Abstract
Background
Glioblastomas (GBMs) are the most aggressive type of glial brain tumors. Despite aggressive treatment with surgery and chemoradiation, GBMs invariably relapse and tumors are progressive. Controversy remains on optimal treatment of patients with recurrent GBMs. Data from previous trials have suggested that the addition of bevacizumab (BEV) to lomustine (CCNU) might improve overall survival (OS) as compared with that with monotherapies. The aim of this study was to compare the efficacy of BEV in addition to CCNU versus single-agent therapy in patients with recurrent GBM.
Methods
Electronic databases were searched for eligible literature updated in December 2017. Trials assessing the effectiveness of CCNU and BEV in progressive GBM were included, of which the main outcomes were progression-free survival (PFS) and OS. All the data were pooled with the corresponding 95% confidence intervals (CIs) using RevMan software. Sensitivity and heterogeneity were quantitatively evaluated.
Results
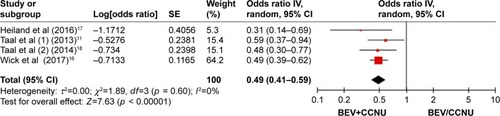
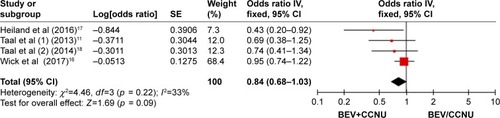
Three randomized clinical trials were identified, including 574 patients (combination group: 358, monotherapies group: 216). The combination group treated with BEV and CCNU showed improvement in PFS (OR = 0.49; 95% CI, 0.41–0.59; p < 0.00001). No significant differences were, however, found in patients in terms of the OS (OR = 0.84; 95% CI, 0.68–1.03; p = 0.09).
Conclusion
Although treatment with CCNU plus BEV prolonged PFS, it did not confer OS advantage over monotherapies in patients with progressive GBM. The encouraging results of the addition of CCNU to BEV warrant investigation in further randomized trials.
Introduction
Glioblastomas (GBMs) are the most common and aggressive type of glial brain tumorsCitation1 and have a poor prognosis. The current standard of treatment for GBM includes surgical resection followed by combined chemoirradiation with concurrent temozolomide (TMZ).Citation2 However, GBMs invariably relapse, and when tumor progresses, treatment options are scarce and with poor effectiveness. Previous studies have identified genetically diverse tumors cells, which are used to classify tumors into different subgroups according to their molecular signature,Citation3–Citation6 to find new targets for specific subgroups of patients.Citation7–Citation9 Because of the extensive endothelial proliferation that characterizes GBM, soon after the discovery of vascular endothelial growth factor (VEGF) and its significance in the angiogenesis of tumor growth, it has been hypothesized that GBM would provide a good target for antiangiogenic treatments.Citation10 Previous studies suggested that bevacizumab (BEV), a monoclonal antibody that targets VEGF, alone or in combination with cytotoxic agents, showed interesting results in terms of treatment for recurrent GBM. Preliminary data suggest a beneficial effect of the combination of BEV and lomustine (CCNU) in patients with GBM progressing after TMZ-based chemoradiation.Citation11 Similar to the TMZ therapy, CCNU, an alkylating nitrosurea drug, can be administered at initial diagnosis or at tumor recurrence. However, Piccioni et alCitation12 demonstrated the equal efficacy of BEV monotherapy, but with no additional benefit of CCNU. Whether BEV should be used as a monotherapy for tumor progression or used by adding to another drug has also remained a matter of debate. Given the lack of clear options, we explored the efficacy of adding CCNU to BEV for GBM patients who progressed after initial therapy.
Methods
Search strategy
Two investigators independently searched the electronic databases PubMed, Embase, and Cochrane Library for relevant literature published up to December 2017. The process was established to find all articles with the keywords “Glioblastoma” AND “Bevacizumab” AND “Lomustine”, and relevant Medical Subject Heading terms were utilized. The reference lists of all articles that dealt with the topic of interest were also hand-searched to check for additional relevant publications.
Eligibility criteria
Studies that met the following criteria were included in the meta-analysis: 1) the studies comparing the effectiveness of combination of BEV and CCNU in progressive GBM and 2) the outcomes of interest were survival efficacy, and hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) were provided. When we found duplicated or overlapped data in multiple reports, we just included the one with the most complete information.
Quality assessment
Two investigators separately rated the quality of the retrieved studies. We chose the risk-of-bias items recommended by The Cochrane Handbook for Systematic Reviews of Interventions.
Data extraction
Two authors independently extracted the relevant data from each trial. Disagreement was resolved by consensus. From each of the eligible studies, the following information was extracted: the first author’s family name, publication year, study type, treatment regimen, and end points of interests. We extracted the corresponding HRs and risk ratios to describe the strength of the association for survival data (overall survival [OS] and progression-free survival [PFS], respectively), with corresponding 95% CIs.
Statistical analysis
The end points of interest in the pooled analysis were OS and PFS data, and the end point outcome was considered as a weighted average of individual estimate of the HR in every included study, using the inverse variance method. If HRs and corresponding 95% CIs were reported, logarithm of HRs and the corresponding logarithm of lower limits and logarithm of upper limits were used as data points in pooling the analysis, whereas if a study did not provide HRs or 95% CIs, the only available data were in the form of K–M curves. Survival data were extracted from amplified K–M curves, according to the methods described by Tierney et al.Citation13
A sensitivity analysis was also performed to examine the impact on the overall results, depending on the heterogeneity across the included studies. Heterogeneity across studies was examined using the I2 statistic.Citation14 Studies with an I2 of 25%–50%, 50%–75%, or >75% were considered to have low, moderate, or high heterogeneity, respectively.Citation15 When there was low heterogeneity among the studies, the fixed-effects model was used; otherwise, the random-effects model was used. A p-value less than 0.05 was considered statistically significant. The statistical analyses were performed using the Review Manager Version 5.3 software (RevMan; The Cochrane Collaboration, Oxford, UK). Findings of our meta-analysis are shown in forest plots. The Begg’s and the Egger’s tests were conducted to evaluate publication bias.
Results
Overview of literature search and study characteristics
A total of 213 studies were retrieved initially for evaluation. Based on the criteria described in the “Methods” section, eight publications were evaluated in more detail, but some did not provide enough detail of outcomes of two approaches. Therefore, a final total of three trialsCitation16–Citation18 addressed the combination of BEV and CCNU in treating progressive GBM. The search process is described in . All included studies in this study were based on moderate- to high-quality evidence. describes the primary characteristics of the eligible studies in more detail.
Table 1 Primary characteristics of the eligible studies
Clinical and methodological heterogeneity
Pooled analysis of PFS with the combination of BEV and CCNU in progressive GBM
Pooling the PFS data from all the three studiesCitation16–Citation18 showed that the combination therapy did prolong the PFS (OR = 0.49; 95% CI, 0.41–0.59; p < 0.00001) as compared with that in the monotherapy group ().
Pooled analysis of OS with the combination of BEV and CCNU in progressive GBM
A fixed-effects model was used to pool the OS data.Citation16–Citation18 The pooled data showed that the combination of BEV and CCNU did not improve the OS (OR = 0.84; 95% CI, 0.68–1.03; p = 0.09) as compared with that in the monotherapy group ().
Discussion
GBM is the most common brain cancer in adults. Despite aggressive treatment with surgery and chemoradiation, its prognosis still remains poor.Citation19 Controversy remains on the optimal treatment of patients with recurrent GBM. GBMs are highly vascularized tumors in which the VEGF signaling pathway is upregulated. BEV is a humanized monoclonal antibody against circulating VEGF. Although BEV is commonly used, data on timing of administration and optimal patient management upon further progression remain limited. CCNU has been an approved option for recurrent GBM and has also been frequently administered in clinical trials as the standard treatment.Citation20,Citation21
Randomized Phase II BELOB trial demonstrated a potential benefit of BEV when added to CCNU chemotherapy in patients with recurrent GBM, although no consensus has been reached on how patients who experience further disease progression after a combined treatment with BEV and CCNU salvage therapy should be treated.Citation22,Citation23
In this analysis, we found that the combination of BEV with CCNU did not confer OS advantage over monotherapy alone, but prolonged PFS to some extent. The mechanism by which the combination treatment with BEV and CCNU prolongs PFS still remains undefined. It has been suggested that normalization of the vasculature around the tumor as well as improved regional cerebral blood flow and not necessarily the inhibition of tumor growth are the key components of the antiangiogenic activity.Citation24–Citation26
Moreover, results can be explained by the detailed assessment of the patient group and a selection bias with regard to the crossover design of various studies. Furthermore, biomarkers can also affect the outcome after treatment. Taal et alCitation18 showed that IDH mutation status increased the sensitivity to treatment. This raises the question of whether trials on recurrent GBM should identify a subset of patients with IDH wild-type tumors or should analyze patients according to IDH mutational status. In Erdem-Eraslan et al’s study,Citation10 to identify recurrent GBM patients who benefit from combined CCNU and BEV treatment, gene expression was performed, and it was observed that patients with a specific molecular subtype of glioma, IGS-18, or “classical GBMs” may show more benefit from BEV+CCNU treatment.
The data on adverse effects (AEs) were limited; therefore, it was not possible to assess the AEs in this meta-analysis. In Wick et al’s study,Citation16 the addition of BEV did not improve neurocognitive functioning, and did not lead to poorer neurocognitive function as compared with that observed with CCNU use alone. Heiland et alCitation17 showed a slight increase in myelosuppression (thrombocytopenia and leukopenia) after the combination therapy. Both the trials showed that the combination therapy can be administered with an acceptable toxicity and no significant negative impact on the clinical performance of the patients compared to monotherapy, and the higher numbers of AEs should be assessed relative to the longer treatment period in the combination group.
Our study still has several limitations. First and the foremost, as this study was a study-level meta-analysis, there is publication bias leading to heterogeneity among the included studies. The inclusion of retrospective studies was an inherent limitation, and differences in patient comorbidities could not be incorporated in such an analysis. Second, there are only two studies that reported available data on AEs, so we could not predict efficacy in AEs.
Conclusion
Although treatment with CCNU plus BEV prolonged PFS, it did not confer OS advantage over monotherapies in patients with progressive GBM. The future of antiangiogenic therapy remains unclear; it is hypothesized that combining immunotherapy with antiangiogenic treatment may have a synergistic effect and enhance the efficacy of both the treatments. The encouraging results of the addition of CCNU to BEV warrant investigation in further randomized trials.
Disclosure
The authors report no conflicts of interest in this work.
References
- FullerGNThe WHO Classification of Tumours of the Central Nervous System, 4th editionArch Pathol Lab Med2008132690618517270
- StuppRMasonWPvan den BentMJEuropean Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials GroupRadiotherapy plus concomitant and adjuvant temozolomide for glioblastomaN Engl J Med20053521098799615758009
- BrennanCWVerhaakRGMcKennaATCGA Research NetworkThe somatic genomic landscape of glioblastomaCell2013155246247724120142
- NoushmehrHWeisenbergerDJDiefesKCancer Genome Atlas Research NetworkIdentification of a CpG island methylator phenotype that defines a distinct subgroup of gliomaCancer Cell201017551052220399149
- ParsonsDWJonesSZhangXAn integrated genomic analysis of human glioblastoma multiformeScience200832158971807181218772396
- VerhaakRGHoadleyKAPurdomECancer Genome Atlas Research NetworkIntegrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1Cancer Cell20101719811020129251
- Cancer Genome Atlas Research NetworkBratDJVerhaakRGAldapeKDComprehensive, integrative genomic analysis of diffuse lower-grade gliomasN Engl J Med2015372262481249826061751
- AgnihotriSBurrellKEWolfAGlioblastoma, a brief review of history, molecular genetics, animal models and novel therapeutic strategiesArch Immunol Ther Exp (Warsz)2013611254123224339
- Wild-BodeCWellerMRimnerADichgansJWickWSublethal irradiation promotes migration and invasiveness of glioma cells: implications for radiotherapy of human glioblastomaCancer Res20016162744275011289157
- Erdem-EraslanLvan den BentMJHoogstrateYIdentification of patients with recurrent glioblastoma who may benefit from combined bevacizumab and CCNU therapy: a report from the BELOB trialCancer Res201676352553426762204
- TaalWOosterkampHMWalenkampAMEA randomized phase II study of bevacizumab versus bevacizumab plus lomustine versus lomustine single agent in recurrent glioblastoma: the Dutch BELOB studyJ Clin Oncol201331152001
- PiccioniDESelfridgeJModyRRDeferred use of bevacizumab for recurrent glioblastoma is not associated with diminished efficacyNeuro Oncol201416681582224627236
- TierneyJFStewartLAGhersiDBurdettSSydesMRPractical methods for incorporating summary time-to-event data into meta-analysisTrials200781617555582
- HigginsJPThompsonSGQuantifying heterogeneity in a meta-analysisStat Med200221111539155812111919
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- WickWGorliaTBendszusMLomustine and bevacizumab in progressive glioblastomaN Engl J Med2017377201954196329141164
- HeilandDHMasalhaWFrancoPMacheinMRWeyerbrockAProgression-free and overall survival in patients with recurrent glioblastoma multiforme treated with last-line bevacizumab versus bevacizumab/lomustineJ Neurooncol2016126356757526614518
- TaalWOosterkampHMWalenkampAMSingle-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trialLancet Oncol201415994395325035291
- LombardiGPambukuABelluLEffectiveness of antiangiogenic drugs in glioblastoma patients: a systematic review and meta-analysis of randomized clinical trialsCrit Rev Oncol Hematol20171119410228259301
- WickWPuduvalliVKChamberlainMCPhase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastomaJ Clin Oncol20102871168117420124186
- BatchelorTTMulhollandPNeynsBPhase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastomaJ Clin Oncol201331263212321823940216
- ReardonDADesjardinsAPetersKPhase II study of metronomic chemotherapy with bevacizumab for recurrent glioblastoma after progression on bevacizumab therapyJ Neurooncol2011103237137920853132
- ReardonDADesjardinsAPetersKBPhase 2 study of carboplatin, irinotecan, and bevacizumab for recurrent glioblastoma after progression on bevacizumab therapyCancer2011117235351535821590689
- BatchelorTTSorensenAGdi TomasoEAZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patientsCancer Cell2007111839517222792
- BrandsmaDvan den BentMJPseudoprogression and pseudoresponse in the treatment of gliomasCurr Opin Neurol200922663363819770760
- FieldKMJordanJTWenPYRosenthalMAReardonDABevacizumab and glioblastoma: scientific review, newly reported updates, and ongoing controversiesCancer20151217997100725263092