Abstract
Purpose
Hepatocellular carcinoma is one of the most predominant malignancies with high fatality rate and its incidence is rising at an alarming rate because of its resistance to radio- and chemotherapy. Indirubin is the major active anti-tumor ingredient of a traditional Chinese herbal medicine. The present study aimed to analyze the effects of indirubin on cell proliferation, migration, invasion, and angiogenesis of tumor-derived endothelial cells (Td-EC).
Methods
Td-EC were derived from human umbilical vein endothelial cells (HUVEC) by treating HUVEC with the conditioned medium of human liver cancer cell line HepG2. Cell proliferation, migration, invasion, and angiogenesis were assessed by MTT, wound healing, in vitro cell invasion, and in vitro tube formation assay.
Results
Td-EC were successfully obtained from HUVEC cultured with 50% culture supernatant from serum-starved HepG2 cells. Indirubin significantly inhibited Td-EC proliferation in a dose- and time-dependent manner. Indirubin also inhibited Td-EC migration, invasion, and angiogenesis. However, indirubin’s effects were weaker on HUVEC than Td-EC.
Conclusion
Indirubin significantly inhibited Td-EC proliferation, migration, invasion, and angiogenesis.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and the second most frequent cause of cancer-related deaths worldwide.Citation1 Tumor occurrence and development are very complicated processes. Cancer cells stimulate angiogenesis in an effort to maintain and allow tumor progression.Citation2 Proliferation, migration, and activation of endothelial cells are involved in tumor angiogenesis.Citation3 Therefore, tumor-derived endothelial cells (Td-EC) are considered to be ideal therapeutic targets in antiangiogenic therapies. Tumor endothelial markers (TEMs) showed elevated expression during tumor angiogenesis than in normal angiogenesis. TEM1 shows overexpression in tumor tissues and is engaged in tumor invasion, growth, angiogenesis, and microvasculature maturation.Citation4–Citation6 TEM8 shows a potential role in cell adhesion, migration, and tubule formation.Citation7,Citation8
Current therapeutic modalities for HCC include curative options such as surgical resection, liver transplantation, and local ablation or palliative procedures such as catheter-directed therapies and systemic therapy.Citation9 However, HCC mortality rate is very high because it is quite resistant to radio- and chemotherapies.Citation10 Thus, new treatment choices are critically required.
Traditional Chinese Medicine (TCM) is a valuable resource that includes many biologically active components that are both safe and effective. Indirubin, extracted from indigo and an active ingredient of Danggui Longhui Wan formula, is the first TCM extract shown to be effective in the treatment of chronic myelogenous leukemia.Citation11 However, the effects of indirubin on Td-EC have not been addressed.
In the present study, we investigated the effects of indirubin on cell proliferation, migration, invasion, and angiogenesis of Td-EC to explore new approaches in cancer therapy.
Materials and methods
Reagents
Indirubin (purity ≥98%) was purchased from Shanghai Yuan Ye Biological Technology Co., Ltd. (Shanghai, China) and dissolved in dimethylsulfoxide (DMSO, Kelong Co, Chengdu, China). Roswell Park Memorial Institute (RPMI) 1640 Medium (PRMI1640, Thermo Fisher Scientific, Waltham, MA, USA) and fetal bovine serum (FBS) were purchased from Thermo Fisher Scientific. The 0.25% trypsin was purchased from HyClone (Shanghai, China). Matrigel was purchased from Becton Drive (BD, Franklin Lakes, NJ, USA).
Cell culture
Human umbilical vein endothelial cells (HUVEC) line was obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM, Thermo Fisher Scientific) containing 20% FBS, 10 ng/L vascular endothelial growth factor (VEGF) (Thermo Fisher Scientific), and 1% antibiotics (100 units/mL penicillin, 100 mg/mL streptomycin, Shanghai Canspec Scientific Instruments Co., Ltd., Shanghai, China) under standard conditions in a humidified incubator at 37°C with 5% CO2.
Human HCC cell line HepG2 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in RPMI1640 medium with 10% FBS and 1% antibiotics under standard conditions. HepG2 cells were cultured in serum-free RPMI1640 for 48 hours when they reached 70% confluence. Then, the medium from the serum-starved HepG2 cells was collected, filtered by 0.22 µm filter membrane, and stored at −80°C as the conditioned medium.
HUVEC were cultured with the complete medium with 50% of the HepG2-conditioned medium for 48 hours to become Td-EC. The identity of Td-EC were validated by checking TEMs that are associated with tumor-specific angiogenesis and are potentially useful to distinguish between tumor and normal endothelium.Citation12 In this study, TEM1 and TEM8 were chosen for Td-EC detection.
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from Td-EC and HUVEC using Trizol Reagent (Thermo Fisher Scientific), and cDNA was synthesized from total RNA using RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). The conditions for PCR were 30 cycles of: denaturation (95°C/30 s), annealing (68°C/30 s), extension (72°C/1 min), and final extension (72°C/10 min) using Deam Taq PCR Master Mix (2X) (Thermo Fisher Scientific). TEM1 and TEM8 gene expression was detected by PCR with normalization by β-actin level as an internal control. Each experiment was performed in triplicate. The sequences of the primers used in this study were: forward primer 5′-ctgatgggtgaggtctggtt-3′ and reverse primer 5′-cattatcccaactgcccagc-3′ for TEM1; forward primer 5′-gtctcctcctggcagaactt-3′ and reverse primer 5′-gctgcaccactggaatgaaa-3′ for TEM8; forward primer 5′-cctctatgccaacacagtgc-3′ and reverse primer 5′-cctgcttgctgatccacatc-3′ for β-actin.
Cell proliferation assay
To test the effect of indirubin on cell proliferation, a quantitative colorimetric assay with MTT (Thermo Fisher Scientific) was applied. Briefly, HUVEC and Td-EC were seeded onto 96-well plates at 5×103 cells in 100 µL of medium per well. After incubation with 5, 10 µM of indirubin for 24, 48, 72 hours, the cells were incubated with 0.5 mg/mL of MTT for 4 hours at 37°C in darkness. After removing the culture medium, 100 µL of DMSO was added to dissolve formazan crystals within the cells. The absorbance was then measured using Synergy™ Microplate Reader (BioTek Instruments, Winooski, VT, USA) at a wavelength of 490 nm. The cell proliferation rate (%) was calculated using the following formula: (absorbance of experimental well/absorbance of control) ×100%. Three replicate wells were used for each analysis.
Cell migration assay
HUVEC and Td-EC (4×105 cells/well) were plated onto 6-well plates to reach 90% confluence. A wound was created by manually scraping the cell monolayer with a p1000 pipette tip. Then, cells were treated with 0, 5, and 10 µmol/L of indirubin for 48 hours. The cells were washed once with 1 mL of DMEM, which was then replaced with 2 mL of DMEM. Dishes were incubated at 37°C in a humidified atmosphere containing 5% CO2. The cells were observed 48 hours later with an inverted fluorescence microscope (Leica DMI4000B, Bensheim, Germany) equipped with an Olympus Qcolor 3 digital camera (the same microscope was used for taking all micrographs). Cell migration was evaluated by migration potential (%), which was the distance the cells migrated from the original wound margin. All the images were processed with ImagePro Plus (Media Cybernetics, Rockville, MD, USA). The experiments were repeated three times.
In vitro tube formation assay
Serum-free culture medium and pre-cooled melted Matrigel (10 mg/mL, BD) were mixed at a ratio of 2:1 (v/v). A 24-well plate was coated with the Matrigel mixture, which was then allowed to polymerize for 1 hour at 37°C. HUVEC and Td-EC (1.5×105 cells/well) were seeded on the surface of the Matrigel at 37°C for 12 hours and tube formation was observed every 4 hours with an inverted fluorescence microscope (Leica DMI4000B) equipped with an Olympus Qcolor 3 digital camera. Changes in tube formation were observed, after adding 5 µM of indirubin for 48 hours, under a microscope, and photographed at 100× magnification. Quantification of the tube formation was evaluated by the number of tube formations. The experiments were repeated three times.
In vitro cell invasion assay
Invasion of endothelial cells was tested using the Matrigel-coated Transwell system (8 µm pore size and 6.5 mm diameter) in 24-well plates (Corning Costar, Cambridge, MA, USA). The lower chamber was filled with 500 µL of DMEM medium with 10% FBS. HUVEC and Td-EC (1×105 cells/well) were seeded into the upper chamber in serum-free media overnight. The cells were then fixed with methanol and stained with H&E (Sigma, Shanghai, China). The cells on the upper filter surface were removed, and cell invasion was determined using an inverted fluorescence microscope (Leica DMI4000B) equipped with an Olympus Qcolor 3 digital camera at 100× magnification, by counting the cells that had migrated to the lower filter side. Samples were assayed twice in triplicate.
Statistical analysis
All experiments were repeated at least three times and all numerical data are presented as mean ± standard error the mean. To evaluate the significant differences between the two groups, the means were compared using Student’s t-test. P<0.05 was considered as statistically significant. These analyses were performed using SPSS 13.0 software (SPSS, Chicago, IL, USA).
Results
Td-EC induction and validation
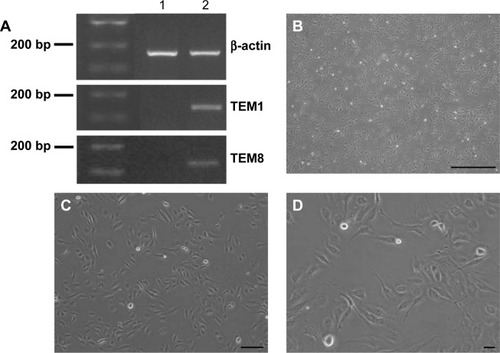
To determine whether HUVEC were induced to become Td-EC by HepG2 conditioned medium, TEM1 and TEM8 were detected by PCR. We found that TEM1 and TEM8 were expressed in Td-EC but not in HUVEC (). Cell morphology showed that Td-EC appeared with endothelial cell morphological features (–D).
Figure 1 Td-EC detection and cell morphology.
Abbreviations: HUVEC, human umbilical vein endothelial cells; PCR, polymerase chain reaction; Td-EC, tumor-derived endothelial cells; TEM, tumor endothelial marker.
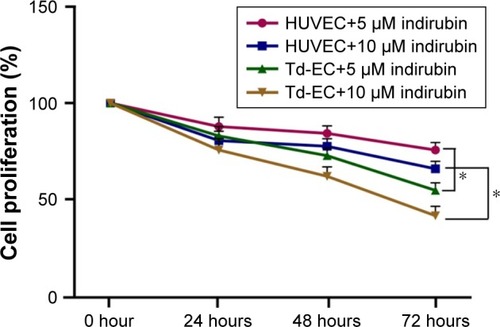
Effects of indirubin on cell proliferation of Td-EC
We found that indirubin inhibited cell proliferation of HUVEC and Td-EC in time- and dose-dependent manners (). Compared to untreated HUVEC and Td-EC, indirubin at doses of 5 and 10 µM significantly inhibited Td-EC proliferation, whereas indirubin’s effects were weaker on HUVEC than Td-EC ().
Figure 2 Effects of indirubin on cell proliferation.
Abbreviations: HUVEC, human umbilical vein endothelial cells; SEM, standard error of the mean; Td-EC, tumor-derived endothelial cells.
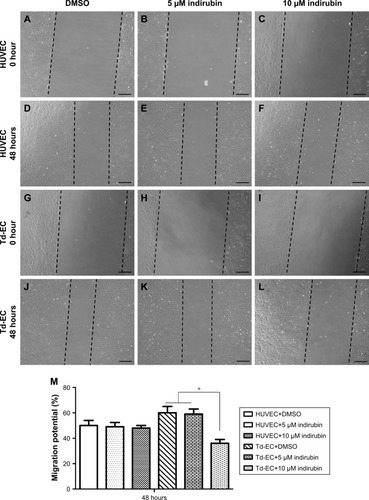
Effects of indirubin on Td-EC migration
Compared to untreated HUVEC and Td-EC, cell migration of Td-EC treated with indirubin for 48 hours was suppressed in a dose-dependent manner (). The migration ability of Td-EC was stronger than HUVEC. However, the inhibitory effects of indirubin were much stronger on Td-EC than HUVEC. Quantification of the wound-healing assay by migration potential (%) is shown in .
Figure 3 Effects of indirubin on Td-EC migration ability in vitro.
Abbreviations: DMSO, dimethylsulfoxide; HUVEC, human umbilical vein endothelial cells; Td-EC, tumor-derived endothelial cells.
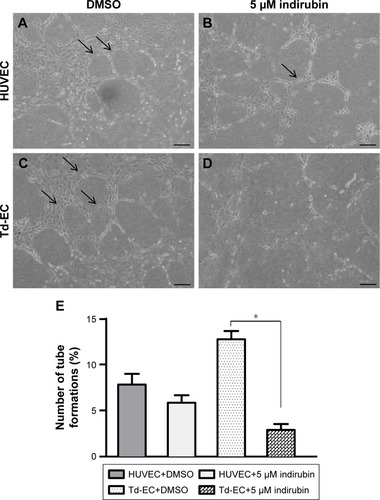
Effects of indirubin on Td-EC angiogenesis in vitro
Vascular tube formation ability was markedly stronger in Td-EC than HUVEC (). The vascular tube formation ability in Td-EC was significantly inhibited by indirubin, but the inhibition was less obvious in HUVEC (). Quantification of tube formation number (%) is shown in .
Figure 4 Effects of indirubin on Td-EC tube formation in vitro.
Abbreviations: DMSO, dimethylsulfoxide; HUVEC, human umbilical vein endothelial cells; Td-EC, tumor-derived endothelial cells.
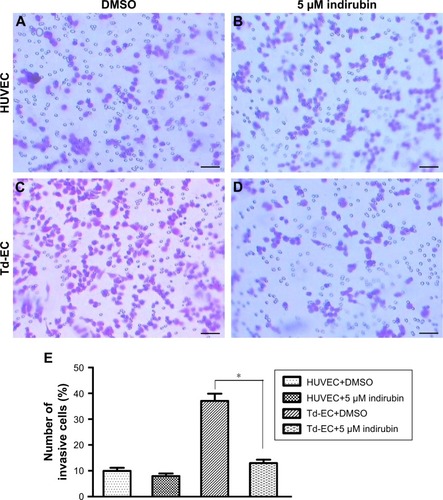
Effects of indirubin on Td-EC invasion in vitro
Transwell assays showed that indirubin had no effects on HUVEC migration (), whereas the invasion cell number of Td-EC was decreased significantly by indirubin treatment (). The invasion cell number of Td-EC was much more than that of HUVEC in the absence of indirubin treatment ().
Figure 5 Effects of indirubin on cell invasion ability in vitro.
Abbreviations: DMSO, dimethylsulfoxide; HUVEC, human umbilical vein endothelial cells; Td-EC, tumor-derived endothelial cells.
Discussion
HCC is one of the most predominant malignancies with high fatality rate, leading to over 600,000 deaths annually.Citation13 The treatment modalities for HCC include surgical resection, radiofrequency ablation, and liver transplantation. Unfortunately, HCC frequently recurs.Citation14 Therefore, a growing number of researchers are seeking new treatments for HCC and focus on TCM. TCM, a complete system of healing developed in ancient China, has been receiving more and more attention in China, and throughout the world in recent decades.Citation15 TCM is considered a medical system for the prevention and treatment of diseases that focuses on the patient rather than the disease, when compared to the Western medicine.Citation16 In this study, we focus on indirubin, the major active anti-tumor component of a traditional Chinese herbal medicine used for treatment of chronic myelogenous leukemia. However, there is little research about indirubin’s effects on HCC.
The stroma is known to maintain the tissue homeostasis and acts as a barrier toward tumor formation. However, when a cell becomes malignant, its surrounding matrix changes in a way to support cancer development.Citation17 Thus, understanding the tumor microenvironment (TME) has become as important as the modulation of cancer cell progression itself.Citation18 The architecture of a typical TME is composed of fibroblasts, myofibro-blasts, endothelial cells, pericytes, adipose cells, immune and inflammatory cells, and the extracellular matrix elements.Citation19 In this research, we pay close attention to Td-EC, which are surrounded by other cells such as pericytes that make them more stable and control the vessel diameter and elasticity as well.Citation20 Metastasis and angiogenesis are among the hallmarks of malignant behavior of cancer cells. Metastasis is responsible for the rapid progress and death by HCC. Angiogenesis and tumor progression are very closely linked to each other. Tumor cells are dependent on angiogenesis because their growth and expansion require oxygen and nutrients.Citation21
TEM1 expressed in tumor endothelium but not in normal endothelium, was recently identified as a novel tumor endothelial cell surface marker potentially involved in angiogenesis.Citation22 TEM8 is considered as a novel extracellular tumor marker among the other cell surface TEMs. TEM8 expression pattern is tumor-specific and has not been detected in physiologic angiogenesis.Citation23 Thus, in this study, we chose TEM1 and TEM8 for Td-EC validation.
In our study, we found that indirubin inhibited cell proliferation, migration, invasion and angiogenesis of Td-EC. All these data suggest that indirubin may be a novel anti-angiogenic treatment for HCC. There are many molecular mechanisms for Td-EC angiogenesis such as tumor hypoxia, epithelial–mesenchymal transition, cancer stem-like cells, and some transcription factors. More molecular mechanisms shall be identified in future indirubin studies.
Conclusion
Our findings indicate that indirubin can inhibit Td-EC proliferation, migration, invasion, and angiogenesis. All these suggest that indirubin can be used in the anti-angiogenic treatment of human HCC.
Acknowledgments
The present study was supported by a Scientific and Technological Development Fund (#2013-0-yy-02) from the Affiliated Hospital of Chengdu University of TCM.
Disclosure
The authors report no conflicts of interest in this work.
References
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365E359E38625220842
- ShojaeiFAnti-angiogenesis therapy in cancer: current challenges and future perspectivesCancer Lett2012320213013722425960
- WeiYYangQZhangYPlumbagin restrains hepatocellular carcinoma angiogenesis by suppressing the migration and invasion of tumor-derived vascular endothelial cellsOncotarget201789152301524128122355
- RmaliKAPuntisMCJiangWGPrognostic values of tumor endothelial markers in patients with colorectal cancerWorld J Gastroenterol20051191283128615761964
- TomkowiczBRybinskiKFoleyBInteraction of endosialin/TEM1 with extracellular matrix proteins mediates cell adhesion and migrationProc Natl Acad Sci U S A200710446179651797017986615
- NandaAKarimBPengZTumor endothelial marker 1 (Tem1) functions in the growth and progression of abdominal tumorsProc Natl Acad Sci U S A200610393351335616492758
- YangMChaudharyASeamanSThe cell surface structure of tumor endothelial marker 8 (TEM8) is regulated by the actin cytoskeletonBiochim Biophys Acta201118131394921129411
- FuSTongXCaiCThe structure of tumor endothelial marker 8 (TEM8) extracellular domain and implications for its receptor function for recognizing anthrax toxinPLoS One201056e1120320585457
- TomitaYDorwardHYoolAJRole of aquaporin 1 signalling in cancer development and progressionInt J Mol Sci2017182E29928146084
- WahidBAliARafiqueSIdreesMNew insights into the epigenetics of hepatocellular carcinomaBiomed Res Int20172017160957528401148
- XiaoZHaoYLiuBQianLIndirubin and meisoindigo in the treatment of chronic myelogenous leukemia in ChinaLeuk Lymphoma20024391763176812685829
- PietrzykŁBiomarkers discovery for colorectal cancer: a review on tumor endothelial markers as perspective candidatesDis Markers20162016491240527965519
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- GrandhiMSKimAKRonnekleiv-KellySMKamelIRGhasebehMAPawlikTMHepatocellular carcinoma: from diagnosis to treatmentSurg Oncol2016252748527312032
- YuFTakahashiTMoriyaJTraditional Chinese medicine and Kampo: a review from the distant past for the futureJ Int Med Res200634323123916866016
- Sánchez-VidañaDIRajwaniRWongMSThe Use of Omic Technologies Applied to Traditional Chinese Medicine ResearchEvid Based Complement Alternat Med20172017635973028250795
- ShimodaMMellodyKTOrimoACarcinoma-associated fibroblasts are a rate-limiting determinant for tumour progressionSemin Cell Dev Biol2010211192519857592
- PavlovaNNThompsonCBThe emerging hallmarks of cancer metabolismCell Metab2016231274726771115
- ChenFZhuangXLinLNew horizons in tumor microenvironment biology: challenges and opportunitiesBMC Med2015134525857315
- DudleyACTumor endothelial cellsCold Spring Harb Perspect Med201223a00653622393533
- ChenHFWuKJEndothelial transdifferentiation of tumor cells triggered by the Twist1-Jagged1-KLF4 Axis: relationship between cancer stemness and angiogenesisStem Cells Int20162016643986426823670
- OpavskyRHaviernikPJurkovicovaDMolecular characterization of the mouse Tem1/endosialin gene regulated by cell density in vitro and expressed in normal tissues in vivoJ Biol Chem200127642387953880711489895
- RmaliKAPuntisMCJiangWGTEM-8 and tubule formation in endothelial cells, its potential role of its vW/TM domainsBiochem Biophys Res Commun2005334123123815993844
