?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Context
Previous studies have demonstrated that 3′-azido-3′-deoxythymidine (AZT) and arsenic trioxide (As2O3), traditional chemotherapy agents, can synergically inhibit the growth of hepatocellular carcinoma cells. However, the molecular mechanisms underlying As2O3 and AZT anti-hepatoma activity are unknown.
Objective
This study aimed to investigate the role of early growth response protein 1 (Egr-1) in the process of As2O3 combined with AZT inhibiting proliferation and inducing apoptosis of human hepatocellular carcinoma HepG2 cells, and explore the possible mechanism.
Materials and methods
The expression of Egr-1 was silenced using siRNA, and then HepG2 cells were treated with As2O3 (2 μM) and AZT (20 μM). The rates of cell inhibition and apoptosis were determined by the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) method and flow cytometry, respectively. The mRNA and protein expression of p53, caspase-3, and Egr-1 were detected by real-time quantitative polymerase chain reaction and Western blotting, respectively.
Results
The inhibitory rate of As2O3 (2 μM) combined with AZT (20 μM) on proliferation of HepG2 cells was significantly higher than that of As2O3 alone. The combination index (CI) values were 0.2<CI<0.4, showing strong synergic effect. After silencing Egr-1, the proliferation inhibition and proapoptotic ability of As2O3 combined with AZT on HepG2 cells were decreased, and the CI value was greater than 1, showing antagonistic effect. In addition, the expression of p53 and caspase-3 mRNA/protein was also significantly decreased.
Conclusion
The present results show that AZT could increase the sensitization of As2O3 for inhibiting proliferation and promoting apoptosis of HepG2 cells through regulating the expression of Egr-1, which may control the expression of p53 and caspase-3.
Introduction
Human hepatocellular carcinoma (HCC) is the most common malignant tumor in the liver and the third leading fatal cancer.Citation1 The prognosis for HCC remains poor, and the latency period is long, mainly due to the propensity for metastatic progression and poor response to pharmacological treatment.Citation2 At present, surgical resection remains the only curative method of treatment but is applicable to only 10%–20% of cases, with a 13% survival rate at 3 years.Citation3 Chemotherapy and radiotherapy are still the most important regimens in HCC therapy. However, these two regimens are either not effective enough to destroy the cancer cells or cause significant side effects; therefore, the development of new regimen is desirable.
Arsenic trioxide (As2O3), which is an active ingredient in traditional Chinese medicine, has been used successfully for treating acute promyelocytic leukemia (APL)Citation4 and is also effective in the treatment of solid tumors, including human hepatoma and breast cancer.Citation5,Citation6 However, this compound has not been widely used because of its toxicity. It is necessary to minimize the dosage of As2O3 without weakening its anticancer effects.
3′-Azido-3′-deoxythymidine (AZT), a powerful inhibitor of reverse transcriptase, has been used in Phase I and II clinical trials, either alone or in combination with other drugs, in the treatment of gastrointestinal cancers, and some cases of tumor regression have been reported.Citation7,Citation8 In previous studies, we reported that As2O3 combined with AZT has a significantly synergic effect on inhibiting the hepatoma cell proliferation (combination index [CI] <1) and inducing its apoptosis.Citation9 Furthermore, our results showed that As2O3 combined with AZT also synergically inhibited the migration and invasion of HepG2 cells.Citation10,Citation11 However, its anticancer targets are largely unknown.
Early growth response protein 1 (Egr-1) is an important nuclear transcription factor, which belongs to the early gene family. Some studies showed that Egr-1 suppressed tumors by regulating downstream target genes such as TGF-β, cyclin D1, c-jun, PTEN, p53, and P21.Citation12–Citation14 Shan et al reported that Egr-1 expression was downregulated in hepatocellular carcinoma, and reexpression of Egr-1 decreased cell growth and tumorigenicity in nude mice.Citation15 Of note, it has been reported that Egr-1 expression level correlates with sensitivity to chemo-drugs in cancer cells.Citation16
In the present study, we used the electroporation method of Egr-1 siRNA to explore the role of Egr-1 in HepG2 cells treated by As2O3 combined with AZT, and the effect of Egr-1 on some tumor-associated genes was also observed in an attempt to provide the molecular basis for the clinical application of As2O3 combined with AZT in hepatocellular carcinoma.
Materials and methods
Chemicals and reagents
AZT and 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazo-liumbromide (MTT) were purchased from Sigma Chemical Co. (St Louis, MI, USA); As2O3 was obtained from Shui Kou Shan Mining Bureau of Heng Yang Industrial Company (Hengyang, China); other cell culture supplies were purchased from GE Healthcare – HyClone (Logan, UT, USA); the antibodies to caspase-3, p53, and β-actin were purchased from ImmunoWay Biotechnology Co. (Newark, DE, USA); peroxidase-conjugated AffiniPure goat anti-rabbit IgG (H+L) was purchased from ZSGB-BIO Co. (Beijing, China); and real-time reverse transcriptase polymerase chain reaction (RT-PCR)-related reagents were purchased from Promega Corporation (Fitchburg, WI, USA).
Cell culture
The HHC cell lines HepG2 were purchased from JRDUN Biotechnology (Shanghai) Co. Ltd. (Shanghai, China). The HHC cell lines HepG2 was cultured in DMEM with high glucose content containing 10% heat-inactivated fetal calf serum, 100 IU/mL penicillin/streptomycin at 37°C and incubated in a 5% CO2 humidified atmosphere. Cells were harvested in logarithmic phase for experiments.
Cell electrotransfection
HepG2 cells were electrotransfected according to the manufacturer’s recommendations (Nucleofector I nuclear transfection apparatus and Amaxa® Cell Line Nucleofector® Kit L, Lonza, Basel, Switzerland) and were divided into three groups: mock (adding the same amount of culture medium), negative control (NC; transfection of non-specific sequence), and Egr1-siRNA (transfected with Egr-1 siRNA) groups. The Egr-1 siRNA segment sense strand is 5′-CCCGGUUACUACCUCUUAUTT-3′, and the antisense strand is 5′-AUAAGAGGUAGUAACCGGGTT-3′. Furthermore, a GFP group (HepG2/GFP, transfect green fluorescent protein) was designed to observe the transfection efficiency under fluorescence microscope. The transfection efficiency of Egr-1 siRNA was also detected by real-time quantitative polymerase chain reaction (qPCR) and Western blotting.
Cell proliferation assay
Drug treatment was initiated after the cells had begun attaching to the growth surface. The effects of As2O3 and AZT on HepG2 survival were determined using the MTT reduction assay described earlier, with minor modifications. In brief, 5×103 cells were plated in 96-well plates in 100 mL of a regular medium per well. After 24 h growth, the cells were treated with As2O3 (2 μmol/L) and AZT (10, 20 μmol/L) for 72 h. Untreated control and treated cells were incubated with 10 μL of MTT (5 mg/mL in serum-free medium) for 4 h before the end of the incubation period. One hundred microliters of lysis solution (10% SDS in 0.01 mol/L of HCl) were then added and another 24 h culture was made. The cell survival fraction was measured at absorbance (A) 490 nm on a microplate reader (Bio-Rad Laboratories Inc., Hercules, CA, USA). Assays were carried out in triplicate. The proliferation and inhibition rate of cells were calculated, respectively, as follows:
Synergy determination
The synergic effect between AZT and As2O3 was analyzed using the CI method. Concentration effect curves calculated for each drug, both separately and in combination, were applied to determine the amount of each drug, either separately or in combination, required to achieve a given level of effect. The CI was calculated as follows:
Apoptosis assessment
Detection of apoptotic cells was carried out by annexin V-fluorescein isothiocyanate/propidium iodide (PI) staining. Cells of the three group were plated onto six-well plates (1×105 cells/well) and grown overnight to allow for cell attachment. They were then treated in the absence (control) or presence of As2O3 (2 μmol/L) + AZT (20 μmol/L) for 48 h. At the end of the treatment, the cells were harvested, washed with PBS, resuspended in 100 μL of binding buffer, and then incubated with 5 μL of annexin V and 5 μL of PI for 30 min at room temperature in the dark according to the manufacturer’s instructions (BD Biosciences, San Jose, CA, USA). The samples were acquired on a FACScan flow cytometer (BD Biosciences) and analyzed with CellQuest Software (BD Biosciences). The amount of apoptosis was determined as the percentage of annexin V+/PI−.
Real-time PCR analysis
After 48 h of drug treatment, total RNA was extracted from cells using Trizol reagent. Reverse transcription was performed using Promega cDNA Synthesis Master mix (Promega Corporation) with 50 ng RNA per 25 μL of reaction mixture. For real-time PCR, we used the ABI System 7500 fast-real-time PCR (Thermo Fisher Scientific, Waltham, MA, USA) in standard mode. The total 25 μL reaction system contained 12.5 μL qPCR Master Mix, 1 μL forward/reverse primers, 0.25 μL 5-carboxy-X-rhodamine, 8.25 μL ddH2O, and 2 μL templates. The experiments were performed three times. PCR products were normalized against the housekeeping gene β-actin, and measurements between samples were compared by cycle threshold (Ct). The primers used in real-time PCR are as in . PCR was performed for Egr-1 or β-actin transcription as follows: denaturation at 94°C for 2 min was followed by 40 cycles of denaturation at 94°C for 15 s, annealing at 58°C for 45 s, and elongation at 60°C for 30 s, followed by a final elongation step at 60°C for 2 min.
Table 1 Primer sequence
Immunoblot analysis
Western blotting was performed using standard techniques. Three groups of cells were seeded at 1×105 cells/mL in 25 mL culture flasks and then treated with As2O3 (2 μmol/L) and AZT (20 μmol/L) and further incubated for 48 h. Cells were washed twice with PBS buffer and lysed in 1% Triton lysis buffer (1% Triton X-100, 50 mM Tris–HCl pH 7.4, 150 mM NaCl, 10 mM EDTA, 100 mM NaF, 1 mM Na3VO4, 1 mM PMSF, and 2 mg/mL aprotinin) on ice, then centrifuged at 10,000 g for 10 min to collect the supernatant. Protein concentrations were determined with a Bio-Rad protein assay. Total proteins 60 μg were subjected to sodium dodecyl sulfate-polyacrylamide gel ectrophoresis (SDS–PAGE) and transferred to polyvinylidine diflouride filter (PVDF) membranes (Hoffman-La Roche Ltd., Basel, Switzerland). Membranes were blocked with 5% skim milk in Tris-buffered saline + Tween (TBST) (10 mM Tris, pH 7.4, 150 mM NaCl, and 0.1% Tween 20) at room temperature for 2 h and incubated with the corresponding primary antibodies at room temperature for 2 h. After washing with TBST, the membrane was reacted with the appropriate horseradish peroxidase-conjugated secondary antibodies for 2 h at room temperature. After extensive washing with TBST, proteins were visualized by chemiluminescent reagents. Blots were then detected with Image Lab software.
Statistical analysis
Each experiment was performed in triplicate, and all the data were processed with SPSS 17.0 software. The results were analyzed by two-way ANOVA and Tukey’s b test. P-value <0.05 was considered statistically significant.
Results
Transfection efficiency
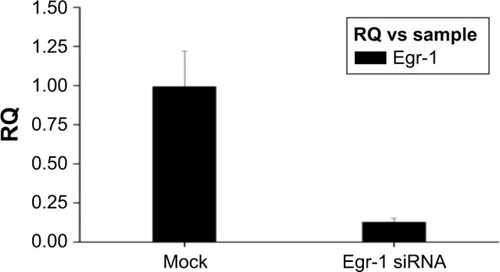
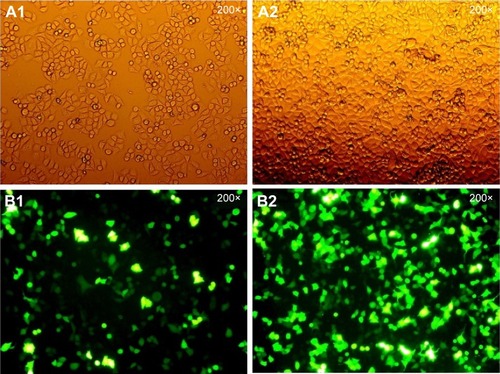
Since the GFP gene is transfected into the cell, the GFP protein can be expressed in the cell and emit green fluorescence. Therefore, the transfection efficiency can be judged by observing the number of green fluorescent cells under fluorescence microscopy. As shown in , the green fluorescent cells were significantly increased at 10 h, so the transfection efficiency could be detected at 24 h. The qPCR result showed that the transfection efficiency was over 75% (), which could meet the experimental requirements.
Figure 1 The HepG2 cells were transfected with Egr-1 siRNA.
Abbreviation: Egr-1, early growth response protein 1.
The effects of Egr-1 on HepG2 cell proliferation
After Egr-1 siRNA transfection, an MTT assay was used to detect the proliferation of the three groups. shows that the cell proliferation rate of HepG2/si-RNA group was significantly increased at 24 and 48 h compared with the NC group (HepG2/NC) and the mock group (P<0.01). The cell proliferation rate of the NC group at 24, 48, and 72 h decreased slightly, but the difference was not statistically significant compared with the mock group (P>0.05). However, the cell proliferation rate of HepG2/si-RNA group at 72 h was not significantly different compared with the NC group (HepG2/NC) and the mock group, which could be due to the decrease in the effect of silencing Egr-1 ().
Table 2 Cell proliferation rate after transfection
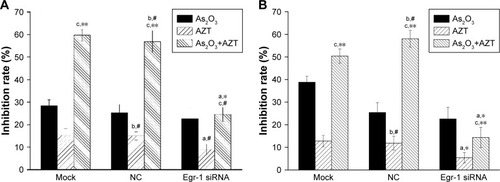
The effect of the combination of As2O3 and AZT on growth of HepG2 cells
The MTT assay results showed that 2 μmol/L As2O3 alone had slightly inhibitory effect on the proliferation of HepG2 cells, but there was no statistically significant difference between three groups (P>0.05). Compared with the NC and the mock groups, the inhibition effect of 10 and 20 μmol/L AZT on HepG2/siRNA group was decreased (P<0.05). Compared with the single drug group, 2 μmol/L As2O3 combined with 10 or 20 μmol/L AZT significantly enhanced the inhibition effect of HepG2 cells of mock and NC groups. The difference was statistically significant (P<0.01). However, after transfection of Egr-1 siRNA, the inhibition effect of the combinations of As2O3 and AZT on the proliferation of HepG2 cells was dramatically decreased (P<0.05) ().
Figure 3 Effect of As2O3 combined with AZT on cell proliferation inhibition.
Abbreviations: As2O3, arsenic trioxide; AZT, 3′-azido-3′-deoxythymidine; Egr-1, early growth response protein 1; NC, negative control.
Synergic effect of the combination of As2O3 and AZT
The synergic effect of combination of As2O3 and AZT at 72 h was analyzed by Calcusyn 2.0 software (). The results showed that after the HepG2 cells of the non-specific control group and the control group were treated with As2O3 combined with AZT, the CI values were 0.2<CI<0.4, showing strong synergic effect. However, the CI value of the siRNA group was greater than 1, showing antagonistic effect.
Table 3 CI value of combination of As2O3 and AZT in the three groups of cells (72 h)
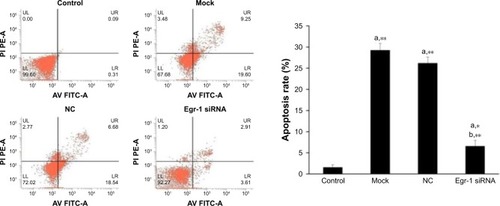
Effects of As2O3 and AZT on apoptosis of HepG2 cells
The results of flow cytometry showed that the apoptotic rate of HepG2 cells treated by As2O3 (2 μmol/L) combined with AZT (20 μmol/L) was significantly higher than that of the control group (P<0.05). Compared with the mock and NC groups, the cell apoptotic rate of HepG2/siRNA group was significantly decreased (P<0.01). The difference between blank control group and NC group was not significant. The results indicated that As2O3 combined with AZT could promote the apoptosis of HepG2 cells. After silencing Egr-1, the effect was attenuated ().
Figure 4 Effects of As2O3 combined with AZT on cell apoptosis.
Abbreviations: As2O3, arsenic trioxide; AV FITC-A, Annexin V fluorescein isothiocyanate-area; AZT, 3′-azido-3′-deoxythymidine; Egr-1, early growth response protein 1; LL, lower left; LR, lower right; NC, negative control; PE-A, phycoerythrin-area under the curve; PI, propidium iodide; UL, upper left; UR, upper right.
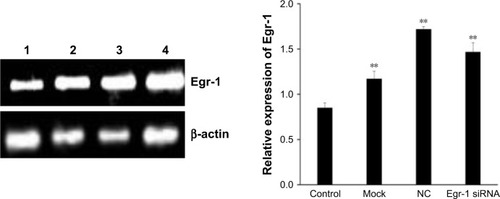
The combination of As2O3 and AZT promotes the expression of Egr-1 mRNA/protein expression
As shown in , Egr-1 mRNA expression level of Egr-1 siRNA group was significantly lower than that of the NC group and blank control group after electrotransfection with Egr-1 siRNA for 24 h (P<0.01). Furthermore, the Egr-1 mRNA expression level of the Egr-1 siRNA group is only equivalent to about one-tenth before transfection.
Figure 5 Relative expression of Egr-1 mRNA in HepG2 cells.
Abbreviations: Egr-1, early growth response protein 1; NC, negative control.
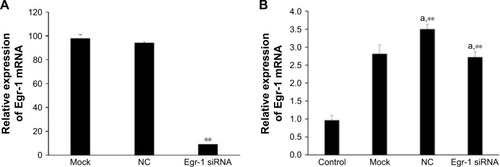
As shown in , the combination of As2O3 (2 μmol/L) and AZT (20 μmol/L) significantly upregulated Egr-1 mRNA expression of HepG2 cell compared with the control group at 48 h (P<0.05). There was no significant difference between the NC and mock groups. However, the expression level of Egr-1 mRNA in the Egr-1 siRNA group was also significantly increased under the action of the drug, which may be because the blocking effect of Egr-1 siRNA is attenuated at 48 h. The Western blotting results showed that the protein level of Egr-1 was consistent with the mRNA level ().
The expression of Egr-1, p53, and caspase-3 in HepG2 cells treated with As2O3 combined with AZT following Egr-1 siRNA
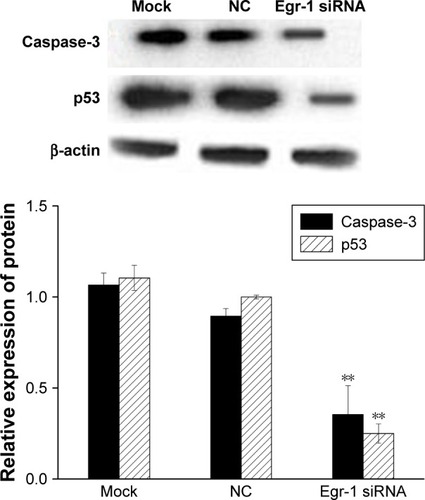
The results of qPCR showed that the expression of p53 and caspase-3 mRNA in the Egr-1 siRNA group was significantly lower than that in mock group or HepG2/NC group (P<0.01). There was no significant difference between the mock group and the NC group (P>0.05) (). The Western blotting results showed that the protein level of p53 and caspase-3 was consistent with the mRNA level ().
Figure 7 The effect of Egr-1 on expression of p53 and caspase-3 mRNA in HepG2 cells.
Abbreviations: Egr-1, early growth response protein 1; NC, negative control.
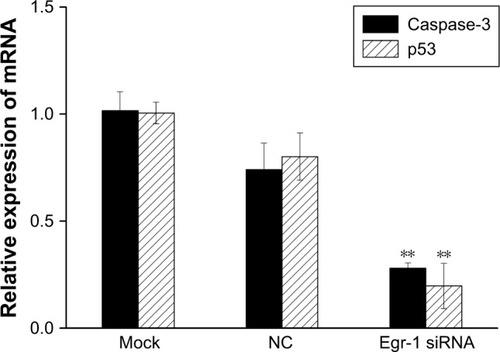
Figure 8 The effect of Egr-1 on expression of p53 and caspase-3 protein in HepG2 cells.
Abbreviations: Egr-1, early growth response protein 1; NC, negative control.
Discussion
Arsenic, a metalloid that is universally distributed in nature, is considered to be one of the most toxic xenobiotics. In the 1970s, arsenic was proven to be a highly effective agent in the treatment of APL, and there has been significant progress since then. Recent in vitro experiments have indicated that As2O3 might be effective in the treatment of solid tumors, such as hepatoma, gastric carcinoma, and breast carcinoma.Citation17–Citation19 In addition, As2O3 can promote apoptosis and differentiation and inhibit proliferation of cancer cells and telomerase activity.Citation20,Citation21 However, several reports demonstrated that malignant solid tumors are insensitive to low dose of As2O3.Citation22 Furthermore, toxic effects of high-dose As2O3 also seriously limits its clinical application. Of note, more and more studies have shown that the combination of As2O3 with other drugs is effective in enhancing efficacy and reducing the use of doses so as to reduce side effects. In an As2O3 and tretinoin integrated treatment protocol for patients newly diagnosed with APL, joint treatment showed a synergistic effect and leaded to a high clinical complete remission (CR) rates.Citation23,Citation24
It has been proven that AZT has excellent inhibition effects and chemo-therapeutic sensitization on a variety of tumors;Citation25–Citation27 one possible mechanism is the inhibition of telomerase activity.Citation28 Moreover, it has been reported that Pt-AZT could downregulate Bcl-2 expression and inhibit the growth of hepatocellular carcinoma in mice.Citation29 However, the dosage of AZT alone needed in treatment of tumors can cause severe toxic side effects; furthermore, the problem of multiple drug resistance occurs when a large dose is used, so restricting the further application of AZT as a clinical antitumor agent.Citation30,Citation31 In some recent studies, AZT exhibited significant synergistic therapeutic with other drugs for tumors in a safe dosage range.Citation32–Citation34 Chau et alCitation35 suggested that the combination of azacytidine with As2O3 would be a logical drug combination for treatment of AML in the future. Wang et alCitation36 reported that the combination of AZT with emodin could co-inhibit the proliferation of leukemia KG-1a cells.
Our previous studies have found that the combination of AZT and As2O3 has a significant synergistic effect (co-index CI<1) on hepatocellular carcinoma, which can reduce the side effects and significantly improve the therapeutic effect of the two compounds. According to some clues, single low doses of As2O3 and AZT did not promote activity caspase-3, but the combination of the two compounds significantly promoted the activity of caspase-3,Citation9 the most important mediators of apoptosis.
Egr-1, a zinc finger transcription factor, has been found to be rapidly induced with a broad range of extracellular stimuli, thus mediating growth, proliferation, differentiation, or apoptosis.Citation37 Studies have shown that Egr-1 is low in most human malignant tumor cells and the level of Egr-1 expression is associated with a 5-year survival of cancer patients. In addition, Egr-1 is able to increase the sensitivity of cancer patients to radiotherapy and chemotherapy.Citation38 However, the role of Egr-1 in HCC is a controversial topic. Peng et alCitation16 reported that Egr-1 promotes hypoxia-induced autophagy to enhance chemo-resistance of hepatocellular carcinoma cells. Conversely, Shan et alCitation15 reported that reexpression of Egr-1 decreased cell growth and tumorigenicity in nude mice.
In the present study, we found that, after silencing Egr-1, the proliferation rate of HepG2 cells was significantly increased, and the effect of inhibition proliferation and promoting apoptosis of the combinations of As2O3 and AZT on HepG2 cells was attenuated. In addition, the CI value was greater than 1, showing antagonistic effect. Our further results showed that the combination of As2O3 with AZT could significantly enhance the expression of p53 and caspase-3, but the effect was decreased after silencing Egr-1. These results suggested that Egr-1, which could control the expression of p53 and caspase-3, may be the key target of the combination of As2O3 with AZT on treating hepatocellular carcinoma. Although these findings have potential clinical significance, it is still crucial to further investigate interconnections of Egr-1 among associated signaling nets of hepatocellular carcinoma.
Acknowledgments
This study was supported by National Natural Science Foundation of China (number 81460456) and Natural Science Foundation of Gansu Province (number 1308RJZA169).
Disclosure
The authors reports no conflicts of interest in this work.
References
- WangXZhangASunHPower of metabolomics in diagnosis and biomarker discovery of hepatocellular carcinomaHepatology2013572072207723150189
- ChafferCLWeinbergRAA perspective on cancer cell metastasisScience20113311559156421436443
- EzakiTHepatocellular carcinoma: referral to a specialist centre is recommendedBMJ199230468211961971310880
- FoxERazzoukBIWidemannBCPhase 1 trial and pharmacokinetic study of arsenic trioxide in children and adolescents with refractory or relapsed acute leukemia, including acute promyelocytic leukemia or lymphomaBlood200811156657317959855
- WangHLiuYWangXRandomized clinical control study of locoregional therapy combined with arsenic trioxide for the treatment of hepatocellular carcinomaCancer2015121172917292526033499
- HoffmanEAGizelskaKMirowskiMMielickiWArsenic trioxide downregulates cancer procoagulant activity in MCF-7 and WM-115 cell lines in vitroContemp Oncol (Pozn)201519210811226034387
- FalconeALencioniMBrunettiIMaximum tolerable doses of intravenous zidovudine in combination with 5-fluorouracil and leucovorin in metastatic colorectal cancer patients. Clinical evidence of significant antitumor activity and enhancement of zidovudine-induced DNA single strand breaks in peripheral nuclear blood cellsAnn Oncol199785395459261522
- FalconeADanesiRDargenioFIntravenous azidothymidine with fluorouracil and leucovorin: a phase I–II study in previously untreated metastatic colorectal cancer patientsJ Clin Oncol1996147297368622018
- ChenCZhangYWangYHuangDXiYQiYSynergic effect of 3-azido-3-deoxythymidine and arsenic trioxide in suppressing hepatoma cellsAnticancer Drugs20112243544321502917
- LiuYYuanLYChuHYChenCAs2O3 in combination with AZT suppresses the proliferation of hepatoma HepG2 cells through activating caspase-3 pathwayTumor2014348705711
- HanLChenCChuHYLiuYLiangYLiJEffects of As2O3 combined with AZT on migration and invasion of liver cancer HepG2 cellsTumor2015359982989
- CuiWHuJLKongLLIncreased chemotherapy sensitivity on adriamycin resistance cells of breast cancer by up-regulated EGR-1Chin J Cancer Prevent Treat20091610744747 Chinese
- BaronVAdamsonEDCalogeroARagonaGMercolaDThe transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGF-β1, PTEN, p53, and fibronectinCancer Gene Ther20061311512416138117
- RagioneFDCucciollaVCrinitiVIndacoSBorrielloAZappiaVp21Cip1 gene expression is modulated by Egr-1: a novel regulatory mechanism involved in the resveratrol antiproliferative effectBiol Chem20032782336023368
- ShanJBalasubramanianMNDonelanWA MEK-dependent transcriptional program controls activation of the early growth response 1 (EGR1) gene during amino acid limitationJ Biol Chem201428935246652467925028509
- PengWXXiongEMGeLEgr-1 promotes hypoxia-induced autophagy to enhance chemo-resistance of hepatocellular carcinoma cellsExp Cell Res20163401627026708617
- MaYWangJLiuLGenistein potentiates the effect of arsenic trioxide against human hepatocellular carcinoma: role of Akt and nuclear factor-κBCancer Lett20113011758421078540
- ShaoQSYeZYLingZQKeJJCell cycle arrest and apoptotic cell death in cultured human gastric carcinoma cells mediated by arsenic trioxideWorld J Gastroenterol200511223451345615948253
- WangXGaoPLongMEssential role of cell cycle regulatory genes p21 and p27 expression in inhibition of breast cancer cells by arsenic trioxideMed Oncol20112841225125420458559
- ZhaoJWangCSongYFangBArsenic trioxide and microRNA-204 display contrary effects on regulating adipogenic and osteogenic differentiation of mesenchymal stem cells in aplastic anemiaActa Biochim Biophys Sin (Shanghai)2014461088589325187411
- ZhangYSunMShiWYangQArsenic trioxide suppresses transcription of hTERT through down-regulation of multiple transcription factors in HL-60 leukemia cellsToxicol Lett2015232248148925436934
- YangXAnLLiXArsenic trioxide induced endoplasmic reticulum stress in laryngeal squamous cell line Hep-2 cellsAuris Nasus Larynx2014411818323880367
- Lo-CocoFAvvisatiGVignettiMRetinoic acid and arsenic trioxide for acute promyelocytic leukemiaN Engl J Med2013369211112123841729
- ShenZXShiZZFangJAll-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemiaProc Natl Acad Sci USA2004101155328533515044693
- JinRRChaoRXiYMEffects of AZT on leukemia cell line KG-1a proliferation and telomerase activityZhongguo Shi Yan Xue Ye Xue Za Zhi201220002277281 Chinese22541081
- HeMJiangYYZhuMEffects of 3′-azido-deoxythymidine on telomerase activity and protein expression of hepatocarcinoma cell line SMMC-7721Ai Zheng2006255543548 Chinese16687071
- ChenWZhangQWuDDengLStudy on tumor cell lines and their telomerase activity by telomerase inhibitorsTumor20006009 Chinese
- MatteucciCMinutoloAMarino-MerloFCharacterization of the enhanced apoptotic response to azidothymidine by pharmacological inhibition of NF-kBLife Sci2015127909725744407
- SabokrouhAVaisirayganiAGoodarziMTComparison between platinum-azidothymidine and azidothymidine effects on Bcl-2 and telomerase gene expression in rats with hepatocellular carcinomaAvicenna J Med Biotechnol201572505626140181
- SunRErikssonSWangLIdentification and characterization of mitochondrial factors modulating thymidine kinase 2 activityNucleosides Nucleotides Nucleic Acids2010294–638238520544523
- ScruggsERDirks NaylorAJMechanisms of zidovudine-induced mitochondrial toxicity and myopathyPharmacology2008822838818504416
- SunYQGuoTKXiYMChenCWangJWangZREffects of AZT and RNA-protein complex (FA-2-b-beta) extracted from Liang Jin mushroom on apoptosis of gastric cancer cellsWorld J Gastroenterol200713314185419117696246
- MattsonDMAhmadIMDayalDCisplatin combined with zidovudine enhances cytotoxicity and oxidative stress in human head and neck cancer cells via a thiol-dependent mechanismFree Radic Biol Med200946223223718983911
- JohnstonJSJohnsonAGanYWientjesMGAuJLSynergy between 3′-azido-3′ deoxythymidine and paclitaxel in human pharynx FaDu cellsPharm Res200320795796112880279
- ChauDNgKChanTSYAzacytidine sensitizes acute myeloid leukemia cells to arsenic trioxide by up-regulating the arsenic transporter aquaglyceroporin 9J Hematol Oncol201584625953102
- WangLNLiZJXiYMEffect of Emodin Combined with AZT on the proliferation and the expression of BCL-2, NF-κB, TGF-β in the leukemia stem cells-KG-1a cellsZhongguo Shi Yan Xue Ye Xue Za Zhi201523512651271 Chinese26524020
- PagelJIDeindlEEarly growth response 1—a transcription factor in the crossfire of signal transduction cascadesIndian J Biochem Biophys2011258226235
- GitenayDBaronVTIs EGR1 a potential target for prostate cancer therapy?Future Oncol200957993100319792968