Abstract
The mutL homolog-1 (MLH1) is a DNA mismatch repair gene and has been reported to be frequently methylated in numerous cancers. However, the association between MLH1 methylation and esophageal cancer (EC), as well as its clinical significance, remains unclear. Hence, we conducted a systematic meta-analysis based on 19 articles (including 1384 ECs, 345 premalignant lesions, and 1244 healthy controls). Our analysis revealed that the frequency of MLH1 methylation was significantly elevated during EC carcinogenesis. In addition, we observed that MLH1 promoter methylation was associated with age (odds ratio [OR]=1.79; 95% CI =1.20–2.66), advanced tumor grade (OR=3.7; 95% CI =2.37–5.77), lymph node metastasis (OR=2.65; 95% CI =1.81–3.88), distant metastasis (OR=7.60; 95% CI =1.23–47.19), advanced clinical stage (OR=4.46; 95% CI =2.88–6.91), and poor prognosis in EC patients (hazard ratio =1.64, 95% CI =1.00–2.69). The pooled sensitivity, specificity, and area under the curve of MLH1 methylation in EC patients versus healthy individuals were 0.15, 0.99, and 0.77, respectively. Our findings indicate that MLH1 methylation is involved in the carcinogenesis, progression, and metastasis of EC. Moreover, methylated MLH1 could be a potential diagnostic and prognostic biomarker for EC.
Introduction
Esophageal cancer (EC) is the eighth most common cancer globally and has the sixth poorest survival rate.Citation1 The worldwide incidence of EC has been increasing for several decades for reasons that are not entirely clear but may be related to the increasing prevalence of risk factors such as smoking, alcohol intake, and obesity.Citation2,Citation3 Esophageal squamous cell carcinoma (ESCC) is the predominant histological type worldwide, with the majority of ESCCs occurring in Asia and southeastern Africa.Citation4 However, in Western Europe and northern America, there is a preponderance of esophageal adenocarcinoma (EAC).Citation5 Despite the development of adequate treatments, including endoscopic resection, surgical resection, chemotherapy, and radiotherapy, treatment outcomes are far from satisfactory, and the 5-year survival rates are ~15%–25%.Citation2,Citation6 Due to the insidious early symptoms of EC and the lack of effective screening techniques, most patients are diagnosed in an advanced stage.Citation7 Therefore, more effective and robust biomarkers are in great demand for the early screening, diagnosis, and prognosis of EC.
EC carcinogenesis is a complex and multifactorial process that includes the accumulation of multiple genetic and epigenetic changes. The precursor lesions for EAC and ESCC are Barrett’s esophagus (BE) and dysplasia, respectively. A minority of individuals with precancerous lesions will develop EC through a progression sequence.Citation8 Encompassing complicated aspects of cancer development, epigenetic modification is considered to have a crucial role in the carcinogenesis of EC.Citation9 As one of the most important epigenetic alterations, abnormal DNA methylation in the promoter region induces transcriptional silencing of tumor suppressor genes (TSGs) and plays an important role in the progression of several cancers, such as cervical cancer,Citation10 hepatocellular carcinoma,Citation11 and head and neck squamous cell carcinoma.Citation12 In addition, aberrant methylation has been shown to occur early in the progression of precancerous lesions to EC.Citation13 Moreover, due to precise and convenient methods of detection, DNA methylation has become a noninvasive biomarker for the early detection and diagnosis of cancer.Citation14
The mutL homolog-1 (MLH1) gene on chromosome 3p22.3 is a key component of the DNA mismatch repair (MMR) pathway, which is a system for recognizing and repairing erroneous insertion, deletion, and misincorporation of bases during DNA replication and is critical for maintaining genomic stability.Citation15 Inactivation of MLH1 was reported to be a contributing factor in the initiation and development of gastrointestinal cancer exhibiting high-frequency microsatellite instability.Citation16,Citation17 Moreover, hypermethylation of the MLH1 gene promoter was shown to be responsible for the loss of MLH1 expression in a wide variety of cancers, including lung cancer,Citation18 colorectal cancer,Citation19 gastric carcinoma,Citation20 ovarian cancer,Citation21 and ECs.Citation22 However, there were inconsistent results among different studies in assessing the association between MLH1 promoter methylation and EC. In addition, the role of MLH1 promoter methylation in EC carcinogenesis and its clinical application for EC diagnosis and prognosis remain less intensely investigated.
Therefore, we carried out a meta-analysis to evaluate the association between MLH1 promoter methylation and EC risk and its role in EC carcinogenesis. We also determined whether MLH1 promoter methylation was correlated with clinicopathological characteristics and overall survival (OS) of EC patients. In addition, we assessed the diagnostic value of MLH1 methylation for EC.
Materials and methods
Literature search
PubMed, Google Scholar, Web of Science, Embase, and China National Knowledge Infrastructure, and Wanfang databases were systematically searched to find eligible studies without language restrictions published prior to May 5, 2017. We used the following key words and search terms, individually as well as in various combinations: “MLH1,” “hMLH1,” “MutL homolog-1,” “methylation,” “DNA methylation,” “promoter methylation,” “esophageal carcinoma,” “esophagus cancer,” “esophageal tumor,” and “esophageal malignancy.” Furthermore, we manually reviewed the reference lists of the initially identified articles to find more potentially relevant articles.
Selection criteria
For the studies to be included in the meta-analysis, they had to meet the following criteria: 1) study samples were confirmed by pathology, including ECs, esophageal precancerous lesions (dysplasia or BE), and normal controls; 2) studies that evaluated the methylation frequency of the MLH1 promoter in the progression of EC carcinogenesis or assessed the association between MLH1 methylation status and the prognosis of EC patients; 3) studies that were of case–control or cohort designs; and 4) studies that provided sufficient data regarding the methylation frequency of the MLH1 promoter to enable the calculation of odds ratios (ORs) and 95% CIs or have reported hazard ratios (HRs) and corresponding 95% CIs. If the authors published several articles using the same (or overlapping) data, only the study with the most complete or up-to-date information was included in the meta-analysis.
Data quality assessment
The quality of studies was assessed according to the Newcastle–Ottawa Scale (NOS) criteria.Citation23 The NOS evaluation system includes three aspects: 1) subject selection: 0–4 points; 2) comparability of subjects: 0–2 points; and 3) clinical outcome: 0–3 points. NOS scores range from 0 to 9, and a score ≥7 indicates a good quality. Only studies with scores ≥7 were included in the analysis.
Data extraction
Three reviewers (JL, DY, and CCZ) independently extracted relevant data from eligible articles using a standardized form. The following information was extracted: first author, publication year, countries, the ethnicity of subjects, the number of samples, control source, methods to detect MLH1 methylation, frequency of MLH1 methylation, HR and the corresponding 95% CI for EC patients with methylated MLH1, and clinicopathological characteristics (including age, gender, smoking history, alcohol consumption, tumor location, differentiation grade, tumor stage, lymph node metastasis, distant metastasis, and clinical stage). The three reviewers discussed any discrepancies and eventually reached consensus.
Statistical analyses
All the analyses were conducted using Stata statistical software, Version 12.0 (Stata Corporation, College Station, TX, USA). The pooled ORs and corresponding 95% CIs were used to evaluate the strengths of the associations between MLH1 methylation and the development of EC carcinogenesis, along with clinicopathological features of EC patients. The assessment of potential heterogeneity was quantified based on Cochran’s Q-testsCitation24 and I-squared (I2) tests,Citation25 with statistically significant heterogeneity defined as P<0.05 or I2>50%. A random-effects modelCitation26 was used to calculate the pooled OR when significant heterogeneity was observed; otherwise, a fixed-effects model was applied.Citation27 Subgroup analyses stratified by ethnicity, histology, control source, detection method, sample size, and publication year were performed to detect potential sources of heterogeneity and lower the between-study heterogeneity. We also performed a sensitivity analysis to assess the robustness of the results and determine the influence of individual studies on the pooled results.Citation28 Publication bias was quantitatively assessed by using Begg’s linear regression testsCitation29 and Begg’s rank correlation.Citation30 HRs with 95% CIs were calculated to evaluate the association between MLH1 methylation and the prognosis of EC patients. Pooled sensitivity and specificity were used to assess the diagnostic power of MLH1 methylation test for EC. Moreover, to evaluate the overall accuracy and stability of the diagnostic test, the summary receiver operating characteristic (SROC) curve and the area under the SROC curve (AUC) were also calculated.Citation31 Fagan plot analysis was performed with 25%, 50%, and 75% pretest probability to assess the diagnostic power of MLH1 methylation in clinical practice for EC diagnosis.Citation32 All P-values were two-sided, and a P-value <0.05 was considered statistically significant.
Results
Study characteristics
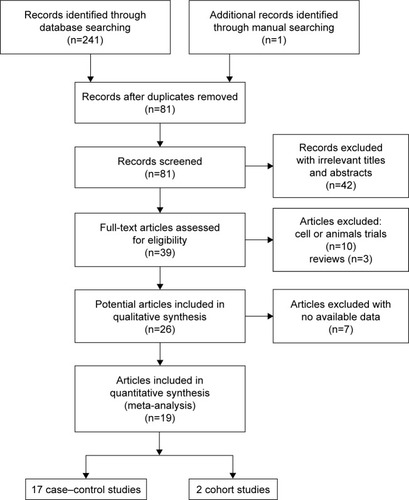
presents the strategy for the selection of included studies as well as the final studies included in the meta-analysis. A total of 242 articles were initially retrieved from a search of six databases. After reading the titles and abstracts, 161 duplicate articles were removed, and 42 articles were excluded due to unrelated content. Based on our search criteria, 39 of the remaining articles were retrieved for detailed evaluation. Among these articles, 10 were excluded due to their focus on cell lines or animal trials, and 7 articles were eliminated for having insufficient data on MLH1 promoter methylation. Finally, 19 articles (including 17 case–control and two cohort studies) fulfilled our inclusion criteria and were included in the meta-analysis.Citation22,Citation33–Citation50 presents the basic characteristics of all eligible studies.
Table 1 The basic characteristics of all eligible studies
Association between MLH1 promoter methylation and EC carcinogenesis
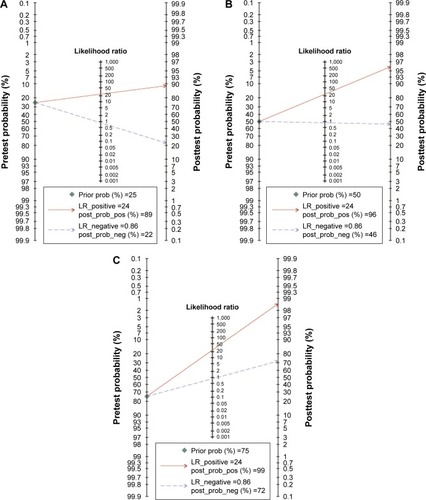
With no obvious evidence of heterogeneity between studies (cancer vs controls: I2=0%, P=0.45; cancer vs precancerous lesions: I2=0%, P=0.41; precancerous lesions vs controls: I2=0%, P=0.92), the association between MLH1 promoter methylation and carcinogenesis of EC was evaluated by using a fixed-effects model. A total of 15 case–control studies, the samples of which collectively included 1,237 ECs and 1,223 normal controls, were included in the current meta-analysis. Our results indicated that the frequency of methylation of the MLH1 promoter was significantly higher in ECs than in normal controls (OR=8.40, 95% CI =5.75–12.28, P<0.01; ). A subgroup analysis was conducted by ethnicity, histology, control source, detection method, sample size, and publication year. The results of this subgroup analysis showed that MLH1 hypermethylation was significantly associated with EC in all subgroups (). Subgroup analysis by ethnicity revealed an OR of 11.90 (95% CI =2.75–51.51, P<0.01) for Caucasian populations and 8.15 (95% CI =5.75–12.08, P<0.01) for Asian populations. The histology subgroup analysis showed that the OR was 8.32 (95% CI =5.64–12.29, P<0.01) for the ESCC subgroup, and it was 9.98 (95% CI =1.83–54.32, P<0.01) for the EAC subgroup. To test the robustness of our results, a sensitivity analysis was performed to determine the influence of an individual study on overall pooled ORs. The omission of individual studies did not significantly change the pooled OR, suggesting that the results were stable and credible (). Furthermore, a total of six studies involving 191 ECs and 262 precancerous lesions were included to evaluate the association between the methylation of MLH1 in ECs and precancerous lesions. We observed that the methylation frequency of MLH1 was markedly elevated in ECs compared with precancerous lesion samples (OR=3.08; 95% CI =1.64–5.92; P<0.01; ). The analysis of the association between MLH1 methylation and esophageal precancerous lesions included 196 precancerous samples and 192 normal controls from five studies. As demonstrated in , the methylation frequency of MLH1 was significantly higher in precancerous lesions than in controls (OR=3.75; 95% CI =1.08–13.02; P=0.04). The potential publication bias was assessed by Begg’s funnel plot analysis and Egger’s test. The results indicated no significant publication bias among the studies under analysis ().
Table 2 Subgroup analyses of MLH1 promoter methylation in esophageal cancer
Figure 2 Pooled forest plot of MLH1 methylation frequency during the carcinogenesis of esophageal cancer.
Abbreviations: MLH1, mutL homolog-1; OR, odds ratio.
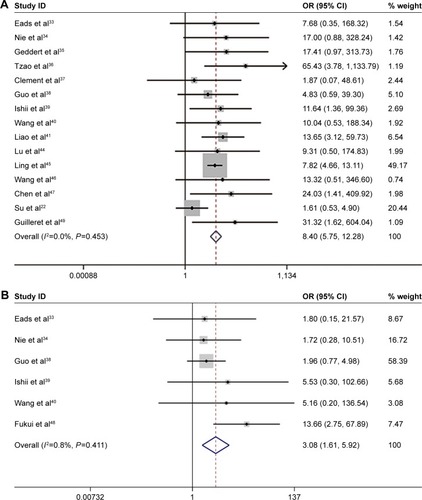
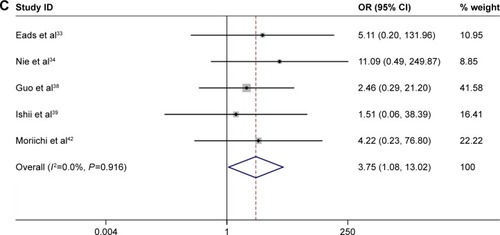
Figure 3 Sensitivity analysis of pooled ORs for the association between MLH1 methylation and esophageal cancer.
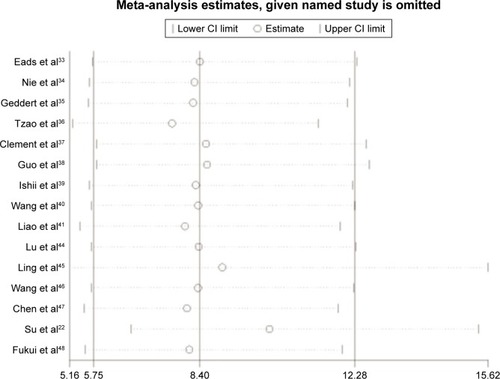
Figure 4 Begg’s funnel plots and Egger’s test of publication bias for MLH1 methylation during the carcinogenesis of esophageal cancer.
Abbreviations: EC, esophageal cancer; MLH1, mutL homolog-1; NC, normal control; PC, precancerous lesions.
MLH1 promoter methylation and clinicopathological features of EC patients
We also evaluated the association between MLH1 methylation and clinicopathological features of EC patients, including age, gender, smoking behavior, alcohol consumption, differentiation grade, location, T stage, lymph node metastasis, distant metastasis, and clinical stage. Our analyses demonstrated that MLH1 methylation was significantly associated with age (OR=1.79; 95% CI =1.20–2.66; P<0.01), T grade (OR=3.7; 95% CI =2.37–5.77; P<0.01), lymph node metastasis (OR=2.65; 95% CI =1.81–3.88; P<0.01), distant metastasis (OR=7.60; 95% CI =1.23–47.19; P=0.03), and clinical stage (OR=4.46; 95% CI =2.88–6.91; P<0.01). However, there was no correlation between MLH1 promoter methylation and other clinicopathological characteristics of EC patients ().
Table 3 Association between MLH1 promoter methylation and clinicopathological features of esophageal cancer patients
Prognostic value of MLH1 promoter methylation for EC patients
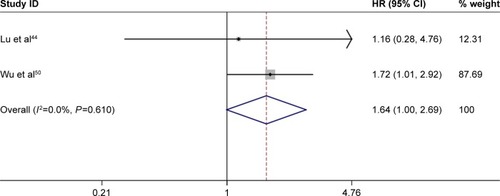
A total of 207 EC patients from two studiesCitation44,Citation50 were involved to assess the prognostic value of MLH1 promoter methylation. The results revealed that MLH1 methylation was significantly associated with poor OS of EC patients (HR =1.64; 95% CI =1.00–2.69; P<0.05; ). More studies with larger sample sizes are necessary to further validate the prognostic value of MLH1 promoter methylation in the future.
Diagnostic value of MLH1 promoter methylation for EC patients
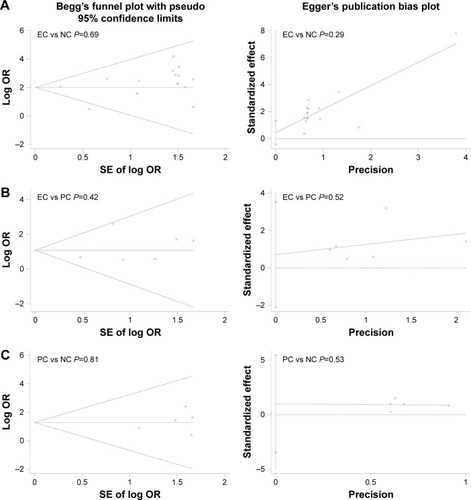
We further calculated the pooled sensitivity, specificity, and AUC and performed Fagan plot analysis from 15 case–control studies to evaluate the diagnostic value of MLH1 promoter methylation for EC patients. The pooled sensitivity, specificity, and AUC values were 0.15 (95% CI =0.08–0.25), 0.99 (95% CI =0.97–1.00), and 0.77 (95% CI =0.73–0.81), respectively (). As shown in , the pretest probability values of being diagnosed with EC were defined as 25%, 50%, and 75%, respectively. The analysis demonstrated that the probabilities of a patient being diagnosed with EC were 89%, 96%, and 99%, respectively, if the MLH1 promoter methylation detection result was positive. When the test was negative, the patient had a 22%, 46%, and 72% possibility of having EC, respectively.
Figure 6 SROC plots of methylated MLH1 for the diagnosis of esophageal cancer.
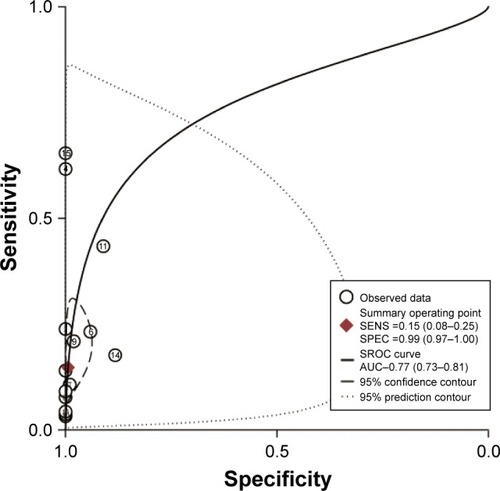
Figure 7 Fagan plot analysis to evaluate the diagnostic power of methylated MLH1 for esophageal cancer.
Abbreviations: LR, likelihood ratio; MLH1, mutL homolog-1.
Discussion
EC is one of the most fatal digestive tract malignancies, representing the eighth leading cause of cancer-related deaths worldwide.Citation51 Without effective early diagnosis biomarkers and therapeutic strategies, the prognosis of EC is relatively poor, with a 5-year survival rate of <20%, although it may be variable in different histotypes.Citation52 Aberrant DNA methylation in the TSG promoter has been identified as an important mechanism of tumorigenesis and progression of several cancers, including cervical cancer,Citation10 oral cancer,Citation53 and colorectal cancer.Citation54 In addition, as a relatively early molecular change, aberrant methylation was reported to be a potential biomarker for early cancer diagnosis.Citation55
The MLH1 gene is a critical component of the DNA MMR system and has been considered to play an essential role in maintaining genomic stability.Citation15 Several previous studies evaluated whether MLH1 promoter methylation is associated with EC risk, but the results of these studies were inconsistent because of the use of different histotypes, control types, population ethnicities, and detection methods.Citation40,Citation45 Therefore, we conducted a meta-analysis to assess the association between MLH1 promoter methylation and EC carcinogenesis. The overall OR of MLH1 promoter methylation frequency was higher in EC tissues than in control tissues, which is consistent with the results found for other types of carcinomas.Citation56 The sensitivity analysis and the absence of heterogeneity indicate that our results were stable and credible. Moreover, the subgroup analysis based on ethnicity revealed that the OR of the association between MLH1 methylation and EC was higher for Caucasian populations than for Asian populations, indicating that the Caucasian population may be more susceptible to MLH1 promoter methylation. The majority of ECs can be subdivided into two main histological subtypes: adenocarcinomas and squamous cell carcinomas. Previous studies have demonstrated the molecular separation between ESCC and EAC, showing that ESCC has a stronger resemblance to head and neck squamous cell carcinoma than to EAC and that EAC more closely resembled gastric cancer than ESCC.Citation57 In this study, the subgroup analysis of histology indicated that the OR of the EAC subgroup was greater than that of the ESCC subgroup, indicating that the methylated MLH1 gene may be used to distinguish the histotype of EC. EC is a complicated and progressive disease. EAC originates predominantly from BE, and dysplasia is the precursor for both EAC and ESCC.Citation5 Our analysis showed that the methylation level of the MLH1 promoter was also significantly higher in EC than in premalignant lesions. Moreover, the frequency of MLH1 methylation was markedly higher in premalignant lesions than in healthy controls. These results collectively indicate that hypermethylation of the MLH1 promoter is involved in the onset and carcinogenesis of EC.
Furthermore, we evaluated the relationship between MLH1 methylation and the clinicopathological parameters of EC. Age is believed to be an important cancer-related risk factor, and EC occurs mostly in patients aged >50 years, with a median age of 68 years.Citation7 Our findings revealed that MLH1 promoter methylation was more likely to occur in elderly patients, which may account for the finding that patients aged >60 years showed a rapid increase in EC.Citation7 In addition, our analysis demonstrated that the frequency of MLH1 promoter methylation was significantly elevated in advanced T grade, lymph node metastasis, distant metastasis, and advanced clinical stage EC patients, suggesting that MLH1 promoter methylation may play a critical role in EC progression and metastasis. However, there was no correlation between MLH1 promoter methylation and other clinicopathological characteristics of EC patients. We also investigated whether MLH1 promoter hypermethylation was correlated with the prognosis of EC patients, based on the prediction of OS using multivariate analysis. The results revealed that compared with EC patients with MLH1 promoter hypomethylation, those with MLH1 promoter hypermethylation had a 1.64-fold higher risk of poor OS, indicating that hypermethylation of the MLH1 promoter is a potential prognosis biomarker for EC patients. However, more studies are needed to confirm and further clarify this finding, as only 207 EC patients were analyzed in the present study.
Abnormal methylation biomarkers have proven to be useful in diagnosing numerous cancers.Citation58,Citation59 Hence, we evaluated the diagnostic effect of MLH1 promoter methylation for EC based on 15 studies of EC versus healthy subjects. The MLH1 methylation test exhibited a pooled sensitivity of 0.15, a specificity of 0.99, and an AUC of 0.77, indicating that MLH1 promoter methylation has a moderate diagnostic accuracy for EC. MLH1 promoter methylation alone may not be suitable for screening and diagnosing EC, due to its low sensitivity. However, given its near-perfect specificity, MLH1 promoter methylation is a potential diagnostic biomarker for EC if combined with other diagnostic technologies, which we confirmed using Fagan plot analysis. The Fagan plot analysis demonstrated that if the pretest probabilities were assumed to be 25%, 50%, and 75%, then 89%, 96%, and 99% of patients would be correctly diagnosed with EC following positive MLH1 methylation tests, suggesting that methylated MLH1 has effective diagnostic power to distinguish EC patients from healthy individuals. More rigorously designed studies with larger sample sizes are essential to validate our findings.
However, there were several limitations of our meta-analysis that should be noted. First, only articles published in English and Chinese were included in the study, which may have contributed to selection bias. Second, most studies were conducted in Asian and Caucasian populations, while other ethnic groups, such as Africans, were underrepresented. Third, because there were relatively few studies describing the association between MLH1 promoter methylation and clinicopathological parameters and OS, studies with larger sample sizes are needed to validate our findings. These studies should also be included in a future-updated meta-analysis to support the findings of the present study.
Conclusion
This integrated analysis provided a strong evidence that MLH1 methylation is significantly associated with the carcinogenesis, progression, and metastasis of EC. In addition, methylated MLH1 is a promising biomarker for the diagnosis and prognosis of EC. Future research with larger sample sizes and strong study designs is essential to confirm our results.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This research was supported by grants from the Zhejiang Provincial Natural Science Foundation of China (No. LY16H160005), the Project of Scientific Innovation Team of Ningbo (No. 2015B11050), and the Ningbo Natural Science Foundation (No. 2014A610235, 2015A610221, and 2017A610236).
Disclosure
The authors report no conflicts of interest in this work.
References
- ZhangYEpidemiology of esophageal cancerWorld J Gastroenterol201319345598560624039351
- Domper ArnalMJFerrandez ArenasALanas ArbeloaAEsophageal cancer: risk factors, screening and endoscopic treatment in Western and Eastern countriesWorld J Gastroenterol201521267933794326185366
- GrundyAPoirierAEKhandwalaFMcFaddenAFriedenreichCMBrennerDRCancer incidence attributable to alcohol consumption in Alberta in 2012CMAJ Open201643E507E514
- LiangHFanJHQiaoYLEpidemiology, etiology, and prevention of esophageal squamous cell carcinoma in ChinaCancer Biol Med2017141334128443201
- ArnoldMSoerjomataramIFerlayJFormanDGlobal incidence of oesophageal cancer by histological subtype in 2012Gut201564338138725320104
- RutegardMCharonisKLuYLagergrenPLagergrenJRouvelasIPopulation-based esophageal cancer survival after resection without neoadjuvant therapy: an updateSurgery2012152590391022657730
- BollschweilerEPlumPMonigSPHolscherAHCurrent and future treatment options for esophageal cancer in the elderlyExpert Opin Pharmacother2017181001101028540761
- SpechlerSJClinical practice. Barrett’s EsophagusN Engl J Med20023461183684211893796
- AhrensTDWernerMLassmannSEpigenetics in esophageal cancersCell Tissue Res2014356364365524816987
- LiJZhouCZhouHThe association between methylated CDKN2A and cervical carcinogenesis, and its diagnostic value in cervical cancer: a meta-analysisTher Clin Risk Manag2016121249126027574435
- ZhangCLiJHuangTMeta-analysis of DNA methylation biomarkers in hepatocellular carcinomaOncotarget2016749812558126727835605
- ShenZZhouCLiJDengHLiQWangJThe association, clinicopathological significance, and diagnostic value of CDH1 promoter methylation in head and neck squamous cell carcinoma: a meta-analysis of 23 studiesOnco Targets Ther201696763677327826202
- KazAMWongCJLuoYDNA methylation profiling in Barrett’s esophagus and esophageal adenocarcinoma reveals unique methylation signatures and molecular subclassesEpigenetics20116121403141222139570
- ArantesLMde CarvalhoACMelendezMECarvalhoALGoloni-BertolloEMMethylation as a biomarker for head and neck cancerOral Oncol201450658759224656975
- LarreaAALujanSAKunkelTASnapShot: DNA mismatch repairCell20101414730.e120478261
- KenyonJNickel-MeesterGQingYEpigenetic loss of MLH1 expression in normal human hematopoietic stem cell clones is defined by the promoter CpG methylation pattern observed by high-throughput methylation specific sequencingInt J Stem Cell Res Ther201632 pii:031
- ZhaoJXLiXWShiBYEffect of histone modifications on hMLH1 alternative splicing in gastric cancerTumour Biol2017394 1010428317697546
- Pastuszak-LewandoskaDKordiakJAntczakAExpression level and methylation status of three tumor suppressor genes, DLEC1, ITGA9 and MLH1, in non-small cell lung cancerMed Oncol20163377527287342
- MaYChenYPetersenIExpression and promoter DNA methylation of MLH1 in colorectal cancer and lung cancerPathol Res Pract2017213433333828214209
- LiuLYangXImplication of Reprimo and hMLH1 gene methylation in early diagnosis of gastric carcinomaInt J Clin Exp Pathol2015811149771498226823831
- SinhaSChunderNMukherjeeNFrequent deletion and methylation in SH3GL2 and CDKN2A loci are associated with early- and late-onset breast carcinomaAnn Surg Oncol20081541070108018239974
- SuYYinLLiuRPromoter methylation status of MGMT, hMSH2, and hMLH1 and its relationship to corresponding protein expression and TP53 mutations in human esophageal squamous cell carcinomaMed Oncol201431278424366688
- StangACritical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analysesEur J Epidemiol201025960360520652370
- HigginsJPThompsonSGQuantifying heterogeneity in a meta-analysisStat Med200221111539155812111919
- ZintzarasEIoannidisJPHEGESMA: genome search meta-analysis and heterogeneity testingBioinformatics200521183672367315955784
- DerSimonianRLairdNMeta-analysis in clinical trials revisitedContemp Clin Trials201545Pt A13914526343745
- MantelNHaenszelWStatistical aspects of the analysis of data from retrospective studies of diseaseJ Natl Cancer Inst195922471974813655060
- LauJIoannidisJPSchmidCHQuantitative synthesis in systematic reviewsAnn Intern Med199712798208269382404
- PetersJLSuttonAJJonesDRAbramsKRRushtonLComparison of two methods to detect publication bias in meta-analysisJAMA2006295667668016467236
- BeggCBMazumdarMOperating characteristics of a rank correlation test for publication biasBiometrics1994504108811017786990
- DevilleWLBuntinxFBouterLMConducting systematic reviews of diagnostic studies: didactic guidelinesBMC Med Res Methodol20022912097142
- FaganTJLetter: nomogram for Bayes theoremN Engl J Med197529352571143310
- EadsCALordRVWickramasingheKEpigenetic patterns in the progression of esophageal adenocarcinomaCancer Res20016183410341811309301
- NieYLiaoJZhaoXDetection of multiple gene hypermethylation in the development of esophageal squamous cell carcinomaCarcinogenesis200223101713172012376481
- GeddertHKielSIskenderECorrelation of hMLH1 and HPP1 hypermethylation in gastric, but not in esophageal and cardiac adeno-carcinomaInt J Cancer2004110220821115069683
- TzaoCHsuHSSunGHPromoter methylation of the hMLH1 gene and protein expression of human mutL homolog 1 and human mutS homolog 2 in resected esophageal squamous cell carcinomaJ Thorac Cardiovasc Surg20051305137116256791
- ClementGBraunschweigRPasquierNBosmanFTBenhattarJMethylation of APC, TIMP3, and TERT: a new predictive marker to distinguish Barrett’s oesophagus patients at risk for malignant transformationJ Pathol2006208110010716278815
- GuoMRenJHouseMGQiYBrockMVHermanJGAccumulation of promoter methylation suggests epigenetic progression in squamous cell carcinoma of the esophagusClin Cancer Res200612154515452216899597
- IshiiTMurakamiJNotoharaKOesophageal squamous cell carcinoma may develop within a background of accumulating DNA methylation in normal and dysplastic mucosaGut2007561131916785283
- WangJSascoAJFuCAberrant DNA methylation of P16, MGMT, and hMLH1 genes in combination with MTHFR C677T genetic polymorphism in esophageal squamous cell carcinomaCancer Epidemiol Biomarkers Prev200817111812518199718
- LiaoLXiangJZhangYHuangDShenZPromoter methylation and its significance of hMLH1, E-cadherin and p16INK4a gene in esophageal squamous cell carcinomaChina Oncol20095340346
- MoriichiKWatariJDasKMEffects of Helicobacter pylori infection on genetic instability, the aberrant CpG island methylation status and the cellular phenotype in Barrett’s esophagus in a Japanese populationInt J Cancer200912461263126919048617
- VasaviMKiranVRavishankarBPrabhakarBAhujaYRHasanQMicrosatellite instability analysis and its correlation with hMLH1 repair gene hypermethylation status in esophageal pathologies including cancersCancer Biomark20107111021045259
- LuCXieHWangFShenHWangJDiet folate, DNA methylation and genetic polymorphisms of MTHFR C677T in association with the prognosis of esophageal squamous cell carcinomaBMC Cancer2011119121375764
- LingZQLiPGeMHAberrant methylation of different DNA repair genes demonstrates distinct prognostic value for esophageal cancerDig Dis Sci201156102992300421674174
- WangFXieXJPiaoYSLiuBWangLDMethylation of p16 and hMLH1 genes in esophageal squamous cell carcinoma and reflux esophagitisZhonghua Bing Li Xue Za Zhi2011408537541 Chinese22169643
- ChenJHuangZJDuanYQXiaoXRJiangJQZhangRAberrant DNA methylation of P16, MGMT, and hMLH1 genes in combination with MTHFR C677T genetic polymorphism and folate intake in esophageal squamous cell carcinomaAsian Pac J Cancer Prev201213105303530623244153
- FukuiSWatariJTomitaTLocalization of specialized intestinal metaplasia and the molecular alterations in Barrett esophagus in a Japanese population: an analysis of biopsy samples based on the “Seattle” biopsy protocolHuman Pathol201651324027067780
- GuilleretILosiLChelbiSTDNA methylation profiling of esophageal adenocarcinoma using Methylation Ligation-dependent Macroarray (MLM)Biochem Biophys Res Commun2016479223123727634218
- WuDChenXXuYPrognostic value of MLH1 promoter methylation in male patients with esophageal squamous cell carcinomaOncol Lett20171342745275028454461
- SiegelRLMillerKDJemalACancer statistics, 2016CA Cancer J Clin201666173026742998
- MillerKDSiegelRLLinCCCancer treatment and survivorship statistics, 2016CA Cancer J Clin201666427128927253694
- JayaprakashCVargheseVKBellampalliRHypermethylation of death-associated protein kinase (DAPK1) and its association with oral carcinogenesis – an experimental and meta-analysis studyArch Oral Biol20178011712928412611
- YeMHuangTLiJRole of CDH13 promoter methylation in the carcinogenesis, progression, and prognosis of colorectal cancer: A systematic meta-analysis under PRISMA guidelinesMedicine2017964e595628121942
- TrujilloKAJonesACGriffithJKBisoffiMMarkers of field cancerization: proposed clinical applications in prostate biopsiesProstate Cancer2012201230289422666601
- LiXYaoXWangYMLH1 promoter methylation frequency in colorectal cancer patients and related clinicopathological and molecular featuresPLoS One201383e5906423555617
- Cancer Genome Atlas Research NetworkAnalysis Working Group: Asan UniversityBC Cancer AgencyIntegrated genomic characterization of oesophageal carcinomaNature2017541763616917528052061
- ShanMYinHLiJDetection of aberrant methylation of a six-gene panel in serum DNA for diagnosis of breast cancerOncotarget2016714184851849426918343
- YangQHuangTYeGWangBZhangXMethylation of SFRP2 gene as a promising noninvasive biomarker using feces in colorectal cancer diagnosis: a systematic meta-analysisSci Rep201663333927659069