Abstract
Background
Although there have been great advances in mechanisms and therapeutic methods of prostate cancer, the mortality rate of prostate cancer remains high. The castration-resistant prostate cancer (CRPC), which develops from hormone-sensitive prostate cancer, foreshadows a more dismal outcome. Concomitant with the researches in the mechanism of CRPC and therapy for CRPC, more and more landmark progress has been made in recent years.
Methods
A number of clinical and experimental studies were reviewed to indicate the novel advancement in the progressive mechanism and therapy of CRPC.
Results
The androgen receptor (AR) is still a vital driver in the progression of CRPC, while other multiple mechanisms also contribute to this progression, such as tumor immunity, cancer stem cells, epithelial–mesenchymal transition and DNA repair disorder. In terms of the therapeutic methods of CRPC, chemotherapy with drugs, such as docetaxel, has been the first-line therapy for CRPC for many years. Besides, newer agents, which target some of the above mechanisms, show additional overall survival benefits for CRPC patients. These therapies include drugs targeting the androgen axis pathway (androgen synthesis, androgen receptor splice variants, coactivators of AR and so on), PI3K-AKT pathway, WNT pathway, DNA repair, rearrangement of ETS gene, novel chemotherapy and immunotherapy, bone metastasis therapy and so on. Understanding these novel findings on the mechanisms of CRPC and the latest potential CRPC therapies will direct us for further exploration of CRPC.
Conclusion
Through comprehensive consideration, the predominant mechanism of CRPC might be the AR signal axis concomitant with tumor microenvironment, stress, immunity, tumor microenvironment and so on. For CRPC therapy, targeting the AR axis pathway and chemotherapy are the first-line treatments at present. However, with the advancements in CRPC therapy made by the researchers, other novel potential methods will occupy more and more important position in the treatment of CRPC, especially the therapies targeting the tumor microenviroment, tumor immunity and DNA repair and so on.
Introduction
Prostate cancer, which has the highest incidence among male malignancies in European and American countries, seriously threatens human health and affects the patients’ life quality.Citation1 According to the statistics, the incidence and mortality rates of prostate cancer have been increasing every year with the rapid increase in Chinese aging society. Specifically, the castration-resistant prostate cancer (CRPC), which develops from hormone-sensitive prostate cancer, has a much higher mortality rate compared with prostate cancer.Citation2
The clinical prognosis of CRPC patients is still unsatisfactory despite the fact that several important mechanisms and therapy advancements of CRPC have been reported. New drugs such as docetaxel, abiraterone and enzalutamide that could delay, to a certain extent, the development of CRPC are still the main therapy agents for CRPC in clinical practice.Citation3 In recent years, numerous progressive mechanisms and therapeutic targets of CRPC have been found due to the efforts of the researchers. In this paper, we will review the novel research advancements of the progressive mechanisms and therapy in CRPC.
The progressive mechanisms of CRPC
At present, gene alteration in the androgen axis and the kinase-dependent signal pathways are the most important mechanisms of CRPC genesis and development. However, accumulated evidences have indicated that tumor immunity, cancer stem cells (CSCs), epithelial–mesenchymal transition (EMT), DNA repairing disorder and other mechanisms might also participate in the progress of CRPC ().
Figure 1 Main progressive mechanisms of CRPC.
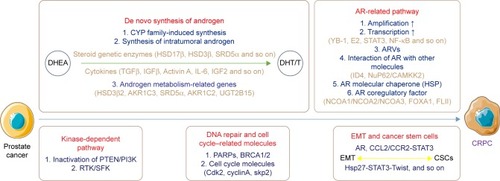
De novo synthesis of androgen
The levels of intratumoral androgen in some CRPC patients were higher than in the primary prostate cancer patients, although serum testosterone (T) was at the castration level (<1.7 nmol/L) in these CRPC patients.Citation4 According to the recent research, the mechanisms of de novo synthesis of androgen are discussed next.
Cytochrome P450 family-induced synthesis
The synthesis of T and dihydrotestosterone depends on the cytochrome P450 (CYP) family. CYP17, a member of the CYP family, has been proved to play a vital role in CRPC. Abiraterone acetate, one of the most common medicines for CRPC, is a kind of inhibitor of CYP17 and has been proved to improve the prognosis of CRPC patients.Citation5
The synthesis of intratumoral androgen
According to previous researches, the levels of T and dihydrotestosterone are mainly dependent on the synthesis of intratumoral androgen, which is based on the conversion of dehydroepiandrosterone in the prostate cancer tissues after androgen deprivation therapy (ADT). This kind of intratumoral androgen synthesis mostly relies on some steroidogenic enzymes such as hydroxysteroid dehydrogenase (HSD)17β, HSD3β and steroid-5α-reductase.Citation6
The activities of steroidogenic enzymes are also correlated with the expression of some other cytokines. Transforming growth factor beta could regulate the synthesis of the intratumoral androgen by upregulating HSD3β and downregulating HSD17β; insulin-like growth factor (IGF)-β could promote the synthesis of intratumoral androgen by increasing the expression of CYP17, AKR1C3 and HSD17β, so as to improve the development of CRPC.Citation7 Moreover, activin A, interleukin (IL)-6 and IGF2 could also regulate the activities of the steroidogenic enzymes.Citation8–Citation10
Recently, it was indicated that arachidonic acid could induce the synthesis of StAR, which is bound to hormone-sensitive lipase and transforms the free cholesterol into androgen in the steroid synthesis-related cells.Citation11
Elevation of the expression level of androgen metabolism–related genes
The upregulation of the expression level of the androgen metabolism–related genes, such as HSD3β2, AKR1C3, SRD5α, AKR1C2 and UGT2B15, also plays an important role in the development of CRPC.Citation12
The regulatory effect of tumor microenvironment in CRPC
Recent studies have demonstrated that the synthesis of intratumoral androgen is related to the tumor microenvironment to a certain extent. The expression levels of steroidogenesis enzymes in the stromal cells are much higher in the CRPC cells. Meanwhile, the activity of prostate specific antigen (PSA) promoter, which is induced by dehydroepiandrosterone through androgen receptor (AR) activation in CRPC cells, could also be enhanced by prostate stromal cells.Citation13 Besides, the anti-angiogenesis effect induced by vascular endothelial growth factor also plays a vital role in the progress of CRPC, while the vascular endothelial growth factor–targeted therapy was suggested to be useful for CRPC according to a study.Citation14
The adaptive variation of AR-related pathway
During the progress of CRPC, the adaptive variation of AR-related pathway plays a vital role and the variation mainly focuses on the following parts.
The amplification of AR gene
The depletion of AR ligands could lead to the amplification of AR gene via feedback regulation.Citation15 The overexpression and the importance of AR signaling in androgen-independent prostate cancer have been proved by reports.Citation16,Citation17 The amplification of AR gene could directly increase the expression level of AR and accelerate the development of CRPC. According to studies, glucocorticoid receptor could regulate some of AR targets in CRPC cells, and endostatin could inhibit glucocorticoid receptor–induced resistance upon AR antagonism.Citation18,Citation19
The transcription activity of AR gene
The regulation of AR transcription via cytokine plays an important role in the development of CRPC. YB-1 protein could combine with the Y-box domain in AR promoter and regulate the transcription of AR gene.Citation20 The estrogen E2 could enhance the expression of SOX4, which is a member of the AR transcription factors in CRPC cells.Citation21 Besides, STAT3, NF-κB and NF-κB/p52 could also activate the transcription of AR and increase its expression level through different pathways.Citation22–Citation24
The mutation of AR gene
The mutation of ligand-binding domain (LBD) or co-effective areas in AR gene could decrease the specificity of the combination between AR and its ligands, while the mutation of F876L region in AR gene has been demonstrated to be correlated with enzalutamide resistance in CRPC.Citation25 Furthermore, the generation of AR splice variants (ARVs) is reported to play an important role in the development of CRPC, especially the AR-V7, which is transferred from AR due to a deficiency of LBD ligand. The expression of AR-V7 could activate the androgen synthesis–related genes and promote the progression of CRPC.Citation26
The interactions between AR and other molecules
Besides the above signal pathways, the activity of AR is also correlated with some other molecules. The inactivation of inhibitor of differentiation 4 could activate the AR and promote CRPC.Citation27 Nucleoporin62 and calcium/calmodulin-dependent protein kinase kinase 2 could impact the activity and development of CRPC.Citation28
The regulator effect of AR molecular chaperone
As a molecular chaperone of AR, the expression of heat shock proteins (HSPs), especially HSP27 and HSP90, could be induced by ADT. It was reported that the activation of HSP27 and HSP90 might contribute to the resistance of ADT.Citation29
The modulation of AR co-regulatory factor
The implementation of AR function was also associated with some co-regulatory factors. The nuclear receptor coactivators (NCOAs)1, NCOA2 and NCOA3, which are the members of non-receptor tyrosine kinase (SRC) family and coactivators of AR, have attracted special attention of the researchers.Citation30 Meanwhile, forkhead box A1 (FOXA1), another cofactor, could modulate the transcription of AR and regulate the development of CRPC via GAT 3A2 gene.Citation31
A recent study demonstrated that the Flightless I Homolog, FLII, could activate as a kind of transcriptional co-regulatory factor of AR and suppress the development of CRPC by regulating the cellular localization of AR in prostate cancer cells and inhibiting the expression of AR. This study revealed that FLII might be an important therapy target of CRPC.Citation32
The regulator effect of kinase-dependent pathway
The kinase-dependent signal pathway, which is modulated by AR axis during ADT, also plays a vital role in the development of CRPC. The inactivation of PTEN gene in CRPC patients could activate PI3K resulting in AR activation.Citation33 In recent years, it was reported that the receptor tyrosine kinases and SRC family kinases could regulate the AR signal and the development of CRPC by inducing the phosphorylation of AR and inhibiting the expressions of co-regulatory factors of AR.Citation34
Modulation of DNA repair genes and cell cycle–related molecules
Mutation of DNA repair genes might play a vital role in CRPC.Citation35 Poly (ADP-ribose) polymerase, which is a kind of DNA repair enzyme, could maintain the function of AR.Citation36 Meanwhile, the mutation of BRCA1/2 gene, which is a member of DNA repair gene, was closely correlated with the development of CRPC.Citation37 Furthermore, the androgen could inhibit the proliferation of AR-positive CRPC cells through the suppression of Cdk2, CyclinA and Skp2, which are typical members of cell cycle–related cytokines.Citation38
The regulation of EMT and CSCs
In recent years, the importance of EMT and CSCs in the progressive CRPC has been demonstrated by various studies. The expression levels of E-cadherin, N-cadherin, Snail, Twist and other EMT markers are altered significantly during ADT. According to previous studies, ADT could trigger the appearance of EMT, which could further accelerate the development of CRPC.
CSC, a kind of potential malignant epithelial stem cell, might effect as an important regulatory target in CRPC. Some representative markers such as Nkx3.1, CD166, PSA−/LO, Nanog, Bmi-1 and SOX2 are shown to be correlated with the progress of CRPC on the grounds of recent studies.Citation39,Citation40
Furthermore, according to a recent study, the interaction between EMT and CSCs might also play a vital role in the recurrence and drug resistance of CRPC through AR, CCL2/CCR2-STAT3, Hsp27-STAT3-Twist and other pathways.Citation41,Citation42
The regulation of inflammation cytokines
The roles of inflammation factors in prostate cancer were proved by accumulating evidences during recent years (). IL-6 could induce the drug resistance of prostate cancer by activating SRC-1, regulating the expression of genes GRB2, SHC and JAK-1 and inhibiting the apoptosis of CRPC cells.Citation43 IL-4, another important inflammation factor, could affect the coactivators of AR, such as CBP/P300 and NF-κB, and regulate the development of CRPC.Citation44 IL-8, which could interact with NF-κB, is overexpressed in CRPC and could promote the angiogenesis and metastasis of CRPC through the SRC and FAK pathway.Citation45 Furthermore, CXCR4, CXCR2/CXCR3, CXCR6 and CXCR7, which belong to the CXCR family, also play an important role in the development of CRPC via inflammation pathways according to recent studies.Citation46–Citation50 The roles of inflammation factors in cancers have been a hot issue in recent years, and a deeper and broader discussion is expected in future.
Table 1 The regulation of inflammation factors in CRPC
The regulation of ncRNAs in CRPC
The ncRNAs serve as a transcription regulator in CRPC (). It was known that miRNA could modulate the development of CRPC by regulating the cell cycle, cell differentiation and cell proliferation.Citation51 Among the miRNAs, miR-1 and miR-206 could regulate the expression levels of glucose metabolism-related genes such as G6PD, TKT, PGD and GPD2 to affect the development of CRPC. Meanwhile, miR-185, miR-342, miR-17/92 and miR101 could regulate the development of CRPC through influencing SREBP, PPARA, COX-2 and other lipid metabolism-related pathways.Citation52,Citation53 Besides, miR-32, miR-148a, miR-99a, miR-2 and miR-221 also play an important role in CRPC via AR signals.Citation54
Table 2 The regulation of ncRNA in CRPC
According to studies, lncRNAs such as PRNCR1, PCGEM1 and CTBP-AS could promote the development of prostate cancer by activating and promoting the transcription of AR.Citation55 lncRNA-p21 could promote the survival of prostate cancer cells through the Warburg effect and hypoxia-inducible factor pathway.Citation56 In addition, HOTAIR, which is a member of lncRNA, could promote the proliferation, invasion and metastasis of CRPC via the modulation of epigenetics and transcription of AR.Citation57 Similarly, the lncRNAs PCAT1 and ANRIL could modulate the expression of AR through interacting with the PGC-1-related coactivator family.Citation58,Citation59
The transformation of prostate cancer into neuroendocrine prostate cancer
Neuroendocrine prostate cancer (NEPC) is insensitive to the ADT. According to recent studies, the loss of RB1 and TP53 and the increase in MYCN and AURKA might be the reasons for the formation of NEPC.Citation60 As a result, these genes related with the formation of NEPC are considered as a potential therapy target of NEPC.
The new advancement of CRPC therapy
Drugs targeting the androgen axis, cytotoxic drugs, immune drugs and drugs targeting bone metastasis (zoledronic acid, 233Ra and denosumab) are the main choices for clinical doctors for CRPC therapy.Citation61,Citation62 Moreover, the drugs targeting PI3K-AKT, WNT, DNA repair and other molecular signaling pathways were demonstrated to be essential in CRPC therapy, while the advances in prostate cancer immunity researches also indicated that immunotherapy might be indispensable in CRPC therapy in the futureCitation63,Citation64 ().
Table 3 Novel potential targets of CRPC therapy
Targeting the androgen axis pathway
As a nuclear receptor related to steroidogenesis, AR is constituted by DNA-binding domain (DBD), LBD, N-terminal domain (NTD) and hinge region. The alteration in any component of AR structure might change the activity of AR and influence the development of CRPC.Citation65 As a result, targeting the structural domain of AR is also a hotspot in CRPC.
Inhibition of androgen synthesis
The inhibitors of the key enzymes in androgen synthesis were indicated to have a satisfactory effect in clinical trials. The therapeutic effect of the combination of abiraterone acetate and prednisone for CRPC has been proved in clinical practice.Citation66,Citation67 Besides, VT-464, acting on CYP17, could suppress the proliferation of tumor and decrease the PSA level through inhibition of AR-axis.Citation68 However, according to a clinical trial, TAK-700 (Orteronel), which also acts on CYP17, showed no significant effect on the overall survival time of CRPC patients.Citation69
Although many new drugs might be effective for CRPC, administration of drugs that act upon the AR axis would still be the most essential and important method in CRPC clinical therapy for some time.
Targeting HSP protein
HSP protein is a very important target in CRPC therapy as mentioned before.Citation29 The application of inhibitors targeting the HSP90 protein has been considered as a novel therapeutic strategy for CRPC patients with mutant AR.Citation70 Knockdown of TCTP that interacts with HSP27 could suppress the survival and proliferation of CRPC cells.Citation71 In recent years, some preclinical studies have indicated that silencing HSP27 or HSP90 could sensitize the prostate cancer cells to chemotherapy and radiation treatments, and several HSP protein inhibitors (tanespimycin, ganetespib, OGX-427) could delay castration resistance or prolong survival in CRPC.Citation72–Citation75 Thus, the combination of HSP blockage and other chemotherapy drugs could be a potential therapeutic strategy in patients with metastasis (m)CRPC.
Targeting the AR-LBD or ARVs
Enzalutamide and ARN-509 (apalutamide) show a great affinity for AR and are effective in CRPC that is resistant to bicalutamide.Citation76 Recently, new drugs targeting AR-LBD and ARVs mainly focus on the following types.
Niclosamide, an antiparasitic drug approved by the US Food and Drug Administration was demonstrated to suppress the expression level of AR-V7, but not androgen receptor full length (AR-FL).Citation77 It was also reported that niclosamide combined with current antiandrogen agents might possess a satisfactory effect in CRPC patients. Besides, drugs such as ASC-J9 (dimethylcurcumin) and inhibitors of calpain (CUDC-101 and TAS3681) might be useful for CRPC therapy by inhibiting ARVs, according to some studies.Citation78–Citation81
Targeting the AR-DBD
Some researchers believed that the alteration of AR-DBD might be correlated with the production of ARVs, and targeting AR-DBD could be a potential therapeutic strategy for CRPC.Citation82
Targeting the AR-NTD
According to a study, the NTD of AR is responsible for AR transcriptional activity.Citation83 As NTD is very important for the activity of all kinds of AR, targeting AR-NTD is expected to be used in CRPC. Several AR-NTD inhibitors (EPI-506, sintokamides) have been under preclinical experiments.Citation82,Citation84 As for EPI-506, it is the first agent that could suppress both canonical and variant-related AR signaling, and the ongoing Phase I/II study of EPI-506 (NCT2606123) will evaluate the benefit of EPI-506 in mCRPC patients. According to a study,Citation82 EPI-001, the novel depressant of AR-NTD, showed a favorable effect on eliminating the castration resistance which was caused by ARVs.
Antagonist of AR
In clinic, bicalutamide, flutamide, nilutamide and other anti-androgen drugs are still considered as the first-line treatment for CRPC. However, the curative effects of these drugs are not everlasting, and the prostate cancer treated with ADT might advance to CRPC after 6 months.Citation85
The core ligand of cyclobutane, which was used as the fourth-generation antiandrogen drug, might be of great importance in CRPC therapy through inhibition of nuclear translocation of AR.Citation86
Targeting the coactivators of AR
According to a clinical trial, depressors of SRC kinase, such as dasatinib and saracatinib, showed a satisfactory effect in CRPC therapy by acting upon AR coactivators.Citation87
Inhibiting AR expression
It might be possible to decrease the AR level by interfering with the expression of AR at the mRNA level. ENZ-4716 is a drug that confirms this hypothesis and is currently in Phase I clinical trial.Citation88
Targeting the PI3K-AKT and WNT pathways
According to previous researches, both PI3K-AKT and WNT pathways play a vital role in the development of CRPC. BEZ235, one of the inhibitors of PI3K-mTOR, and GDC0068, one of the inhibitors of AKT, are both in Phase I clinical trials at present.Citation89 Inhibition of WNT signal could potentiate the antitumor activity of the Plk1 inhibitor for CRPC.Citation90 Besides, targeting PORCN (porcupine), which is essential for WNT pathway, might be a potential treatment method for CRPC.Citation91
Targeting DNA repair
As mentioned earlier, mutations in DNA repair genes, such as BRCA2, homologous recombination, and nucleotide excision repair and mismatch repair, are common therapy targets in oncotherapy. For instance, inhibitors of poly (ADP-ribose) polymerase, especially olaparib (AZD-2281), showed satisfactory efficacy in CRPC patients according to the clinical trials.Citation82
Targeting the rearrangement of ETS gene
ERG, ETV1, ETV6 and FLI1, which belong to the E twenty-six transcriptional factor family, are considered as the important oncogenes in different cancers.Citation92 As one of the most important gene rearrangements in E twenty-six family, the gene rearrangement in TMPRSS2-ERG was deemed to be a potential target in CRPC therapy. Compounds targeting the rearrangement of TMPRSS2-ERG, such as DB1255 and PLA2G7, could regulate the biological function of prostate cells.Citation93,Citation94 Besides targeting the classical ERG pathway, YK-4-279 might be useful in inhibiting the proliferation and survival of CRPC cells by suppressing the FLI1 gene.Citation95
Chemotherapy for CRPC
Although the side effects of chemotherapy are inevitable, chemotherapy in CRPC is nonnegligible because it is perfectly capable of killing prostate cancer cells.
Taxanes and epothilones are the typical drugs targeting the microtubules, while docetaxel and cabazitaxel are classical taxane drugs used in CRPC,Citation96 and pemetrexed could be used for CRPC under docetaxel-resistant condition.Citation97 As a newer trend of CRPC chemotherapy, the effect of applying taxanes in an earlier hormone-naive clinical setting of prostate cancer has been proved by studies.Citation98,Citation99 Cabazitaxel, the next generation of taxane drugs, has been shown to be an effective antitumor agent in docetaxel resistance cell lines.Citation100,Citation101 In recent years, some reports indicate that the taxanes may have cross-resistance with enzalutamide and abiraterone. As a result, the application of enzalutamide in prostate cancer patients has only a modest effect following docetaxel treatment.Citation102–Citation104
Besides the drugs mentioned earlier, several novel chemotherapy drugs have appeared recently. Saikosaponin-d possesses therapeutic potential for CRPC by reversing EMT and inhibiting the expression of MMP2/9 and WNT/β-actin.Citation105 Moreover, the expression of pigment epithelium-derived factor also could enhance the efficacy of low-dose chemotherapy and improve the prognosis in CRPC.Citation106
Immunotherapy in CRPC
Although immunotherapy in CRPC is still in its initial stage, the prospect of immunotherapy has received more attention in recent decades. Immunotherapy might be of significant importance in both therapy and prevention of CRPC.Citation107
Vaccines for prostate cancer
After the idea of tumor vaccine came up, much effort was put into this field. Up to now, the application of vaccines in prostate cancer has mainly focused on the following:
Peptide vaccine: PROSTVAC-VF is a kind of peptide vaccine using PSA as the target antigen and is integrated with avipoxvirus, vaccinia and TRICOM. PROSTVAC-VF could prolong the survival time of the CRPC patients with a satisfactory tolerance according to the clinical trials.Citation108
Nucleic acid vaccine: Nucleic acid vaccine is a kind of vaccine that could express the related target antigen and induce the related immunoreaction. PTVG-HP is a typical nucleic acid vaccine, and its target antigen is prostatic acid phosphatase. According to reports, PTVG-HP could induce the related immunoreaction and has shown a potential usage for CRPC.Citation109
Whole-cell vaccine (WCV): The WCV could induce multiple kinds of antibodies, but the application of WCV is limited because the immunogenicity of WCV is poor in general. For prostate cancer and CRPC, GVAX, which is modified with costimulators, was effective in Phase I and II clinical trials, but was unsatisfactory in Phase III clinical trial.Citation110 Under this condition, the application of WCV in prostate cancer still needs more research.
Dendritic cell (DC) vaccine: As the most effective antigen-presenting cell, DC is the first choice for preparation of autogenous cell vaccine. For CRPC, Sipulencel-T is the representative of DC vaccine and is able to induce CD4 and CD8 contraposing prostate cancer cells.Citation111 According to studies, Sipulencel-T could improve the prognosis of the CRPC patients as well.
Immune checkpoints in prostate cancer
In the immune system, the immune checkpoint is a kind of inhibitory signal pathway, which is important for maintaining self-tolerance and regulating the duration. For prostate cancer, targeting cytotoxic T-lymphocyte-associated antigen-4 and programmed death protein-1 is the most effective strategy for immune checkpoint-related therapies.Citation112 Ipilimumab, which could block or inhibit the cytotoxic T-lymphocyte-associated antigen-4, might prolong the progression-free survival time of CRPC patients, according to clinical trials.Citation113 Nivolumab and pembrotizumab represent the immune drugs targeting the programmed death protein-1, but the therapeutic effect of them is not obvious.Citation114
Recently, tasquinimod and lenalidomide that target the myeloid-derived suppressor cell showed the antitumor ability by the regulation of immune system and inhibition of angiogenesis in CRPC.Citation115
Targeting the prostate-specific membrane antigen (PSMA)
PSMA, which is upregulated after ADT, has received more attention in recent decades. J591 is a monoclonal antibody of PSMA and might decrease the PSA level and prolong the survival time of CRPC patients.Citation116
The therapy of bone metastasis in CRPC
Bisphosphonates and denosumab, which are the typical drugs for bone metastasis, also show their effectiveness in CRPC by targeting the osteoclasts, while denosumab might inhibit the metastasis of CRPC through RANKL pathway.Citation117
Besides chemotherapy, radiotherapy could improve the prognosis of CRPC patients with bone metastasis. Radium-223, a kind of alpha particle radiopharmaceutical, has been proved to be effective in bone metastasis patients and prognosis with low clinical risks.Citation118 According to the reports, ACK1/AR pathway could induce the phosphorylation of AR, as well as the upregulation of ataxia-telangiectasia mutated and the regulation of DNA damage, to accelerate the resistance to radiotherapy. AIM-100, an inhibitor of ACK1, could improve the effect of radiotherapy in CRPC.Citation119
Other drugs for CRPC
Inhibitors of tyrosine kinase
IGF-1R, a member of tyrosine kinase, could interact with MAPK, PI3K/AKT and other signaling pathways to modulate the development of CRPC. It has been reported that figitumumab, an antibody of IGF-1R, could decrease the expression level of PSA and AR.Citation120 Cabozantinib, another oral tyrosine kinase inhibitor, has a significant effect on decreasing the PSA level and suppressing the development of CRPC.Citation121
Inhibitors of BET protein
As mentioned before, BER protein plays a vital role in CRPC through the AR signal pathway. GSK525762, which is the first inhibitor for BRD4 in clinical trial, shows satisfactory therapeutic effects for CRPC patients, while the clinical therapeutic effect of another inhibitor for BRD4, OTX015, still needs to be confirmed by more researches in the future.Citation122,Citation123
Inhibitors for NOTCH pathway
According to the recent research, NOTCH pathway is correlated with the development of CRPC. A novel inhibitor for NOTCH, RO4929097, could suppress the development of CRPC and might be a potential therapeutic target for CRPC.Citation124
Apoptosis and cell cycle pathway
Escin, a natural compound that is extracted from buckeye, has been used for anti-inflammation and detumescence for a long time.Citation125 A latest study demonstrated that escin could suppress the proliferation and survival of CRPC cells by regulating cell cycle and inducing apoptosis.Citation126
Conclusion
Concomitant with the researches in the mechanism of CRPC and therapy for CRPC, more breakthrough progress has been made. Through comprehensive consideration, the predominant mechanism of CRPC might be the AR signal axis concomitant with other carcinogenesis signals, stress, immunity, tumor microenvironment and so on. With the latest findings in tumor microenvironment, tumor immunity and tumor metabolism, these fields might play indispensable roles in CRPC. However, the interactions between the above mechanisms and the other novel potential mechanisms are still uncertain.
Meanwhile, the clinical guidelines that were established by the European Association of Urology or the American Urological Association are the authoritative references for making therapeutic strategy for CRPC in clinic up to now. With the guidance of clinical guidelines for CRPC, the specific state of each patient, the affordability of the patients and other factors also need to be considered when formulating the therapeutic strategy. Unfortunately, the therapeutic effect is not satisfactory in some CRPC patients nowadays. Therefore, novel and effective therapeutic strategy for CRPC patients is very necessary and exigent in clinic. We hold that the detection of vital gene of CRPC and the application of targeting drugs for CRPC might play an important part in future, while targeting the individual gene expression will bring big rewards for the therapeutic effect and avoid the side effect. Furthermore, the immunotherapy and tumor vaccine of CRPC might be the focus of research in CRPC therapy in future. Although there are still numerous unsolved difficulties in the research upon the mechanism and therapy of CRPC, we believe that great progress would be made with the efforts of researchers all over the world in the future.
Author contributions
KC contributed to designing this study. KSW and HLR contributed to the collection and analysis of data, discussion, and writing of the manuscript. TBX, DL, HMY and XPZ contributed to the correction of grammatical errors in this article. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No 81272560, 81672524 and 81672528) and the Program for New Century Excellent Talents in University from the Department of Education of China (NCET-08-0223).
Disclosure
The authors report no conflicts of interest in this work.
References
- MathasSMisteliTThe dangers of transcriptionCell200913961047104920005797
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- PetrylakDPPractical guide to the use of chemotherapy in castration resistant prostate cancerCan J Urol2014212 Supp 1778324775728
- MontgomeryRBMostaghelEAVessellaRMaintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growthCancer Res200868114447445418519708
- FizaziKScherHIMolinaACOU-AA-301 InvestigatorsAbiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 studyLancet Oncol2012131098399222995653
- LabrieFLuu-TheVBelangerAIs dehydroepiandrosterone a hormone?J Endocrinol2005187216919616293766
- LubikAAGunterJHHollierBGIGF2 increases de novo steroidogenesis in prostate cancer cellsEndocr Relat Cancer201320217318623319492
- HoflandJvan WeerdenWMSteenbergenJDitsNFJensterGde JongFHActivin A stimulates AKR1C3 expression and growth in human prostate cancerEndocrinology2012153125726573423024260
- ChunJYNadimintyNDuttSInterleukin-6 regulates androgen synthesis in prostate cancer cellsClin Cancer Res200915154815482219638459
- LubikAAGunterJHHollierBGIGF2 increases de novo steroidogenesis in prostate cancer cellsEndocr Relat Cancer201320217318623319492
- LockeJAGunsESLehmanMLArachidonic acid activation of intratumoral steroid synthesis during prostate cancer progression to castration resistanceProstate201070323925119790237
- StanbroughMBubleyGJRossKIncreased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancerCancer Res20066652815282516510604
- MizokamiAKohEIzumiKProstate cancer stromal cells and LNCaP cells coordinately activate the androgen receptor through synthesis of testosterone and dihydrotestosterone from dehydroepiandrosteroneEndocr Relat Cancer20091641139115519608712
- KellyWKHalabiSCarducciMRandomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401J Clin Oncol201230131534154022454414
- MathasSMisteliTThe dangers of transcriptionCell200913961047104920005797
- LinjaMJSavinainenKJSaramakiORTammelaTLVessellaRLVisakorpiTAmplification and overexpression of androgen receptor gene in hormone-refractory prostate cancerCancer Res20016193550355511325816
- HaapalaKKuukasjarviTHyytinenERantalaIHelinHJKoivistoPAAndrogen receptor amplification is associated with increased cell proliferation in prostate cancerHum Pathol200738347447817217995
- LeeJHKangMWangHEndostatin inhibits androgen- independent prostate cancer growth by suppressing nuclear receptor-mediated oxidative stressFASEB J20173141608161928069826
- AroraVKSchenkeinEMuraliRGlucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockadeCell201315561309132224315100
- ShiotaMTakeuchiASongYY-box binding protein-1 promotes castration-resistant prostate cancer growth via androgen receptor expressionEndocr Relat Cancer201118450551721652770
- YangMWangJWangLEstrogen induces androgen-repressed SOX4 expression to promote progression of prostate cancer cellsProstate201575131363137526015225
- De MiguelFLeeSOOnateSAGaoACStat3 enhances transactivation of steroid hormone receptorsNucl Recept200311312904256
- JinRJLhoYConnellyLThe nuclear factor-kappaB pathway controls the progression of prostate cancer to androgen-independent growthCancer Res200868166762676918701501
- NadimintyNLouWSunMAberrant activation of the androgen receptor by NF-kappaB2/p52 in prostate cancer cellsCancer Res20107083309331920388792
- BalbasMDEvansMJHosfieldDJOvercoming mutation-based resistance to antiandrogens with rational drug designElife20132e0049923580326
- DehmSMTindallDJAlternatively spliced androgen receptor variantsEndocr Relat Cancer2011185R183R19621778211
- JoshiJBPatelDMortonDJInactivation of ID4 promotes a CRPC phenotype with constitutive AR activation through FKBP52Mol Oncol201711433735728252832
- KaracostaLGKuroskiLAHofmannWANucleoporin 62 and Ca(2+)/calmodulin dependent kinase kinase 2 regulate androgen receptor activity in castrate resistant prostate cancer cellsProstate201676329430626552607
- AzadAAEiglBJMurrayRNKollmannsbergerCChiKNEfficacy of enzalutamide following abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer patientsEur Urol2015671232925018038
- ZhouHJYanJLuoWSRC-3 is required for prostate cancer cell proliferation and survivalCancer Res200565177976798316140970
- JozwikKMCarrollJSPioneer factors in hormone-dependent cancersNat Rev Cancer201212638138522555282
- WangTSongWChenYFlightless I homolog represses prostate cancer progression through targeting androgen receptor signalingClin Cancer Res20162261531154426527749
- WangSGaoJLeiQProstate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancerCancer Cell20034320922114522255
- GelmanIHAndrogen receptor activation in castration-recurrent prostate cancer: the role of Src-family and Ack1 tyrosine kinasesInt J Biol Sci201410662062624948875
- MateoJBoysenGBarbieriCEDNA repair in prostate cancer: biology and clinical implicationsEur Urol201771341742527590317
- SchiewerMJGoodwinJFHanSDual roles of PARP-1 promote cancer growth and progressionCancer Discov20122121134114922993403
- Kote-JaraiZLeongamornlertDSaundersEBRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patientsBr J Cancer201110581230123421952622
- KokontisJMLinHPJiangSSAndrogen suppresses the proliferation of androgen receptor-positive castration-resistant prostate cancer cells via inhibition of Cdk2, CyclinA, and Skp2PLoS One2014910e10917025271736
- LevesqueEHuangSPAudet-WalshEMolecular markers in key steroidogenic pathways, circulating steroid levels, and prostate cancer progressionClin Cancer Res201319369970923186779
- MitsiadesNSungCCSchultzNDistinct patterns of dysregulated expression of enzymes involved in androgen synthesis and metabolism in metastatic prostate cancer tumorsCancer Res201272236142615222971343
- ShiotaMBishopJLNipKMHsp27 regulates epithelial mesenchymal transition, metastasis, and circulating tumor cells in prostate cancerCancer Res201373103109311923492367
- IzumiKFangLYMizokamiATargeting the androgen receptor with siRNA promotes prostate cancer metastasis through enhanced macrophage recruitment via CCL2/CCR2-induced STAT3 activationEmbo Mol Med2013591383140123982944
- KarkeraJSteinerHLiWThe anti-interleukin-6 antibody siltuximab down-regulates genes implicated in tumorigenesis in prostate cancer patients from a phase I studyProstate201171131455146521321981
- LeeSOLouWNadimintyNLinXGaoACRequirement for NF-(kappa)B in interleukin-4-induced androgen receptor activation in prostate cancer cellsProstate200564216016715678501
- WaughDJWilsonCThe interleukin-8 pathway in cancerClin Cancer Res200814216735674118980965
- TaichmanRSCooperCKellerETPientaKJTaichmanNSMcCauleyLKUse of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to boneCancer Res20026261832183711912162
- ShenHSchusterRLuBWaltzSELentschABCritical and opposing roles of the chemokine receptors CXCR2 and CXCR3 in prostate tumor growthProstate200666161721172816941672
- WangJLuYWangJKochAEZhangJTaichmanRSCXCR6 induces prostate cancer progression by the AKT/mammalian target of rapamycin signaling pathwayCancer Res20086824103671037619074906
- SinghRKLokeshwarBLThe IL-8-regulated chemokine receptor CXCR7 stimulates EGFR signaling to promote prostate cancer growthCancer Res20117193268327721398406
- WangJShiozawaYWangJThe role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancerJ Biol Chem200828374283429418057003
- PasquinelliAEMicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationshipNat Rev Genet201213427128222411466
- SinghAHappelCMannaSKTranscription factor NRF2 regulates miR-1 and miR-206 to drive tumorigenesisJ Clin Invest201312372921293423921124
- HirokiEAkahiraJSuzukiFChanges in microRNA expression levels correlate with clinicopathological features and prognoses in endometrial serous adenocarcinomasCancer Sci2010101124124919891660
- JalavaSEUrbanucciALatonenLAndrogen-regulated miR-32 targets BTG2 and is overexpressed in castration-resistant prostate cancerOncogene201231414460447122266859
- YangLLinCJinClncRNA-dependent mechanisms of androgen-receptor-regulated gene activation programsNature2013500746459860223945587
- YangFZhangHMeiYWuMReciprocal regulation of HIF-1alpha and lincRNA-p21 modulates the Warburg effectMol Cell20145318810024316222
- ScaravilliMPorkkaKPBrofeldtAMiR-1247-5p is overexpressed in castration resistant prostate cancer and targets MYCBP2Prostate201575879880525731699
- PrensnerJRIyerMKBalbinOATranscriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progressionNat Biotechnol201129874274921804560
- YapKLLiSMunoz-CabelloAMMolecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4aMol Cell201038566267420541999
- BeltranHRickmanDSParkKMolecular characterization of neuroendocrine prostate cancer and identification of new drug targetsCancer Discov20111648749522389870
- ArsovCWinterCRabenaltRAlbersPCurrent second-line treatment options for patients with castration resistant prostate cancer (CRPC) resistant to docetaxelUrol Oncol201230676277120884252
- RyanCJSaylorPJEverlyJJSartorOBone-targeting radiopharmaceuticals for the treatment of bone-metastatic castration-resistant prostate cancer: exploring the implications of new dataOncologist201419101012101825232039
- MassariFMainesFModenaACastration resistant prostate cancer (CRPC): state of the art, perspectives and new challengesAnticancer Agents Med Chem201313687288623272970
- SuarezCMorales-BarreraRRamosVRole of immunotherapy in castration-resistant prostate cancer (CRPC)BJU Int2014113336737523650874
- DarJAEisermannKMasoodiKZN-terminal domain of the androgen receptor contains a region that can promote cytoplasmic localizationJ Steroid Biochem Mol Biol2014139162424099702
- NarayananSSrinivasSFeldmanDAndrogen-glucocorticoid interactions in the era of novel prostate cancer therapyNat Rev Urol2016131476026643568
- YeDHuangYZhouFA phase 3, double-blind, randomized placebo-controlled efficacy and safety study of abiraterone acetate in chemotherapy-naïve patients with mCRPC in China, Malaysia, Thailand and RussiaAsian J Urol201742758529264210
- TorenPJKimSPhamSAnticancer activity of a novel selective CYP17A1 inhibitor in preclinical models of castrate-resistant prostate cancerMol Cancer Ther2015141596925351916
- FizaziKJonesROudardSPhase III, randomized, double-blind, multicenter trial comparing orteronel (TAK-700) plus prednisone with placebo plus prednisone in patients with metastatic castration-resistant prostate cancer that has progressed during or after docetaxel-based therapy: ELM-PC 5J Clin Oncol201533772373125624429
- SolitDBScherHIRosenNHsp90 as a therapeutic target in prostate cancerSemin Oncol200330570971614571418
- BaylotVKatsogiannouMAndrieuCTargeting TCTP as a new therapeutic strategy in castration-resistant prostate cancerMol Ther201220122244225622893039
- O’MalleyKJLangmannGAiJRamos-GarciaRVessellaRLWangZHsp90 inhibitor 17-AAG inhibits progression of LuCaP35 xenograft prostate tumors to castration resistanceProstate201272101117112322161776
- HeSZhangCShafiAAPotent activity of the Hsp90 inhibitor ganetespib in prostate cancer cells irrespective of androgen receptor status or variant receptor expressionInt J Oncol2013421354323152004
- ZoubeidiAZardanABeraldiECooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activityCancer Res20076721104551046517974989
- IschiaJSaadFGleaveMThe promise of heat shock protein inhibitors in the treatment of castration resistant prostate cancerCurr Opin Urol201323319420023385973
- TranCOukSCleggNJDevelopment of a second-generation antiandrogen for treatment of advanced prostate cancerScience2009324592878779019359544
- LiuCLouWZhuYNiclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancerClin Cancer Res201420123198321024740322
- YamashitaSLaiKPChuangKLASC-J9 suppresses castration-resistant prostate cancer growth through degradation of full-length and splice variant androgen receptorsNeoplasia2012141748322355276
- LibertiniSJTepperCGRodriguezVAsmuthDMKungHJMudryjMEvidence for calpain-mediated androgen receptor cleavage as a mechanism for androgen independenceCancer Res200767199001900517909000
- SunHMediwalaSNSzafranATManciniMAMarcelliMCUDC-101, a novel inhibitor of full-length Androgen Receptor (flAR) and Androgen Receptor Variant 7 (AR-V7) Activity: mechanism of action and in vivo efficacyHorm Cancer20167319621026957440
- TeplyBAAntonarakisESNovel mechanism-based therapeutics for androgen axis blockade in castration-resistant prostate cancerCurr Opin Endocrinol Diabetes Obes201623327929026978733
- AndersenRJMawjiNRWangJRegression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptorCancer Cell201017653554620541699
- BanuelosCALalATienAHCharacterization of niphatenones that inhibit androgen receptor N-terminal domainPLoS One201499e10799125268119
- SadarMDWilliamsDEMawjiNRSintokamides A to E, chlorinated peptides from the sponge Dysidea sp. that inhibit transactivation of the N-terminus of the androgen receptor in prostate cancer cellsOrg Lett200810214947495018834139
- MichelsJEnzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE trial-there is no significant reduction in death (Yet)J Clin Oncol2017351123
- PollockJAWardellSEParentAAInhibiting androgen receptor nuclear entry in castration-resistant prostate cancerNat Chem Biol2016121079580127501397
- YuEYWildingGPosadasEPhase II study of dasatinib in patients with metastatic castration-resistant prostate cancerClin Cancer Res200915237421742819920114
- BianchiniDOmlinAPezaroCFirst-in-human Phase I study of EZN-4176, a locked nucleic acid antisense oligonucleotide to exon 4 of the androgen receptor mRNA in patients with castration-resistant prostate cancerBr J Cancer2013109102579258624169353
- CarverBSChapinskiCWongvipatJReciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancerCancer Cell201119557558621575859
- LiJKarkiAHodgesKBCotargeting polo-like kinase 1 and the Wnt/beta-catenin signaling pathway in castration-resistant prostate cancerMol Cell Biol201535244185419826438599
- ZhangLSLumLDelivery of the porcupine inhibitor WNT974 in miceMethods Mol Biol2016148111111727590157
- HollenhorstPCMcIntoshLPGravesBJGenomic and biochemical insights into the specificity of ETS transcription factorsAnnu Rev Biochem20118043747121548782
- NhiliRPeixotoPDepauwSTargeting the DNA-binding activity of the human ERG transcription factor using new heterocyclic dithiophene diamidinesNucleic Acids Res201341112513823093599
- VainioPGuptaSKetolaKArachidonic acid pathway members PLA2G7, HPGD, EPHX2, and CYP4F8 identified as putative novel therapeutic targets in prostate cancerAm J Pathol2011178252553621281786
- RahimSBeauchampEMKongYBrownMLToretskyJAUrenAYK-4-279 inhibits ERG and ETV1 mediated prostate cancer cell invasionPLos One201164e1934321559405
- KimSJKimSICurrent treatment strategies for castration-resistant prostate cancerKorean J Urol201152315716521461278
- CaffoOFratinoLBarbieriRPemetrexed as second-line chemotherapy for castration-resistant prostate cancer after docetaxel failure: results from a phase II studyUrol Oncol201331218018621803618
- ShiotaMYokomizoAEtoMTaxane chemotherapy for hormone-naive prostate cancer with its expanding role as breakthrough strategyFront Oncol2015530426793621
- BotrelTEClarkOLima PompeoACEfficacy and safety of combined Androgen Deprivation Therapy (ADT) and docetaxel compared with ADT alone for metastatic hormone-naive prostate cancer: a systematic review and meta-analysisPLoS One2016116e015766027308831
- MitaACDenisLJRowinskyEKPhase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumorsClin Cancer Res200915272373019147780
- YapTAPezaroCJde BonoJSCabazitaxel in metastatic castration-resistant prostate cancerExpert Rev Anticancer Ther20121291129113623098113
- DarshanMSLoftusMSThadani-MuleroMTaxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancerCancer Res201171186019602921799031
- BadrisingSvan der NoortVvan OortIMClinical activity and tolerability of enzalutamide (MDV3100) in patients with metastatic, castration-resistant prostate cancer who progress after docetaxel and abiraterone treatmentCancer2014120796897524382803
- BianchiniDLorenteDRodriguez-VidaAAntitumour activity of enzalutamide (MDV3100) in patients with metastatic castration-resistant prostate cancer (CRPC) pre-treated with docetaxel and abirateroneEur J Cancer2014501788424074764
- ZhongDZhangHJJiangYDSaikosaponin-d: a potential chemotherapeutics in castration resistant prostate cancer by suppressing cancer metastases and cancer stem cell phenotypesBiochem Biophys Res Commun2016474472272927155154
- NeliusTMartinez-MarinDHirschJPigment epithelium-derived factor expression prolongs survival and enhances the cytotoxicity of low-dose chemotherapy in castration-refractory prostate cancerCell Death Dis20145e121024810046
- GulleyJLMadanRADeveloping immunotherapy strategies in the treatment of prostate cancerAsian J Urol20163427828529264196
- KantoffPWSchuetzTJBlumensteinBAOverall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancerJ Clin Oncol20102871099110520100959
- BeckerJTOlsonBMJohnsonLEDaviesJGDunphyEJMcNeelDGDNA vaccine encoding prostatic acid phosphatase (PAP) elicits long-term T-cell responses in patients with recurrent prostate cancerJ Immunother201033663964720551832
- KaranDVan VeldhuizenPCombination immunotherapy with prostate GVAX and ipilimumab: safety and toxicityImmunotherapy20124657758022788125
- ShoreNDMantzCADosoretzDEBuilding on sipuleucel-T for immunologic treatment of castration-resistant prostate cancerCancer Control201320171623302902
- AntonarakisESCombining active immunotherapy with immune checkpoint blockade for the treatment of advanced prostate cancerAsian J Androl201214452052122580638
- KwonEDDrakeCGScherHICA184-043 InvestigatorsIpilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trialLancet Oncol201415770071224831977
- SanlorenzoMVujicIDaudAPembrolizumab cutaneous adverse events and their association with disease progressionJAMA Dermatol2015151111206121226222619
- MadanRAKarzaiFHNingYMPhase II trial of docetaxel, bevacizumab, lenalidomide and prednisone in patients with metastatic castration-resistant prostate cancerBJU Int2016118459059726780387
- MadanRAKarzaiFHNingYMPhase II trial of docetaxel, bevacizumab, lenalidomide and prednisone in patients with metastatic castration-resistant prostate cancerBJU Int2016118459059726780387
- ChuGCZhauHEWangRRANK- and c-Met-mediated signal network promotes prostate cancer metastatic colonizationEndocr Relat Cancer201421231132624478054
- RyanCJSaylorPJEverlyJJSartorOBone-targeting radiopharmaceuticals for the treatment of bone-metastatic castration-resistant prostate cancer: exploring the implications of new dataOncologist201419101012101825232039
- MahajanKCoppolaDRawalBAck1-mediated androgen receptor phosphorylation modulates radiation resistance in castration-resistant prostate cancerJ Biol Chem201228726221122212222566699
- ChiKNGleaveMEFazliLA phase II pharmacodynamic study of preoperative figitumumab in patients with localized prostate cancerClin Cancer Res201218123407341322553344
- SmithDCSmithMRSweeneyCCabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trialJ Clin Oncol201331441241923169517
- WyceADegenhardtYBaiYInhibition of BET bromodomain proteins as a therapeutic approach in prostate cancerOncotarget20134122419242924293458
- CoudeMMBraunTBerrouJBET inhibitor OTX015 targets BRD2 and BRD4 and decreases c-MYC in acute leukemia cellsOncotarget2015619176981771225989842
- WyceADegenhardtYBaiYInhibition of BET bromodomain proteins as a therapeutic approach in prostate cancerOncotarget20134122419242924293458
- SirtoriCRAescin: pharmacology, pharmacokinetics and therapeutic profilePharmacol Res200144318319311529685
- PiaoSKangMLeeYJCytotoxic effects of escin on human castration-resistant prostate cancer cells through the induction of apoptosis and G2/M cell cycle arrestUrology2014844982.e1982.e7
