Abstract
Cisplatin (CDDP) is one of the most commonly used chemotherapy drugs for the treatment of various cancers. Although platinum-based therapies are highly efficacious against rapidly proliferating malignant tumors, the development of CDDP resistance results in significant relapse as well as decreased overall survival rates, which is a significant obstacle in CDDP-based cancer therapy. Long non-coding RNAs (lncRNAs) are involved in cancer development and progression by the regulation of processes related to chromatin remodeling, transcription, and posttranscriptional processing. Emerging evidence has recently highlighted the roles of lncRNAs in the development of CDDP resistance. In this review, we discuss the roles and mechanisms of lncRNAs in CDDP chemoresistance, including changes in cellular uptake or efflux of a drug, intracellular detoxification, DNA repair, apoptosis, autophagy, cell stemness, and the related signaling pathways, aiming to provide potential lncRNA-targeted strategies for overcoming drug resistance in cancer therapy.
Keywords:
Introduction
Cancer significantly affects the quality of life and is a leading cause of death worldwide. It has been reported that about 14.1 million new cancer cases and 8.2 million deaths occurred in 2012 worldwide.Citation1 In 2018, 1,735,350 new cancer cases and 609,640 cancer deaths are projected to occur in the USA.Citation2 Increasing national investment in cancer research contributes to accelerating progress in the prevention and treatment of cancer. Currently, the gold standard for antitumor therapeutic strategies is a combination of chemotherapy and surgery. However, chemotherapeutic anticancer agents are the standard treatment regimen for patients in whom surgery is not a viable option.Citation3 Cisplatin is one of the most widely used and successful cytotoxic drugs for the treatment of a broad variety of tumors such as ovarian, testicular, bladder, lung, esophageal, and nasopharyngeal carcinoma (NPC). Since the discovery of the antitumor activity of cisplatin, novel platinum-based agents (carboplatin and oxaliplatin) have been developed with reduced side effects and increased efficacy.Citation4 However, as a prototype of platinum-based agent, cisplatin remains widely used as a chemotherapeutic agent. When cisplatin is used in platinum-based chemotherapy, nearly 85% of patients with metastatic testicular cancer can be curedCitation5 and the 5-year survival rate in patients with completely resected non-small-cell lung cancer (NSCLC) tumors is improved.Citation6
Nevertheless, there exist many patients intrinsically resistant to cisplatin-based therapies, especially with colorectal, lung, and prostate cancers. What is more, originally sensitive tumors eventually develop chemoresistance, which is frequently observed in ovarian cancer.Citation7 Chemoresistance allows the cancer cells to become increasingly antagonistic and improves the ability of cancer invasion and migration, leading to tumor relapse and poor prognosis.Citation8,Citation9 Emerging studies have revealed that dysregulated expression of long non-coding RNAs (lncRNAs) plays an essential role in cisplatin resistance.Citation10 The lncRNAs, which are >200 nucleotides (nt) in length and which lack a significant open reading frame, may play major roles in a wide variety of biological pathways and cellular processes at the epigenetic, transcriptional, and posttranscriptional levels.Citation10,Citation11 Here, we briefly review the functions and mechanisms of lncRNAs in the regulation of drug resistance in cancer cells, mainly focusing on cisplatin chemoresistance.
Cisplatin
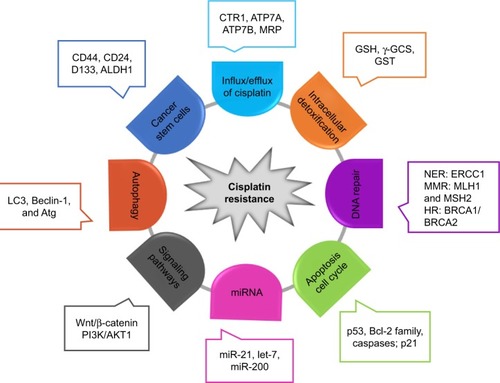
As an alkylating agent, cisplatin was first described by Michele Peyrone in 1845, and its antitumor activity was discovered in the 1970s.Citation12,Citation13 Since its approval by the US Food and Drug Administration for the treatment of testicular and ovarian cancer in 1987,Citation12,Citation14 cisplatin has gradually become a first-line chemotherapeutic agent. The platinum atom of cisplatin interacts with nucleophilic NCitation7-sites of purine in DNA to form inter- and intra-strand DNA crosslinks,Citation8,Citation14 which results in DNA damage, cell cycle arrest, and activation of multiple signal transduction pathways, leading to cell apoptosis.Citation8,Citation15 Moreover, cisplatin-induced production of reactive oxygen species and activation of inflammatory pathways may also contribute to the induction of apoptosis.Citation16 The introduction of cisplatin for the treatment of testicular cancer has improved its cure rate from 10% to 85%.Citation17 Unfortunately, the development of cisplatin resistance limits its efficacy in cancer treatment. Studies over the years have revealed multiple potential mechanisms related to cisplatin resistance (). Cisplatin resistance may occur through reduced intracellular platinum accumulation due to decreased drug uptake or increased drug export in cancer cells. Down-regulation of copper transporter 1 (CTR1) has been associated with resistance to cisplatin by reducing cisplatin uptake.Citation18 On the other hand, the efflux of cisplatin is mediated by transporting P-type adenosine triphosphatases (ATP) 7A and ATP7B, or multidrug-resistance-associated proteins (MRPs) in the cell membrane, and an upregulation of these efflux transporters is one of the major mechanisms of cisplatin resistance.Citation19 Cisplatin scavenging by intracellular detoxification is another major mechanism of cisplatin resistance, in which glutathione (GSH) plays a key role in the overexpression of enzymes involved in GSH synthesis and GSH conjugation has been reported to be associated with cisplatin resistance.Citation20 In addition, activation of the DNA damage systems, such as the nucleotide excision repair system, can attenuate the apoptotic process, leading to cisplatin resistance. Increased expression of nucleotide excision repair proteins, including XPF–ERCC1 complex, is associated with reduced efficacy of platinum-based therapy.Citation21 Since the mismatch repair (MMR) system can detect cisplatin-induced DNA lesion and activate the apoptotic signal, downregulation or a mutation of MMR-related genes such as MLH1 and MSH2 has been reported to contribute to cisplatin resistance.Citation22 Homologous recombination is another mechanism to repair cisplatin-induced DNA damage, and hence, a deficiency of breast cancer susceptibility proteins 1 and 2 (BRCA1/2), two critical components in the homologous recombination system, promotes cell sensitivity to cisplatin in cancer cells.Citation23 The expression of tumor suppressor protein p53 and p53-related nuclear transcription factors in cancer cells has been shown to mediate the cytotoxic effect of cisplatin.Citation24,Citation25 As the cytotoxic effect of cisplatin is associated with apoptotic signaling pathways, the expression levels of Bcl-2 proteins, caspases, and mitochondrial intermembrane proteins are crucial factors in influencing cell sensitivity to cisplatin.Citation26–Citation28 Furthermore, accumulating evidence suggests that the alteration in cell autophagy and PI3K/AKT1 signaling pathway can modulate cell sensitivity to cisplatin through compensating for cisplatin-induced lethal signals.Citation29,Citation30
Figure 1 Molecular mechanisms of cisplatin resistance.
Abbreviations: ALDH1, aldehyde dehydrogenase 1 family member A1; ATG7, autophagy associated gene; BRCA2, breast cancer susceptibility proteins 2; CTR1, copper transporter 1; ERCC1, excision repair cross-complementing rodent repair deficiency, complementation group 1; GSH, glutathione; GST, glutathione S-transferase; HR, homologous recombination; MMR, mismatch repair; MRP, multidrug-resistant-associated protein; NER, nucleotide excision repair; γ-GCS, γ-glutamylcysteine synthetase.
lncRNA
With the rapid development of sequencing technologies, it has been determined that <2% of the human genome encodes proteins, while the remaining transcriptional products are ncRNAs, which are considered as non-functional and transcriptional noise.Citation31 The ncRNAs can be classified into two major groups based on their sizes: small ncRNAs for those with a length <200 nt and lncRNAs for those with a length >200 nt, which includes intronic lncRNAs, intergenic lncRNAs, bidirectional lncRNAs, enhancer lncRNAs, and sense or antisense lncRNAs.Citation32 The lncRNAs can modulate gene expression at epigenetic, transcriptional, and posttranscriptional levels.Citation10,Citation33 In recent years, various studies have suggested that lncRNAs are involved in embryonic development and in the etiology of many human diseases, especially cancer.Citation34 Using advanced sequencing technology, numerous lncRNAs have been found to be dysregulated or aberrantly expressed in multiple types of cancers. The lncRNAs have been reported to act as critical factors in cancer development and progression by regulating cell proliferation, cell death, metastasis, and angiogenesis.Citation35 As lncRNAs play an important role in tumor cell survival and death, it is conceivable that lncRNAs may also alter cell sensitivity to chemotherapy, which is aimed at eradicating tumor cells by inhibiting cell growth and promoting cell apoptosis. It has been reported that lncRNA H19 contributes to doxorubicin resistance through regulating MDR1 expression.Citation36 Du et al have reported that lncRNA-XIST promoted temozolomide resistance in glioma cells through DNA MMR pathway.Citation37 Moreover, lncRNA UCA1 has been shown to promote 5-fluorouracil resistance in colorectal cancer cells by inhibiting miR-204-5p.Citation38 In sum, growing evidence has indicated that dysregulated expression of lncRNAs in cancer cells plays an important role in the development of chemoresistance through altering the mechanisms of drug export, drug metabolism, DNA repair, cell proliferation, apoptosis, and autophagy.Citation3,Citation11
lncRNA and cisplatin resistance
As stated above, numerous studies over the years have demonstrated that lncRNAs play a significant role in chemoresistance.Citation11 Aiming to understand the roles and mechanisms of lncRNAs in cisplatin resistance, we searched PubMed for all articles associated with “lncRNA and cisplatin resistance” and found that 22 lncRNAs have been reported to play an important role in cisplatin resistance through various mechanisms in multiple cancers (; ).
Table 1 Predictive lncRNAs involved in response to cisplatin
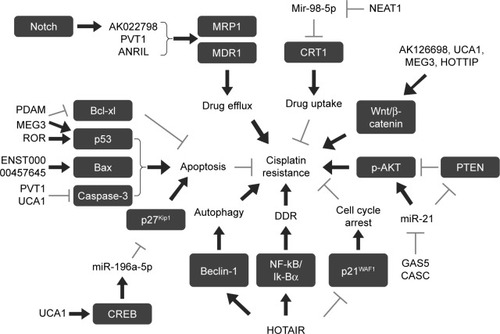
Figure 2 Role of lncRNAs in cisplatin resistance.
Abbreviations: DDR, DNA damage response; lncRNAs, long non-coding RNAs; MDR1, multidrug-resistant protein; MRP1, multidrug-resistant-associated protein 1.
Influx/efflux of cisplatin
Previous studies have indicated that reduced drug uptake or increased drug efflux in cancer cells, which results in a reduced intracellular platinum accumulation, is an important biochemical and cytological mechanism of cisplatin resistance.Citation8 ATP-binding cassette transporters, including P-glycoprotein and MRPs, can increase the drug efflux. Hang et alCitation39 have reported that Notch 1 could promote cisplatin resistance in gastric cancer (GC) through upregulation of lncRNA AK022798 expression. When lncRNA AK022798 was knocked down, the expression of MRP1 and P-glycoprotein MDR1, two membrane drug efflux-porters, was significantly decreased, while cell apoptosis, caspase-3, and caspase-8 activities were significantly increased in SGC7901 and BGC823 cisplatin-resistant cancer cells. These results indicated that lncRNA AK022798 is a crucial mediator in Notch 1-induced cisplatin resistance in cancer cells.Citation39 In GC tissues and cells, high expression of lncRNA PVT1 was significantly associated with the development of cisplatin resistance.Citation40 PVT1 silencing could reverse the cisplatin resistance in cisplatin-resistant cell lines, while upregulation of PVT1 decreased the sensitivity of parental GC cells to cisplatin, which was mediated through upregulation of MDR1, MRP, mTOR, and HIF-1a expression.Citation40 The lncRNA ANRIL has also been reported to be highly expressed in cisplatin-resistant and 5-fluorouracil-resistant GC tissues and cells.Citation41 Further studies have revealed that ANRIL knockdown might inhibit cell proliferation and invasion, promote anticancer agent-induced apoptosis, and reverse drug resistance in cisplatin- and 5-fluorouracil-resistant GC cell lines by downregulating MDR-related gene expression, including MDR1 and MRP1.Citation41 CTR1, a copper influx transporter, plays a vital role in platinum drug uptake and the development of cisplatin resistance.Citation42 In lung cancer cells, lncRNA nuclear-enriched abundant transcript 1 (NEAT1) might enhance cisplatin sensitivity by upregulating (−)-epigallocatechin-3-gallate (EGCG)-induced CTR1 expression.Citation43 Furthermore, NEAT1 might act as a competing endogenous RNA (ceRNA) of hsa-mir-98-5p to regulate CTR1 expression.Citation43
Intracellular detoxification
GSH is a kind of metallothionein, which shows a much higher affinity to cisplatin than DNA.Citation44 Increased GSH synthesis was associated with cisplatin resistance, and GSH depletion increased sensitivity to cisplatin.Citation45 As such, overexpression of enzymes involved in GSH synthesis and metabolism participates in the process of cisplatin resistance. The lncRNA H19 was overexpressed in ovarian cancer tissues and correlated with cancer recurrence, whereas H19 knockdown in A2780-DR cells increased their sensitivity to cisplatin treatment with a lower GSH level. H19 contributed to cisplatin resistance by regulating NRF2 and its target proteins including NQO1, GSR, G6PD, GCLC, GCLM, and GSTP1, which are involved in the GSH metabolism pathway.Citation46
DNA repair and cell cycle
Nuclear factor-κB (NF-κB) signaling-mediated activation of DNA damage response plays a role in the development of cell resistance to cisplatin.Citation47 The lncRNA HOTAIR overexpression induced cisplatin resistance in ovarian cancer cells and resulted in sustained activation of DNA damage response after cisplatin treatment through NF-κB activation due to Iκ-Bα (NF-κB inhibitor) downregulation. Collectively, these data suggests that HOTAIR contributes to chemoresistance through DNA damage-induced NF-κB signaling pathways.Citation48 HOTAIR has also been reported to promote cisplatin resistance by regulating p21WAF1 (p21), a cyclin-dependent kinase inhibitor which inhibits cell proliferation by inducing G0/G1 arrest, in lung adenocarcinoma (LAD) cells.Citation49 In nasopharyngeal carcinoma (NPC) cells, knockdown of lncRNA ANRIL inhibited cell proliferation, while it induced cell apoptosis and potentiated cisplatin-induced DNA damage by regulating microRNA let-7a expression.Citation50
Apoptosis
As cisplatin-induced DNA damage causes cell apoptosis, inhibition of apoptosis may also be involved in the acquired cisplatin resistance. p53, a tumor suppressor gene, plays a critical role in the apoptosis pathway. Several studies have shown that lncRNAs were associated with the cisplatin chemoresistance by downregulating p53-induced cell apoptosis. The lncRNA p53-dependent apoptosis modulator (PDAM) silencing induced cisplatin resistance in glioma cells by harboring wild-type p53, while BCL2L1 knockdown in PDAM-suppressed cells abrogated the cisplatin-resistant phenotype.Citation51 These data indicate that PDAM regulated cisplatin resistance by regulation of p53-dependent antiapoptotic genes (OTs).Citation51 The long non-coding RNA regulator of reprogramming (lncRNA-ROR), which played a crucial role in cell proliferation, migration, and apoptosis of NPC, promoted cisplatin resistance in NPC by improving cell proliferation and reducing cell apoptosis mediated by p53 signaling pathways.Citation52 In A549 cisplatin-resistant cells, lncRNA MEG3 expression was significantly downregulated and overexpression of MEG3 restored cell sensitivity to cisplatin by suppressing cell proliferation and inducing apoptosis and cell cycle arrest.Citation53,Citation54 Further studies elucidated that MEG3-mediated chemosensitivity was associated with the WNT/β-catenin signaling pathway by regulation of p53, as well as with the mitochondrial apoptosis pathway.Citation55 In addition, Ma et al have revealed that downregulation of lncRNA TRPM2-AS inhibited cisplatin resistance, induced cell apoptosis, and altered cell cycle distribution in NSCLC through activating the p53-p66shc pathway.Citation56
The Bcl-2 family is a key member in mitochondrial apoptosis pathway, which consists of the antiapoptotic family (such as BCL-2 and BCL-XL), the proapoptotic family (BAX and BAK), and the proapoptotic BH3-only protein family (such as BAD, BIK).Citation57 The lncRNA H19 contributed to cisplatin resistance in LAD by promoting cell migration via vimentin and reducing apoptosis via FAS, BAK, and BAX. The clinical study has shown that in patients with LAD, a high tumor H19 expression was negatively correlated with cisplatin-based chemotherapy response and a significantly shorter median progression-free survival, which were consistent with the data in in vitro experiment.Citation58 The lncRNA ENST000457645 could remarkably reverse cisplatin resistance by promoting apoptosis of cisplatin-resistant CP70 cells, which was associated with altered levels of apoptosis proteins such as Bax and cleaved caspase-3.Citation59
The lncRNA PVT1 was upregulated in ovarian cancer tissues from cisplatin-resistant patients and in cisplatin-resistant cells. PVT1 overexpression promoted cisplatin resistance through regulating the expression of TGF-β1, p-Smad4, and caspase-3, molecules related to the apoptotic pathways.Citation60 The upregulation of UCA1 lncRNA contributed to cisplatin resistance by promoting cancer cell proliferation while inhibiting apoptosis in bladder cancer and cervical cancer cells.Citation61,Citation62 In human bladder cancer cells, UCA1-mediated chemosensitivity was associated with the apoptosis pathway by upregulating miR-196a-5p targeting p27Kip1.Citation61 In cervical cancer cells, UCA1 suppressed cell apoptosis by downregulating caspase-3 and upregulating CDK2, whereas cell proliferation was enhanced through inducing survivin and decreasing p21 expression.Citation62 In ovarian cancer cells, curcumin-induced MEG3 lncRNA expression due to demethylation was directly associated with a decrease in miR-214 and extracellular vesicle-mediated transfer of miR-214, resulting in an elevation of cisplatin-induced cell apoptosis and cell sensitivity to cisplatin-based chemotherapy.Citation63 The lncRNA SFTA1P increased cisplatin chemosensitivity by enhancing cisplatin-induced apoptosis by increasing the expression of hnRNP-U and GADD45A in lung squamous cell carcinoma.Citation64
It has also been reported that lncRNAs CUDR, HOTAIR, and HULC modulated cisplatin resistance through alteration of cell apoptosis, but their exact molecular mechanisms remain to be elucidated.Citation65–Citation67 Wang et alCitation65 have reported that lncRNA CUDR (UCA1a) played a pivotal role in bladder cancer progression, and promoted cell proliferation, migration, and invasion in UM-UC-2 cells. In addition, CUDR overexpression might contribute to cisplatin resistance by antagonizing apoptosis.Citation65 HOTAIR also promoted cisplatin resistance in ovarian carcinoma. The knockdown of HOTAIR suppressed cell proliferation and invasion, and notably increased chemosensitivity to cisplatin specifically by promoting cisplatin-induced apoptosis in SKOV-3 cisplatin-resistance cells.Citation66 Patients with a high expression of HULC lncRNA in GC showed a significantly worse prognosis, and HULC knockdown enhanced the sensitivity of GC cells to cisplatin by enhancing cisplatin-induced apoptosis.Citation67
Signaling pathways
Studies over the years have demonstrated that diverse signaling pathways are involved in the development of drug resistance.Citation68 Analysis of mRNA, lncRNA, and miRNA expression profiles by microarray in cisplatin-resistant A549 cells and parental A549 cells revealed that 1,471 mRNAs, 1,380 lncRNAs, and 25 miRNAs were differentially expressed.Citation69 Gene coexpression network analysis identified many genes including lncRNA AK126698 that potentially play a significant role in cisplatin resistance.Citation69 Pathway analysis showed that the Wnt pathway was targeted by both miRNAs and lncRNAs including lncRNA AK126698. Moreover, in vitro cell culture experiments confirmed that AK126698 lncRNA induced cisplatin resistance in NSCLC through activating Wnt/β-catenin pathway.Citation69 UCA1 lncRNA expression levels were significantly higher in T24-resistant cells and bladder cancer tissues from patients treated with cisplatin, and overexpression of UCA1 lncRNA promoted cisplatin resistance in bladder cancer cells through upregulating Wnt6 expression, which consequently activated Wnt signaling.Citation70 The lncRNA HOTTIP could promote cell proliferation, cell cycle progression, and induce cell resistance to cisplatin by activating the Wnt/β-catenin pathway in osteosarcoma and ovarian cancer cells.Citation71
Autophagy
Autophagy plays an important role in the maintenance of cell hemostasis, and LC3, Beclin-1, and Atg family members are important factors in autophagosome formation.Citation72 Recently, several studies have reported that autophagy could act as a protective mechanism against cisplatin treatment in cancer cells.Citation29 Like 3-MA, an autophagy inhibitor, lncRNA GAS5 was shown to inhibit autophagy and, therefore, enhance cell sensitivity to cisplatin in NSCLC cells.Citation73 In human endometrial cancer cells, HOTAIR lncRNA contributed to cisplatin resistance by regulating autophagy mediated through the regulation of Beclin-1 expression.Citation74 The lncRNA XIST was upregulated in NSCLC cells and promoted the progression of NSCLC through regulating autophagy. Knockdown of XIST enhanced the chemosensitivity to cisplatin in NSCLC cells, which was reversed by the administration of a miR-17 inhibitor and overexpression of ATG7, a key factor in autophagosome formation. These data suggest that lncRNA XIST enhanced the chemoresistance of NSCLC cells to cisplatin by regulating autophagy via the miR-17/ATG7 pathway.Citation75 In human glioblastoma cells, the upregulation of lncRNA AC023115.3 promoted chemosensitivity to cisplatin by decreasing autophagy. Further mechanism experiments showed that AC02115.3 acted as a miR-26a sponge and increased its target gene GSK3β expression.Citation76
Cancer stem cells (CSCs)
CSCs are a small population of specialized cells that have the potential to self-renew and differentiate into other tumor cell subtypes and are involved in tumor initiation, progression, distant metastasis, and chemoresistance. Emerging evidence indicates that lncRNAs play an important role in the maintenance of CSCs, increasing tumor cells’ resistance to chemotherapy.Citation77 HOTAIR lncRNA could promote tumorigenesis and tumor metastasis by affecting the stemness of CSCs. Moreover, Liu et al have found that HOTAIR contributed to cisplatin resistance by regulating the biology of tumor stem cells.Citation78 HOTAIR was overexpressed in tumor tissues from NSCLC patients with drug resistance and in cisplatin-resistant A549 cells, and knockdown of HOTAIR expression increased the sensitivity of A549/cisplatin cells to cisplatin. Further mechanistic studies demonstrated that HOTAIR-induced cisplatin resistance might be associated with the promotion of tumor sphere cell growth through upregulating tumor stem cell-related Klf4 expression.Citation78
ceRNAs
In recent years, accumulating evidence indicates that lncRNAs, such as ceRNAs, could regulate target mRNA levels by combining competitively with common miRNAs, which is a potential mechanism in the regulation of cisplatin resistance. The lncRNA NEAT1-enhanced cisplatin sensitivity was mediated through upregulating EGCG-induced CTR1 expression due to its sponging action on mir-98-5p in lung cancer cells.Citation43 The lncRNA LINC00161 promoted cisplatin-induced apoptosis and decreased cell resistance to cisplatin. Further studies revealed that the effect of LINC00161 was achieved through upregulation of IFIT2 protein expression mediated via competitively sponging miR-645 action on IFIT2 mRNA.Citation79 Moreover, other lncRNAs such as CASC, GAS5, and MEG3 have also been reported to function as ceRNAs of miR-21 and miR-21-5p and upregulate PTEN and SOX7 expression, respectively, in NSCLCCitation81,Citation82 and cervical cancer cells,Citation80,Citation83 resulting in an alteration of cell sensitivity to cisplatin. In glioma cell, lncRNA AC023115.3 acted as a ceRNA for miR-26a and attenuated the inhibitory effect of miR-26a on GSK3β, which impaired cisplatin resistance.Citation76
Conclusion
Cisplatin resistance, either intrinsic or acquired, is a significant burden for successful cancer treatment. Here, we have discussed the roles of lncRNAs in cisplatin chemoresistance () through mechanisms such as alterations in cellular uptake or efflux of the drug, intracellular detoxification, cell apoptosis, autophagy, DNA repair, CSC, and ceRNA action (). Although the study of lncRNAs on chemoresistance is still in its infancy, growing evidence suggests that lncRNAs may serve as biomarkers for cancer diagnosis and prognosis and molecular targets for cancer therapy, including chemoresistance. BC-819 (H19-DTA) is a DNA vector that carries the gene for diphtheria toxin-A under the control of the H19 promoter sequence, which therefore has the potential to treat various malignancies that overexpress H19 lncRNA. Current clinical trials indicate that BC-819 given locally in combination with systemic chemotherapy may provide an additional therapeutic benefit for the treatment of pancreatic, bladder, ovarian, or peritoneal cancer.Citation84–Citation86
Of course, considerable work needs to be done for the lncRNA-based cancer therapy to be applied in clinical practice. First, chemoresistance is a complicated biologic process in which the roles and mechanisms of lncRNAs are still poorly understood. The majority of studies are in in vitro systems. Second, informative functional studies rely on animal experiments. However, establishing lncRNA function model in mice is difficult. Third, the sequence conservation of lncRNAs is much poorer than that of protein-coding genes. Thus, the lncRNAs which have been successfully verified in animal models may be not able to translate into clinical practice. Fourth, as a therapeutic strategy, the technology for either elimination or overexpression of a specific lncRNA at specific target cells in vivo is still in development. Finally, it is currently unclear whether interference of an endogenous lncRNA expression in the body will generate deleterious biologic consequence. Nevertheless, studies over the last decades have provided sufficient data to warrant further investigation of lncRNAs on tumorigenesis, tumor progression, and tumor chemoresistance.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (number 81673516) and a Special Talents Fund from the Central South University of China. We are very grateful to Ms Jale Manzo (Department of Medicine, Weill Cornell Medicine, New York, NY, USA) for editing grammar and spelling of the article.
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- SiegelRLMillerKDJemalACancer statistics, 2018CA Cancer J Clin201868173029313949
- ChenQNWeiCCWangZXSunMLong non-coding RNAs in anti-cancer drug resistanceOncotarget2017811925193627713133
- KellandLThe resurgence of platinum-based cancer chemotherapyNat Rev Cancer20077857358417625587
- HuddartRABirtleAJRecent advances in the treatment of testicular cancerExpert Rev Anticancer Ther20055112313815757445
- Artal CortesACalera UrquizuLHernando CuberoJAdjuvant chemotherapy in non-small cell lung cancer: state-of-the-artTransl Lung Cancer Res20154219119725870801
- KoberleBTomicicMTUsanovaSKainaBCisplatin resistance: preclinical findings and clinical implicationsBiochim Biophys Acta20101806217218220647037
- SiddikZHCisplatin: mode of cytotoxic action and molecular basis of resistanceOncogene200322477265727914576837
- RebucciMMichielsCMolecular aspects of cancer cell resistance to chemotherapyBiochem Pharmacol20138591219122623435357
- XiongXDRenXCaiMYYangJWLiuXYangJMLong non-coding RNAs: an emerging powerhouse in the battle between life and death of tumor cellsDrug Resist Updat201626284227180308
- MajidiniaMYousefiBLong non-coding RNAs in cancer drug resistance developmentDNA Repair (Amst)201645253327427176
- JamiesonERLippardSJStructure, recognition, and processing of cisplatin-DNA adductsChem Rev19999992467249811749487
- RosenbergBVanCampLTroskoJEMansourVHPlatinum compounds: a new class of potent antitumour agentsNature196922251913853865782119
- BasuAKrishnamurthySCellular responses to cisplatin-induced DNA damageJ Nucleic Acids2010201020136720811617
- BrozovicAThe relationship between platinum drug resistance and epithelial-mesenchymal transitionArch Toxicol201791260561928032148
- FerreiraJAPeixotoANevesMMechanisms of cisplatin resistance and targeting of cancer stem cells: adding glycosylation to the equationDrug Resist Updat201624345426830314
- EinhornLHTreatment of testicular cancer: a new and improved modelJ Clin Oncol1990811177717811700077
- HowellSBSafaeiRLarsonCASailorMJCopper transporters and the cellular pharmacology of the platinum-containing cancer drugsMol Pharmacol201077688789420159940
- GuminskiADBalleineRLChiewYEMRP2 (ABCC2) and cisplatin sensitivity in hepatocytes and human ovarian carcinomaGynecol Oncol2006100223924616213010
- GalluzziLSenovillaLVitaleIMolecular mechanisms of cisplatin resistanceOncogene201231151869188321892204
- AroraSKothandapaniATillisonKKalman-MalteseVPatrickSMDownregulation of XPF-ERCC1 enhances cisplatin efficacy in cancer cellsDNA Repair (Amst)20109774575320418188
- FinkDNebelSAebiSThe role of DNA mismatch repair in platinum drug resistanceCancer Res19965621488148868895738
- TurnerNCTuttANPlatinum chemotherapy for BRCA1-related breast cancer: do we need more evidence?Breast Cancer Res201214611523146216
- AsadaNTsuchiyaHTomitaKDe novo deletions of p53 gene and wild-type p53 correlate with acquired cisplatin-resistance in human osteosarcoma OST cell lineAnticancer Res1999196B5131513710697522
- LeongCOVidnovicNDeYoungMPSgroiDEllisenLWThe p63/p73 network mediates chemosensitivity to cisplatin in a biologically defined subset of primary breast cancersJ Clin Invest200711751370138017446929
- van OosterwijkJGHerpersBMeijerDRestoration of chemo-sensitivity for doxorubicin and cisplatin in chondrosarcoma in vitro: BCL-2 family members cause chemoresistanceAnn Oncol20122361617162622112972
- HenkelsKMTurchiJJCisplatin-induced apoptosis proceeds by caspase-3-dependent and -independent pathways in cisplatin-resistant and -sensitive human ovarian cancer cell linesCancer Res199959133077308310397248
- ParkMSDe LeonMDevarajanPCisplatin induces apoptosis in LLC-PK1 cells via activation of mitochondrial pathwaysJ Am Soc Nephrol200213485886511912244
- RenJHHeWSNongLAcquired cisplatin resistance in human lung adenocarcinoma cells is associated with enhanced autophagyCancer Biother Radiopharm2010251758020187799
- MitsuuchiYJohnsonSWSelvakumaranMWilliamsSJHamiltonTCTestaJRThe phosphatidylinositol 3-kinase/AKT signal transduction pathway plays a critical role in the expression of p21WAF1/CIP1/SDI1 induced by cisplatin and paclitaxelCancer Res200060195390539411034077
- AlexanderRPFangGRozowskyJSnyderMGersteinMBAnnotating non-coding regions of the genomeNat Rev Genet201011855957120628352
- MaLBajicVBZhangZOn the classification of long non-coding RNAsRNA Biol201310692593323696037
- TangYWangJLianYLinking long non-coding RNAs and SWI/SNF complexes to chromatin remodeling in cancerMol Cancer20171614228212646
- WuRSuYWuHDaiYZhaoMLuQCharacters, functions and clinical perspectives of long non-coding RNAsMol Genet Genomics201629131013103326885843
- GibbEABrownCJLamWLThe functional role of long non-coding RNA in human carcinomasMol Cancer2011103821489289
- ZhuQNWangGGuoYLncRNA H19 is a major mediator of doxorubicin chemoresistance in breast cancer cells through a cullin4A-MDR1 pathwayOncotarget2017854919909200329190892
- DuPZhaoHPengRLncRNA-XIST interacts with miR-29c to modulate the chemoresistance of glioma cell to TMZ through DNA mismatch repair pathwayBiosci Rep2017375BSR2017069628831025
- BianZJinLZhangJLncRNA-UCA1 enhances cell proliferation and 5-fluorouracil resistance in colorectal cancer by inhibiting miR-204-5pSci Rep201662389227046651
- HangQSunRJiangCLiYNotch 1 promotes cisplatin-resistant gastric cancer formation by upregulating lncRNA AK022798 expressionAnticancer Drugs201526663264025763542
- ZhangXWBuPLiuLZhangXZLiJOverexpression of long non-coding RNA PVT1 in gastric cancer cells promotes the development of multidrug resistanceBiochem Biophys Res Commun2015462322723225956062
- LanWGXuDHXuCSilencing of long non-coding RNA ANRIL inhibits the development of multidrug resistance in gastric cancer cellsOncol Rep201636126327027121324
- XuXRenHZhouBPrediction of copper transport protein 1 (CTR1) genotype on severe cisplatin induced toxicity in non-small cell lung cancer (NSCLC) patientsLung Cancer201277243844222516052
- JiangPWuXWangXHuangWFengQNEAT1 upregulates EGCG-induced CTR1 to enhance cisplatin sensitivity in lung cancer cellsOncotarget2016728433374335127270317
- DabrowiakJCGoodismanJSouidAKKinetic study of the reaction of cisplatin with thiolsDrug Metab Dispos200230121378138412433807
- JamaliBNakhjavaniMHosseinzadehLAmidiSNikounezhadNH ShiraziFIntracellular GSH alterations and its relationship to level of resistance following exposure to cisplatin in cancer cellsIran J Pharm Res201514251351925901159
- ZhengZGXuHSuoSSThe essential role of H19 contributing to cisplatin resistance by regulating glutathione metabolism in high-grade serous ovarian cancerSci Rep201662609327193186
- JanssensSTinelALippensSTschoppJPIDD mediates NF-kappaB activation in response to DNA damageCell200512361079109216360037
- OzesARMillerDFOzesONNF-kappaB-HOTAIR axis links DNA damage response, chemoresistance and cellular senescence in ovarian cancerOncogene201635415350536127041570
- LiuZSunMLuKThe long noncoding RNA HOTAIR contributes to cisplatin resistance of human lung adenocarcinoma cells via downregualtion of p21(WAF1/CIP1) expressionPLoS One2013810e7729324155936
- WangYChengNLuoJDownregulation of lncRNA ANRIL represses tumorigenicity and enhances cisplatin-induced cytotoxicity via regulating microRNA let-7a in nasopharyngeal carcinomaJ Biochem Mol Toxicol2017317e21904
- PangJCLiKKLauKMKIAA0495/PDAM is frequently downregulated in oligodendroglial tumors and its knockdown by siRNA induces cisplatin resistance in glioma cellsBrain Pathol20102061021103220477830
- LiLGuMYouBLong non-coding RNA ROR promotes proliferation, migration and chemoresistance of nasopharyngeal carcinomaCancer Sci201610791215122227311700
- XiaYHeZLiuBWangPChenYDownregulation of Meg3 enhances cisplatin resistance of lung cancer cells through activation of the WNT/beta-catenin signaling pathwayMol Med Rep20151234530453726059239
- LiuJWanLLuKThe long noncoding RNA MEG3 contributes to cisplatin resistance of human lung adenocarcinomaPLoS One2015105e011458625992654
- MaWLiJHuJmiR214-regulated p53-NOX4/p66shc pathway plays a crucial role in the protective effect of Ginkgolide B against cisplatin-induced cytotoxicity in HEI-OC1 cellsChem Biol Interact2016245728126768586
- MaLYXieXWMaLDownregulated long non-coding RNA TRPM2-AS inhibits cisplatin resistance of non-small cell lung cancer cells via activation of p53-p66shc pathwayEur Rev Med Pharmacol Sci201721112626263428678322
- YouleRJStrasserAThe BCL-2 protein family: opposing activities that mediate cell deathNat Rev Mol Cell Biol200891475918097445
- WangQChengNLiXCorrelation of long non-coding RNA H19 expression with cisplatin-resistance and clinical outcome in lung adenocarcinomaOncotarget2017822558256727911863
- YanHXiaJYFengFZLong non-coding RNA ENST000457645 reverses cisplatin resistance in CP70 ovarian cancer cellsGenet Mol Res2017161gmr16019411
- LiuELiuZZhouYMiRWangDOverexpression of long non-coding RNA PVT1 in ovarian cancer cells promotes cisplatin resistance by regulating apoptotic pathwaysInt J Clin Exp Med2015811205652057226884974
- PanJLiXWuWLong non-coding RNA UCA1 promotes cisplatin/gemcitabine resistance through CREB modulating miR-196a-5p in bladder cancer cellsCancer Lett20163821647627591936
- WangBHuangZGaoRExpression of long noncoding RNA urothelial cancer associated 1 promotes cisplatin resistance in cervical cancerCancer Biother Radiopharm201732310111028414550
- ZhangJLiuJXuXLiLCurcumin suppresses cisplatin resistance development partly via modulating extracellular vesicle-mediated transfer of MEG3 and miR-214 in ovarian cancerCancer Chemother Pharmacol201779347948728175963
- LiLYinJYHeFZLong noncoding RNA SFTA1P promoted apoptosis and increased cisplatin chemosensitivity via regulating the hnRNP-U-GADD45A axis in lung squamous cell carcinomaOncotarget2017857974769748929228625
- WangYChenWYangCLong non-coding RNA UCA1a(CUDR) promotes proliferation and tumorigenesis of bladder cancerInt J Oncol201241127628422576688
- WangYWangHSongTHOTAIR is a potential target for the treatment of cisplatinresistant ovarian cancerMol Med Rep20151222211221625824616
- ZhangYSongXWangXHuJJiangLSilencing of LncRNA HULC enhances chemotherapy induced apoptosis in human gastric cancerJ Med Biochem201635213714328356873
- WickstromMDybergCMilosevicJWnt/beta-catenin pathway regulates MGMT gene expression in cancer and inhibition of Wnt signalling prevents chemoresistanceNat Commun20156890426603103
- YangYLiHHouSHuBLiuJWangJThe noncoding RNA expression profile and the effect of lncRNA AK126698 on cisplatin resistance in non-small-cell lung cancer cellPLoS One201385e6530923741487
- FanYShenBTanMLong non-coding RNA UCA1 increases chemoresistance of bladder cancer cells by regulating Wnt signalingFEBS J201428171750175824495014
- LiZZhaoLWangQOverexpression of long non-coding RNA HOTTIP increases chemoresistance of osteosarcoma cell by activating the Wnt/beta-catenin pathwayAm J Transl Res2016852385239327347346
- LevineBKroemerGAutophagy in the pathogenesis of diseaseCell20081321274218191218
- ZhangNYangGQShaoXMWeiLGAS5 modulated autophagy is a mechanism modulating cisplatin sensitivity in NSCLC cellsEur Rev Med Pharmacol Sci201620112271227727338051
- SunMYZhuJYZhangCYAutophagy regulated by lncRNA HOTAIR contributes to the cisplatin-induced resistance in endometrial cancer cellsBiotechnol Lett201739101477148428721581
- SunWZuYFuXDengYKnockdown of lncRNA-XIST enhances the chemosensitivity of NSCLC cells via suppression of autophagyOncol Rep20173863347335429130102
- MaBYuanZZhangLLong non-coding RNA AC023115.3 suppresses chemoresistance of glioblastoma by reducing autophagyBiochim Biophys Acta2017186481393140428499919
- YanHBuPNon-coding RNAs in cancer stem cellsCancer Lett201842112112629331418
- LiuMYLiXQGaoTHElevated HOTAIR expression associated with cisplatin resistance in non-small cell lung cancer patientsJ Thorac Dis20168113314332228066612
- WangYZhangLZhengXLong non-coding RNA LINC00161 sensitises osteosarcoma cells to cisplatin-induced apoptosis by regulating the miR-645-IFIT2 axisCancer Lett2016382213714627609068
- FengYZouWHuCModulation of CASC2/miR-21/PTEN pathway sensitizes cervical cancer to cisplatinArch Biochem Biophys20176236242030
- CaoLChenJOuBLiuCZouYChenQGAS5 knockdown reduces the chemo-sensitivity of non-small cell lung cancer (NSCLC) cell to cisplatin (DDP) through regulating miR-21/PTEN axisBiomed Pharmacother20179357057928686971
- WangPChenDMaHLiYLncRNA MEG3 enhances cisplatin sensitivity in non-small cell lung cancer by regulating miR-21-5p/SOX7 axisOnco Targets Ther2017105137514929123412
- WenQLiuYLyuHLong noncoding RNA GAS5, which acts as a tumor suppressor via microRNA 21, regulates cisplatin resistance expression in cervical cancerInt J Gynecol Cancer20172761096110828472815
- HannaNOhanaPKonikoffFMPhase 1/2a, dose-escalation, safety, pharmacokinetic and preliminary efficacy study of intratumoral administration of BC-819 in patients with unresectable pancreatic cancerCancer Gene Ther201219637438122498722
- GofritONBenjaminSHalachmiSDNA based therapy with diphtheria toxin-A BC-819: a phase 2b marker lesion trial in patients with intermediate risk nonmuscle invasive bladder cancerJ Urol201419161697170224342146
- LavieOEdelmanDLevyTA phase 1/2a, dose-escalation, safety, pharmacokinetic, and preliminary efficacy study of intraperitoneal administration of BC-819 (H19-DTA) in subjects with recurrent ovarian/peritoneal cancerArch Gynecol Obstet2017295375176128154921
