Abstract
Background
Previous studies have reported that nuclear receptor subfamily 5, group A, member 2 (NR5A2) polymorphisms (rs3790843 G>A, rs3790844 T>C, rs12029406 C>T) are associated with the risk of pancreatic cancer. However, the results of epidemiological investigations are still controversial. In order to explore its potential attributing factors, we pooled the updated literatures to evaluate the association between NR5A2 polymorphism and the risk of pancreatic cancer in this meta-analysis.
Materials and methods
Databases such as PubMed, Google Scholar and China National Knowledge Infrastructure were searched for eligible articles following strict inclusion and exclusion criteria (updated to November 18, 2017). Odds ratios (ORs) and 95% CIs were computed to assess the intensity of association. In addition, heterogeneity, sensitivity analysis and publication bias were explored. All statistical analyses were conducted by STATA 14.0.
Results
Our results showed that the rs3790843 (GA vs GG: OR=0.86, CI=0.76–0.98, P=0.992; GA+AA vs GG: OR=0.83, CI=0.73–0.94, P=0.950; A vs G: OR=0.85, CI=0.78–0.93, P=0.802), rs3790844 (CC vs TT: OR=0.65, CI=0.54–0.78, P=0.617; CC vs TT+CT: OR=0.73, CI=0.62–0.85, P=0.742; C vs T: OR=0.78, CI=0.73–0.84, P=0.555) and rs12029406 (TT vs CC: OR=0.73, CI=0.61–0.89, P=0.483; TT vs CC+CT: OR=0.78, CI=0.66–0.92, P=0.648; T vs C: OR=0.87, CI=0.79–0.95, P=0.837) polymorphisms were associated statistically with the risk of pancreatic cancer. Furthermore, the results of subgroup analysis showed that rs3790843 and rs3790844 polymorphisms were especially related to the risk of pancreatic cancer in Caucasian population.
Conclusion
Our results revealed that NR5A2 may have a protective effect on pancreatic cancer. However, more well-designed researches are needed to verify the relationship between NR5A2 polymorphisms and the risk of pancreatic cancer.
Introduction
Pancreatic cancer is a highly aggressive and malignant tumor, in which the most frequent type of tissue is ductal adenocarcinoma.Citation1 In the USA, about 85% of pancreatic cancer patients had already developed metastasized or unresectable lesions at the time of diagnosis and were expected to live 12 months at most.Citation2,Citation3 The mortality rates are still rising in our country (China).Citation4 Although the cause of pancreatic cancer is not yet clear, its high mortality rate is closely related to the biologic characteristics of the tumor and the genetic factors.Citation5 It has been shown that 10% of pancreatic cancer is caused by genetic mutations.Citation6 Meanwhile, levels of molecular markers can be used to predict the prognosis of pancreatic cancer.Citation7
Nuclear receptor subfamily 5, group A, member 2 (NR5A2), also called as liver receptor homolog-1, is a member of orphan nuclear hormone receptors, which is highly expressed in the pancreas, liver, intestine and ovary and is involved in the balance of cholesterol, steroidogenesis and bile acid in the body.Citation8,Citation9 NR5A2 is located on chromosome 1q32.1, which is reported to play an important role in the stability of the pancreatic acinar cell.Citation10 Murtaugh reported that the lack of NR5A2 will promote the mutation of Kras gene which can accelerate deterioration of pancreatic cancer.Citation11 Human genome-wide association studies have suggested that there is a significant association between single-nucleotide polymorphisms (SNPs) of NR5A2 and the risk of pancreatic cancer.Citation12 Among these SNPs, rs3790844 is the focus of our study.
Located in the first intron of NR5A2, the SNP rs3790843 is characterized by G>A, while rs3790844 is characterized by T>C.Citation13 The SNP rs12029406, which is characterized by C>T, might influence the receptor activity, which, in turn, can change the disease risk and survival.Citation20 After searching the databases, we found five articles focusing on the association between their polymorphisms and the risk of pancreatic cancer.Citation18–Citation22 In order to get rid of the limitations of a single trial and gain a result of comprehensive meaning, we combined the five studies using meta-analysis to confirm whether rs3790843, rs3790844 and rs12029406 polymorphisms would affect the risk of pancreatic cancer, which has never been done before.
Materials and methods
Literature search
We searched for relevant publications using the following words and terms: “NR5A2”, “polymorphism/variety” and “pancreatic cancer/pancreatic ductal adenocarcinoma” in databases such as PubMed, Google Scholar and the China National Knowledge Infrastructure, and all literatures were published before November 18, 2017. References to these literatures were also screened in order to prevent loss of any valuable data.
Inclusion and exclusion criteria
Eligible studies were selected according to the following inclusion criteria: 1) focusing on the association between NR5A2 polymorphism and the risk of pancreatic cancer, 2) a case–control study, 3) providing available and sufficient data of genotype frequencies for calculating an odds ratio (OR) with 95% CI and 4) distribution of the genotype in case and control groups was in Hardy–Weinberg equilibrium (HWE). The exclusion criteria were as follows: 1) non-case–control, pure cell or animal studies, 2) not related to the risk of pancreatic cancer and 3) not containing useful genotype frequency data.
Data extraction
Two authors (Q Chen, H Yuan) independently screened the literatures, extracted the relevant information and finally discussed disagreement. All the data were recorded in the standard form: first author’s name, year of publication, country of population, control source, genotypic methods, number of genotypes in case–control groups and result of the HWE test. We set the total data of the case and control groups >1,000 as “sample size Y” and <1,000 as “sample size N”. In addition, we classified the populations from USA, England and Germany as Caucasian, while those from China and Japan were identified as Asian for subgroup analysis.
Genetic model
A is the mutant allele of rs3790844 which includes two alleles G and A, C is the mutant allele of rs3790844 and T is the mutant allele of rs12029406. We selected the heterozygous model (GA vs GG or CT vs TT or CT vs CC), homozygous model (AA vs GG or CC vs TT or TT vs CC), dominant model (GA+AA vs GG or CT+CC vs TT or CT+TT vs CC), recessive model (AA vs GG+GA or CC vs TT+CT or TT vs CC+CT) and allele model (A vs G or C vs T or T vs C) for further meta-analysis.
Statistical analysis
Our meta-analysis was conducted by Stata software (version 14.0; StataCorp LP, College Station, TX, USA). To assess the strength of association between NR5A2 polymorphism and the risk of pancreatic cancer, we calculated the OR together with 95% CI for each genetic model. Furthermore, we evaluated the heterogeneity by Cochran’s Q-statistic.Citation14 A P-value <0.1 indicated significant heterogeneity. Another method to evaluate the heterogeneity is I2, which represents the percentage of variance across the whole study. In general, values of I2 <25% represent “low heterogeneity”, while values >75% represent “high heterogeneity”.Citation15 Statistically, I2 >50% indicates that the heterogeneity is significant and the random-effects model was chosen.Citation16 Otherwise, the fixed-effects model was used.Citation17 Moreover, data with similar characteristics such as source of control (HB/PB), sample size (Y/N) and ethnicity of the population (Asian/Caucasian) were used for subgroup analysis.
Sensitivity analyses were performed to assess the stability of the studies on the pooled ORs. A single study in the analysis was omitted each time to calculate the outcomes again. Publication bias was evaluated by using Begg’s test and Egger’s test, and P<0.05 indicated significant bias. HWE was checked by the goodness-of-fit chi-square test, and P>0.05 indicated the genetic balance of the population and that the data were from the same Mendelian group.
Results
Literature selection and study characteristics
The procedure of literature screening is shown in . We found 24 articles related to NR5A2 polymorphism from PubMed, Google Scholar and China National Knowledge Infrastructure. According to the inclusion and exclusion criteria, 19 articles were excluded. Among the selected articles, 3 articles were reviews, 6 articles were not associated with the risk of pancreatic cancer and the other 10 articles lacked usable data for estimating an OR with 95% CI. Ultimately, a total of five literatures were included in the meta-analysis.Citation18–Citation22 Among them, four studies containing 2,212 cases and 2,932 controls surveyed the association between rs3790843 G>A and pancreatic cancer risk, five articles on the relation between rs3790844 T>C and pancreatic cancer risk included 4,191 cases and 5,133 controls, and three publications containing 1,822 cases and 2,510 controls explored the correlation of rs12029406 C>T with pancreatic cancer risk. One of the articles studied the association between NR5A2 polymorphism and the risk of pancreatic cancer not only from England but also from Germany with different genotyping methods.Citation20 Data for different controls from the same study were considered as a separate data set, which can be used for all analyses. Study characteristics of each literature are shown in .
Table 1 Characteristics of the five studies included in the meta-analysis
NR5A2 rs3790843, rs3790844 and rs12029406 polymorphisms
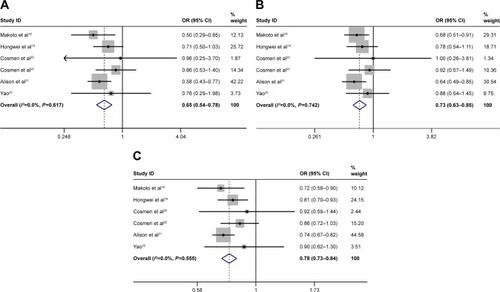
We first analyzed the association between rs3790843, rs3790844 and rs12029406 polymorphisms and the risk of pancreatic cancer in the overall population, and the forest plots are shown in –. Then, in order to explain the relationship between their polymorphisms and the risk of pancreatic cancer, we also performed the subgroup analyses by control source, sample size and ethnicity. All results are presented in in detail.
Table 2 Meta-analysis results of association between NR5A2 polymorphism and pancreatic cancer risk
Figure 2 Forest plots of pancreatic cancer risk associated with NR5A2 rs3790843 G>A polymorphism.
Abbreviation: OR, odds ratio.
Overall effects for meta-analysis
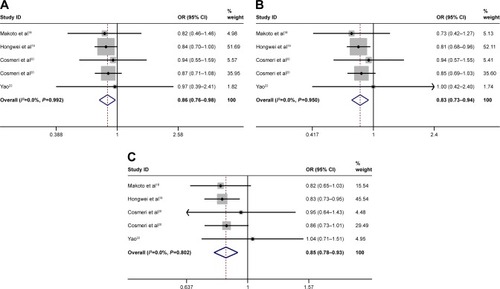
Of the studies, two articles suggested the association between rs3790843 G>A and pancreatic cancer risk, three studies revealed an obvious association between rs3790844 T>C and pancreatic cancer risk and one research showed the association between rs12029406 C>T and pancreatic cancer risk. After analyzing the existing data, we found a significant association between rs3790843 polymorphism (GA vs GG: OR=0.86, CI=0.76–0.98, P=0.992; GA+AA vs GG: OR=0.83, CI=0.73–0.94, P=0.950; A vs G: OR=0.85, CI=0.78–0.93, P=0.802; ), rs3790844 polymorphism (CC vs TT: OR=0.65, CI=0.54–0.78, P=0.617; CC vs TT+CT: OR=0.73, CI=0.62–0.85, P=0.742; C vs T: OR=0.78, CI=0.73–0.84, P=0.555; ) and rs12029406 polymorphism (TT vs CC: OR=0.73, CI=0.61–0.89, P=0.483; TT vs CC+CT: OR=0.78, CI=0.66–0.92, P=0.648; T vs C: OR=0.87, CI=0.79–0.95, P=0.837; ) and the risk of pancreatic cancer susceptibility.
Subgroup analysis for control source
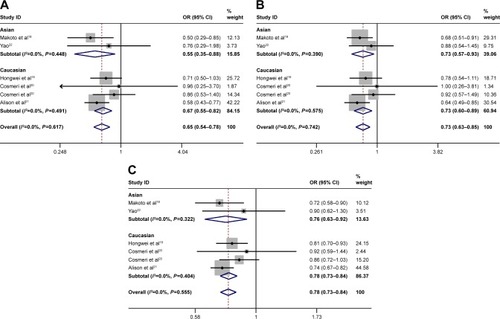
Subgroup analysis was stratified by control sources. Statistically significant association between rs3790843 (GA vs GG: OR=0.84, CI=0.71–0.99, P=0.944; GA+AA vs GG: OR=0.80, CI=0.68–0.94, P=0.737; A vs G: OR=0.83, CI=0.74–0.93, P=0.934; ) and rs3790844 (CC vs TT: OR=0.61, CI=0.49–0.74, P=0.495; CC vs TT+CT: OR=0.69, CI=0.58–0.82, P=0.718; C vs T: OR=0.76, CI=0.70–0.82, P=0.559; ) polymorphisms with the risk of pancreatic cancer was detected in hospital-based studies under five genetic models.
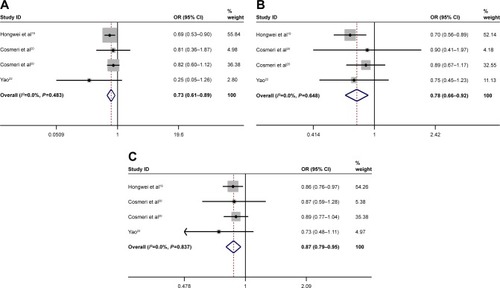
Subgroup analysis for sample size
The sample size with sufficient statistical capacity is critical to the study and for the relationship between polymorphisms and the risk of cancer. We set the total data >1,000 as “Y” and <1,000 as “N”. Statistically significant association between rs3790843 (GA vs GG: OR=0.85, CI=0.75–0.98, P=0.793; GA+AA vs GG: OR=0.82, CI=0.72–0.94, P=0.745; A vs G: OR=0.84, CI=0.76–0.93, P=0.757; ) and rs3790844 (CC vs TT: OR=0.66, CI=0.54–0.82, P=0.344; CC vs TT+CT: OR=0.73, CI=0.63–0.85, P=0.411; C vs T: OR=0.78, CI=0.72–0.85, P=0.300; ) polymorphisms and the risk of pancreatic cancer was also detected in “Y”-based studies under five genetic models.
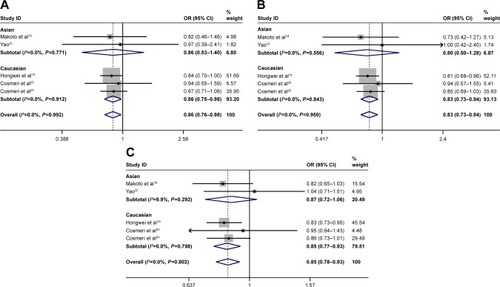
Subgroup analysis for ethnicity
Subgroup analysis was stratified by ethnicity. The relationship between rs3790843 (GA vs GG: OR=0.86, CI=0.76–0.98, P=0.912; GA+AA vs GG: OR=0.83, CI=0.73–0.94, P=0.843; A vs G: OR=0.85, CI=0.77–0.93, P=0.798; ) and rs3790844 (CC vs TT: OR=0.67, CI=0.55–0.82, P=0.491; CC vs TT+CT: OR=0.73, CI=0.60–0.89, P=0.575; C vs T: OR=0.78, CI=0.73–0.85, P=0.404; ) polymorphisms and the risk of pancreatic cancer was detected from the data of Caucasian population.
Heterogeneity test
Based on the Cochran’s Q-statistic, all the data showed nonsignificant heterogeneity under five genetic models ().
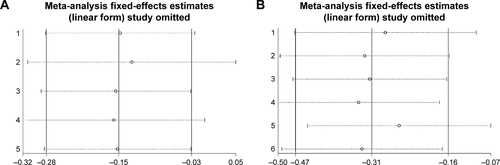
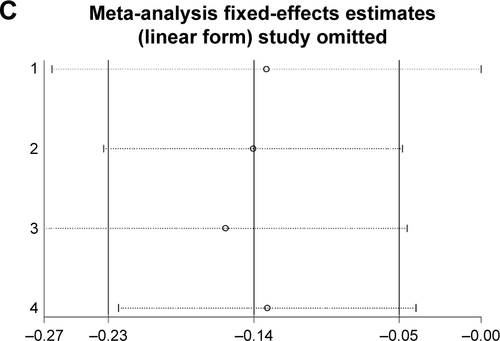
Sensitivity analysis
In order to compare the differences and assess the sensitivity, a single study in the analysis was omitted each time to calculate the outcomes again. The pooled ORs were not influenced significantly, which indicated our results of this meta-analysis were stable ().
Publication bias analysis
To evaluate the publication bias of the publications, Begg’s test and Egger’s test were performed. The funnel plots of five genetic models whose shapes were roughly symmetrical suggested no significant publication bias (figure not shown). Moreover, the results shown in indicate that our results were reliable.
Table 3 The results of Begg’s and Egger’s tests
Discussion
As a highly malignant disease, pancreatic cancer is the fourth leading cause of cancer death in the USA.Citation23 Most patients would have already progressed to an advanced stage with a poor cure rate when diagnosed. Surgical treatment is still the first choice to cure pancreatic cancer up to now, but only 15%–20% of patients have the opportunity to take it up.Citation24,Citation25 What is worse, despite surgical resection, the majority of these patients would die from recurrence or metastasis in 2 years.Citation26 Radiotherapy and chemotherapy, such as gemcitabine (Eli Lilly and Company, Indianapolis, IN, USA) or molecular-targeted drugs, are recommended for patients lacking the opportunity to undergo surgery.Citation27,Citation28 Disappointingly, the efficacy of these drugs to increase the survival rate is barely observed and unexplainable drug resistance comes up. Different from other cancers, the 5-year survival rate of pancreatic cancer has changed just a little in the past four decades (as low as 6%).Citation29 It has been shown that genetic factors play an important role in the development, progression and prognosis of pancreatic cancer.Citation7,Citation30,Citation31 Exploring the genetic background of pancreatic cancer is instructive to find out some targets which can help in early diagnosis and judge the prognosis of pancreatic cancer.
Previous studies have shown that NR5A2, which is also known as liver receptor homolog-1, is an important regulator of pancreatic exocrine function.Citation32 Lack of NR5A2, combined with pancreas-specific transcription factor 1 causes instability of pancreatic acinar cells, which increases the pancreatic injury and then causes mutations in the Kras gene.Citation10,Citation32–Citation34 As we all know, mutation of Kras is the earliest event in deterioration of pancreatic cancer.Citation11 We integrated the results of five studies and found a negative link between NR5A2 rs3790844 polymorphism and the risk of pancreatic cancer. However, histologic examinations show that NR5A2 is overexpressed in tumor tissues, which is quiet puzzling.Citation35 Although the mechanism is not clear, it does not deny our conclusion that NR5A2 is a protective factor for early pancreatic cancer. Here, we put forward several possible explanations for discussion. Overexpression of NR5A2 is also closely related to diabetes and hyperlipidemia, which are the important risk factors for pancreatic cancer, and has a positive effect on mutation of Kras later in pancreatic cancer progression.Citation11,Citation36,Citation37 Moreover, NR5A2 also contributes to other tumors. For instance, NR5A2 was reported to be involved in the metabolism of glutamine to induce hepatocellular carcinoma, while it also induces the expression and transcription of ERα and mediates the secretion of estrogen to affect the progression of breast cancer.Citation38–Citation40 In addition, NR5A2 is overexpressed in colon cancer and osteosarcoma cells.Citation41,Citation42 Recently, many studies have reported the relationship between the NR5A2 rs3790843, rs3790844 and rs12029406 polymorphisms and the risk of cholangiocarcinoma, gastric cancer and pancreatic cancer without consistent results.Citation13,Citation18–Citation22,Citation43 In order to clarify the association, we integrated the data of pancreatic cancer for the meta-analysis.
The polymorphism was observed to be related with pancreatic cancer in hospital-based studies, but not in population-based studies in the subgroup analysis by the control source. Unsurprisingly, a larger sample bounded by 1,000 was more credible than a smaller sample in the relationship between them and cancer, which suggested that future research should require a larger sample size. Moreover, the subgroup analysis by ethnicity was also performed. The association between rs3790843 and rs3790844 polymorphisms and the risk of pancreatic cancer in Caucasian population under all genetic models was statistically significant; however, this was not found in the Asian population. Different genetic background or different lifestyles may lead to this difference between them and the total population.
As far as we know, this is the first meta-analysis of the association between NR5A2 (rs3790843, rs3790844, rs12029406) polymorphisms and the risk of pancreatic cancer. The analysis showed that the GA or AA genotype reduced the risk of pancreatic cancer and the A allele reduced the risk to 0.85 compared with the rs3790843 polymorphism and the TC or CC genotype, which played a similar role in the rs3790844 polymorphism, and the C allele reduced the risk to 0.78. Moreover, the TC or TT genotype of rs12029406 could also protect from pancreatic cancer and the T allele reduced the risk to 0.87. The largest value of I2 was 16.9% (<25%), which means that no heterogeneity was found in all studies. Meanwhile, no abnormality was found in sensitivity analysis.
Limitations
There were some limitations in our meta-analysis which might have affected the final outcomes. First, the study was based on unadjusted OR values, which did not contain the well-known predisposing factors of pancreatic cancer, such as age, sex, alcohol, smoking, diabetes and chronic pancreatitis. Second, we lacked data from non-Caucasian populations in Africa in the subgroup analysis for ethnicity. Finally, different genotypic methods (Fluidgm, TapMan, KASPar and so on) could also have an impact on the outcome. More researches are needed to further analyze the association between NR5A2 polymorphism and the risk of pancreatic cancer.
Conclusion
Our study showed that the NR5RA rs3790843, rs3790844 and rs12029406 polymorphisms were favorable factors in the risk of pancreatic cancer and were more protective in Caucasian population. If the mechanism behind this could be discovered by further research, hopefully, it will become a target for future personalized treatment of pancreatic cancer. Due to the limitations shown in this analysis, larger sample size and diverse studies are needed to confirm our results.
Acknowledgments
This work was supported by the Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX17_1288).
Supplementary materials
Figure S1 Subgroup analysis of type of control source for NR5A2 rs3790843 G>A polymorphism and pancreatic cancer risk.
Abbreviations: HB, hospital-based; OR, odds ratio; PB, population-based.
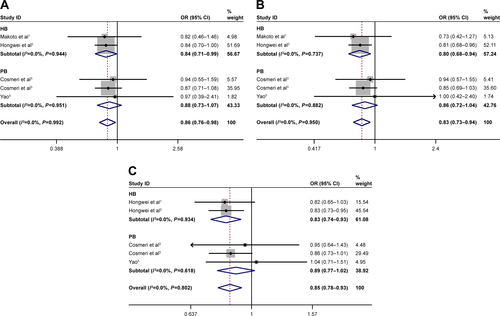
Figure S2 Subgroup analysis of type of control source for NR5A2 rs3790844 T>C polymorphism and pancreatic cancer risk.
Abbreviations: HB, hospital-based; OR, odds ratio; PB, population-based.
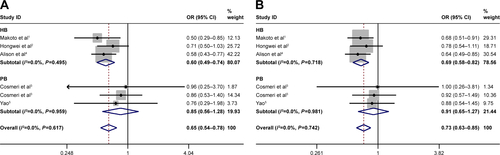
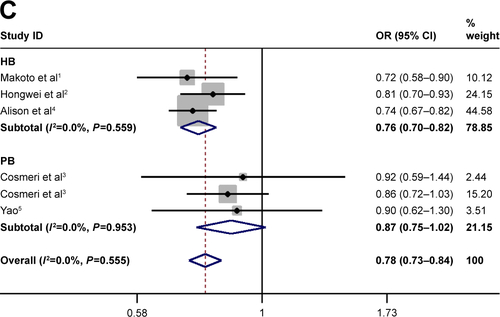
Figure S3 Subgroup analysis of sample size for NR5A2 rs3790843 G>A polymorphism and pancreatic cancer risk.
Abbreviation: OR, odds ratio.
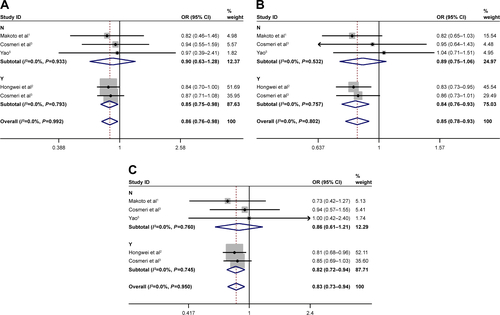
Figure S4 Subgroup analysis of sample size for NR5A2 rs3790844 T>C polymorphism and pancreatic cancer risk.
Abbreviation: OR, odds ratio.
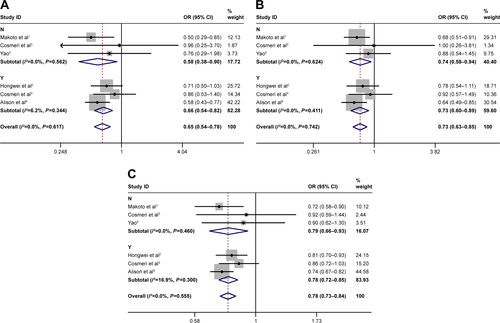
Figure S5 The sensitivity analysis of pancreatic cancer risk associated with NR5A2 polymorphism.
References
- MakotoUShinichiOManabuMGenome-wide association study-identified SNPs (rs3790844, rs3790843) in the NR5A2 gene and risk of pancreatic cancer in JapaneseSci Rep201551701826592175
- HongweiTXiaoqunDManalHBody mass index and obesity- and diabetes-associated genotypes and risk for pancreatic cancerCancer Epidemiol Biomarkers Prev201120577979221357378
- CosmeriRDanieleCNathaliaGPancreatic cancer susceptibility loci and their role in survivalPLoS One2011611e2792122125638
- AlisonPKSaraLJulieBMAn absolute risk model to identify individuals at elevated risk for pancreatic cancer in the general populationPLoS One201389e7231124058443
- YaoYA Case Control Study of Pancreatic Cancer with Environmental, Diease, Psychological, Behavioral Factors and the Preliminary Study of Genetic Predisposition in Pancreatic Cancer.caj [D]PekingPeking Union Medical College2013
Disclosure
The authors report no conflicts of interest in this work.
References
- DucreuxMCuhnaASCaramellaCESMO Guidelines CommitteeCancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-upAnn Oncol201526Suppl 5v56v6826314780
- VincentAHermanJSchulickRHrubanRHGogginsMPancreatic cancerLancet2011378979160762021620466
- SiegelRLMillerKDJemalACancer statistics, 2016CA Cancer J Clin201666173026742998
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- LiaoXHuangKHuangRGenome-scale analysis to identify prognostic markers in patients with early-stage pancreatic ductal adenocarcinoma after pancreaticoduodenectomyOnco Targets Ther2017104493450628979141
- GroverSSyngalSHereditary pancreatic cancerGastroenterology201013941076108020727885
- AnsariDRosendahlAElebroJAnderssonRSystematic review of immunohistochemical biomarkers to identify prognostic subgroups of patients with pancreatic cancerBr J Surg20119881041105521644238
- LazarusKAWijayakumaraDChandALSimpsonERClyneCDTherapeutic potential of liver receptor homolog-1 modulatorsJ Steroid Biochem Mol Biol20121303–513814622266285
- LeeYKMooreDDLiver receptor homolog-1, an emerging metabolic modulatorFront Biosci2008135950595818508634
- GuidoVFJohnPMChristopherWMatthiasHNr5a2 maintains acinar cell differentiation and constrains oncogenic Kras-mediated pancreatic neoplastic initiationGut201463465666423645620
- MurtaughLCPutting GWAS to the functional test: NR5A2 and pancreatic cancer riskGut201463453553623759730
- PetersenGMAmundadottirLFuchsCSA genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33Nat Genet201042322422820101243
- ZhangXGuDDuMAssociations of NR5A2 gene polymorphisms with the clinicopathological characteristics and survival of gastric cancerInt J Mol Sci20141512229022291725514243
- HandollHHSystematic reviews on rehabilitation interventionsArch Phys Med Rehabil20068787516731227
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- DerSimonianRLairdNMeta-analysis in clinical trialsControl Clin Trials1986731771883802833
- MantelNHaenszelWStatistical aspects of the analysis of data from retrospective studies of diseaseJ Natl Cancer Inst195922471974813655060
- MakotoUShinichiOManabuMGenome-wide association study-identified SNPs (rs3790844, rs3790843) in the NR5A2 gene and risk of pancreatic cancer in JapaneseSci Rep201551701826592175
- HongweiTXiaoqunDManalHBody mass index and obesity-and diabetes-associated genotypes and risk for pancreatic cancerCancer Epidemiol Biomarkers Prev201120577979221357378
- CosmeriRDanieleCNathaliaGPancreatic cancer susceptibility loci and their role in survivalPLoS One2011611e2792122125638
- AlisonPKSaraLJulieBMAn absolute risk model to identify individuals at elevated risk for pancreatic cancer in the general populationPLoS One201389e7231124058443
- YaoYA Case Control Study of Pancreatic Cancer with Environmental, Diease, Psychological, Behavioral Factors and the Preliminary Study of Genetic Predisposition in Pancreatic Cancer.caj [D]PekingPeking Union Medical College2013
- SiegelRMaJZouZJemalACancer statistics, 2014CA Cancer J Clin201464192924399786
- SteeleCWKarimSALeachJDGCXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinomaCancer Cell201629683284527265504
- FerroneCRPieretti-VanmarckeRBloomJPPancreatic ductal adenocarcinoma: long-term survival does not equal cureSurgery20121523 Suppl 1S43S4922763261
- SiegelRLMillerKDJemalACancer statistics, 2015CA Cancer J Clin201565152925559415
- Von HoffDDErvinTArenaFPIncreased survival in pancreatic cancer with nab-paclitaxel plus gemcitabineN Engl J Med2013369181691170324131140
- HidalgoMPancreatic cancerN Engl J Med2010362171605161720427809
- ShinEJCantoMIPancreatic cancer screeningGastroenterol Clin North Am201241114315722341255
- KamisawaTWoodLDItoiTTakaoriKPancreatic cancerLancet201638810039738526830752
- BaileyPChangDKNonesKGenomic analyses identify molecular subtypes of pancreatic cancerNature20165317592475226909576
- FlandezMCendrowskiJCanameroMNr5a2 heterozygosity sensitises to, and cooperates with, in ammation in KRasG12V-driven pancreatic tumourigenesisGut201463464765523598351
- HolmstromSRDeeringTSwiftGHLRH-1 and PTF1-L coregu-late an exocrine pancreas-specific transcriptional network for digestive functionGenes Dev201125161674167921852532
- CarriereCYoungALGunnJRLongneckerDSKorcMAcute pancreatitis markedly accelerates pancreatic cancer progression in mice expressing oncogenic KrasBiochem Biophys Res Commun2009382356156519292977
- BenodCVinogradovaMVJouravelNKimGEFletterickRJSablinEPNuclear receptor liver receptor homologue 1 (LRH-1) regulates pancreatic cancer cell growth and proliferationProc Natl Acad Sci U S A201110841169271693121949357
- DuboisMJBergeronSKimHJThe SHP-1 protein tyrosine phosphatase negatively modulates glucose homeostasisNat Med200612554955616617349
- IwakiMMatsudaMMaedaNInduction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptorsDiabetes20035271655166312829629
- XuPOosterveerMHSteinSLRH-1-dependent programming of mitochondrial glutamine processing drives liver cancerGenes Dev201630111255126027298334
- ZhuJZouZNiePDownregulation of microRNA-27b-3p enhances tamoxifen resistance in breast cancer by increasing NR5A2 and CREB1 expressionCell Death Dis2016711e245427809310
- AnnicotteJSChaveyCServantNThe nuclear receptor liver receptor homolog-1 is an estrogen receptor target geneOncogene200524558167817516091743
- YuanQCaoGLiJZhangYYangWMicroRNA-136 inhibits colon cancer cell proliferation and invasion through targeting liver receptor homolog-1/Wnt signalingGene2017628485528710032
- LiZWuSLvSWangHWangYGuoQSuppression of liver receptor homolog-1 by microRNA-451 represses the proliferation of osteosarcoma cellsBiochem Biophys Res Commun2015461345045525869073
- ZimmerVMihalacheFHöblingerAThe frequent NR5A2 gene variant rs3790844 mediates an increased risk of cholangiocarcinoma in a large European cohortZ Gastroenterol201149429