog ascertains an antagonistic mechanism between autophagy and proteasomal degradation pathway in HT-29 cellsBMC Cancer2016162326772983
- NandurdikarRSMaciagAECaoZKeeferLKSaavedraJEDiazeniumdiolated carbamates: a novel class of nitric oxide donorsBioorg Med Chem20122062025202922356735
- HanCHuangZZhengCNovel hybride of (phenylsulfonyl) furoxan and anilinopyrimidine as potent and selective epidermal growth factor receptor inhibitors for intervention of non-small-cell lung cancerJ Med Chem201356114738474823668441
- VanniniFMacKessack-LeitchACEschbachEKChattopadhyayMKodelaRKashfiKSynthesis and anti-cancer potential of the positional isomers of NOSH-aspirin (NBS-1120) a dual nitric oxide and hydrogen sulfide releasing hybridBioorg Med Chem Lett201525204677468226323873
- FuJLiuLHuangZHybrid molecule from O2-(2,4-dinitrophenyl) diazeniumdiolate and oleanolic acid: a glutathione S-transferase π-activated nitric oxide prodrug with selective anti-human hepatocellular carcinoma activity and improved stabilityJ Med Chem201356114641465523617697
- LiuJHTangJZhuZFChenLDesign, synthesis, and anti-tumor activity of novel betulinic acid derivativesJ Asian Nat Prod Res2013161344224350921
- HollandRJKloseJRDeschampsJRCaoZKeeferLKSaavedraJEDirect reaction of amides with nitric oxide to form diazeniumdiolatesJ Org Chem201479199389939325210948
- FukumuraDKashiwagiSJainRKThe role of nitric oxide in tumor progressionNat Rev Cancer20066752153416794635
- RabenderCSAlamASundaresanGThe role of nitric oxide synthase uncoupling in tumor progressionMol Cancer Res20151361034104325724429
- RenZGuXLuBAnticancer efficacy of a nitric oxide-modified derivative of bifendate against multidrug-resistant cancer cellsJ Cell Mol Med20162061095110526864945
- HeigoldSSersSBechtelWIvanovasBSchäferRBauerGNitric oxide mediates apoptosis induction selectively in transformed fibroblasts compared to nontransformed fibroblastsCarcinogenesis200223692994112082014
- HeinzelmannSBauerGMultiple protective functions of catalase against intercellular apoptosis-inducing ROS signaling of human tumor cellsBiol Chem2010391667569320370323
- BauerGIncreasing the endogenous NO level causes catalase inactivation and reactivation of intercellular apoptosis signaling specifically in tumor cellsRedox Biol20156357371
- PotzelLMullauerFBColakSKesslerJHMedemaJPBetulinic acid-induced mitochondria-dependent cell death is counterbalanced by an autophagic salvage responseCell Death Dis20145e116924722294
- LiuYLuoWBetulinic acid induces Bax/Bak-independent cytochrome c release in human nasopharyngeal carcinoma cellsMol Cells201233551752422526391
- RzeskiWStepulakASzymańskiMBetulinic acid decreases expression of bcl-2 and cyclin D1, inhibits proliferation, migration and induces apoptosis in cancer cellsNaunyn Schmiedebergs Arch Pharmacol20063741112016964520
- AshkenaziAFairbrotherWJLeversonJDSouersAJFrom basic apoptosis discoveries to advanced selective BCL-2 family inhibitorsNat Rev Drug Discov201716427328428209992
- AdamsJMCorySBcl-2-regulated apoptosis: mechanism and therapeutic potentialCurr Opin Immunol200719548849617629468
- HorbayRBilyyRMitochondrial dynamics during cell cyclingApoptosis201621121327133527658785