Abstract
Background
Papillary thyroid cancer (PTC) is a common endocrine malignancy with relatively good prognosis. Radioactive iodine (RAI) is considered effective for patients with total or nearly total thyroidectomy, but the beneficial effects of RAI are still controversial.
Materials and methods
To determine whether RAI therapy could improve the survival rates of PTC patients, we conducted a retrospective analysis using data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program. Disease-specific survival (DSS) was obtained using multivariate Cox proportional hazard regressions.
Results
DSS was improved by RAI ablation in patients with tumor >2 cm, age >45 years and gross extrathyroidal or lymph node metastasis. In a further analysis, RAI therapy did not improve the DSS in patients with tumor <2 cm except those with distant metastasis. For patients with tumor >2 cm, those involving gross extrathyroidal extension, age >45 years or disease in the lymph nodes, DSS was improved after RAI therapy. Patients with distant metastasis always benefited from RAI ablation.
Conclusion
RAI ablation should be recommended to patients with tumor <2 cm and distant metastasis or patients with tumor >2 cm and one of the following risk factors: gross extrathyroidal extension, age >45 years, lymph node and distant metastases.
Introduction
Thyroid cancer is a common endocrine malignancy. Over the past 3 decades, the incidence of thyroid cancer increased by 211% in the USA, driven mostly by the increase of papillary thyroid cancer (PTC). With the high incidence of thyroid cancer, mortality rate for advanced-stage PTC has also increased.Citation1,Citation2
It is widely agreed that PTC demonstrates relatively indolent clinical behavior. The improvements in diagnosis and treatment of PTC provide an excellent prognosis, with 90% survival rate at 10 years.Citation3–Citation5 However, it remains a challenge to establish a standard treatment strategy for patients with PTC. To determine whether patients need more aggressive treatment, risk stratification systems were set up to stratify patients into low and high risk of recurrence or death of thyroid cancer.Citation6 In the current American Thyroid Association (ATA) Guidelines, radioactive iodine (RAI) is not routinely recommended after thyroidectomy for the low risk patients, but it is considered for ATA intermediate and high risk patients.Citation7 For patients with low risk factors, total thyroidectomy can remove the remnant, thus postoperative RAI ablation has no further effect on prognosis improvement.Citation8 It was reported that RAI only affected the survival of patients >45 years old with primary tumors >2 cm and lymph node and distant metastases.Citation9 Surgical resection followed by RAI is often advised for high risk tumors. While Mazzaferri and Jhiang demonstrated that RAI therapy significantly reduced recurrence rate and cancer-related death in patients with tumors >1.5 cm,Citation10 Jonklaas et al stated that RAI therapy benefited all patients except those with stage 1 disease.Citation11 No statistic differences of recurrence and death rates were reported between the 131I group and the no-131I group in a study recruiting 1,542 patients from Mayo Clinic.Citation12 RAI did not affect the survival rate in patients with small intrathyroid tumors. Therefore, RAI therapy was only recommended for patients with residual disease.Citation13,Citation14 In most countries, postsurgical RAI ablation is recommended for PTC patients with tumors >1 cm. Due to the lack of long-term randomized trials and inconsistence of previous studies, the beneficial effects of RAI are still controversial.Citation15
To further investigate the effects of RAI therapy on patients with PTC, we conducted a retrospective analysis using data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program. The objective of this study was to determine whether RAI therapy could improve the long-term survival rates. Furthermore, we aimed to determine whether a cohort of patients with specific risk factors could be identified, and whether RAI therapy was associated with an improved survival rates for those patients.
Materials and methods
A retrospective cohort analysis using the SEER database from the National Cancer Institute was performed. Patients were selected between 2003 and 2013. Because of the different clinical behaviors between classical type and follicular variant of PTC, histologic subtypes of PTC were limited using the International Classification of Diseases for Oncology, 3rd editionCitation32 (ICD-O-3) as follows: 8050/3: papillary carcinoma, not otherwise specified (NOS); 8260/3: papillary adenocarcinoma, NOS; 8343/3: papillary carcinoma, encapsulated.
Patients whose information of tumor size, extension, lymph node and distant metastases was blank or unknown were excluded from this study. Cases with or without RAI ablation after total or nearly total thyroidectomy were eligible for further analysis.
Demographic data included sex (male and female), age at diagnosis (<45 or ≥45 years), race (white, black, Asian, other or unknown) and region (East, Northern Plains, Pacific Coast, Southwest or Alaska). Tumor size cutoffs from 1 to 4 cm were examined in 1 cm intervals to determine whether a tumor size threshold could be identified above which RAI therapy was beneficial to survival rates. The cancer characteristic of tumor extension was categorized as intrathyroidal (codes 100, 200, 300, 400), minimal extrathyroidal (code 450) and gross extrathyroidal (codes 480, 500, 520, 550, 600, 620, 650, 700, 720, 730, 800). Lymph node metastasis was classified into 2 groups: negative (code 000) and positive (codes 120, 135, 140, 155, 158, 160). Distant metastasis included no distant metastasis diseases (code 00) and distant metastasis diseases (codes 12, 40, 51, 60). Treatment characteristics included surgery (code 40: subtotal or nearly total thyroidectomy, code 50: total thyroidectomy) and radiation therapy (none or radioactive iodine therapy). All the variables were defined using the SEER specific codes.
To determine the tumor size threshold and assess the association between tumor size and RAI therapy, the analysis was firstly stratified by tumor size (by 1 cm intervals). We further stratified the patients based on tumor extension, age, lymph node and distant metastases to investigate the effects of RAI on patients with specific features.
Patient demographics, cancer- and treatment-related characteristics were compared between no RAI and RAI therapy groups using chi-square or Fisher’s exact tests. In our study, the overall survival (OS) and disease-specific survival (DSS) were obtained using multivariate Cox proportional hazard regressions. A Cox proportion hazards model was used to assess the association between all the variables and survival. Stratified analyses were performed and Cox proportion hazards model was obtained to investigate the effects of RAI therapy. Adjusted hazard ratios (aHR) with 95% confidence intervals were generated to quantify the strength of the relative risk, and aHR>1.0 represented a worse prognosis than those <1.0. All the tests and performed were 2-tailed, and P<0.05 was considered statistically significant. All statistical analyses were performed using SPSS 19.0 (IBM Corporation, Armonk, NY, USA).
Ethics statement
This was a retrospective cohort analysis using data from the SEER database which was designed and maintained by the National Cancer Institute. Research was limited to secondary use of information previously collected in the course of normal care and data were anonymized before the conduction of statistical analyses. This article does not contain any studies with human participants or animals performed by any of the authors.
Results
Patient characteristics
A total of 41,287 cases were eligible for our study. The median follow-up time was 47.2 months. Among the total death rate of 1,423 (3.4%), only 261 patients died from thyroid cancer (0.6%). Characteristics of patients are compared between the treatment subgroups in . Over half of the patients underwent postoperative RAI therapy. Patients with larger tumors, extrathyroidal extension, lymph node or distant metastases were more likely to be given RAI ablation after total or nearly total thyroidectomy.
Table 1 Patient characteristics within subgroups
Survival analysis
The aHRs for OS and DSS are listed in to investigate the clinical significances. The multivariate models demonstrated that older age, larger tumor, extrathyroidal extension, lymph node and distant metastases had negative effects on the survival when controlling for the remaining variables (P<0.05). RAI therapy improved both OS (aHR = 0.586, P<0.001) and DSS (aHR = 0.600, P<0.001).
Table 2 Cox proportional hazards regression model analysis of overall survival (OS) and disease-specific survival (DSS)
Subgroup analysis of RAI therapy
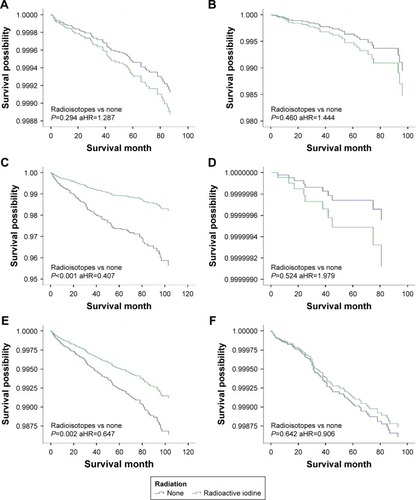
We investigated the beneficial effects of RAI on patients with different features. Patients were stratified based on tumor size, age, extension, and metastasis. To determine the tumor size threshold for which RAI therapy could benefit the survival, tumor size was tested by 1 cm intervals between 1 and 4 cm. For patients with tumors <2 cm, RAI improved OS but did not benefit DSS. However, for those with tumors >2 cm, RAI therapy could significantly improve the OS and DSS. Similar results were found when patients were classified by age (<45 or ≥45 years), extension (intrathyroidal, minimal extrathyroidal or gross extrathyroidal), or lymph nodes metastasis (negative or positive). DSS was improved in older age, gross extrathyroidal, and lymph node groups but not the distant metastasis group ().
Table 3 Cox proportional hazards regression model analysis of overall survival (OS) and disease-specific survival (DSS) of patients stratified by patients’ features
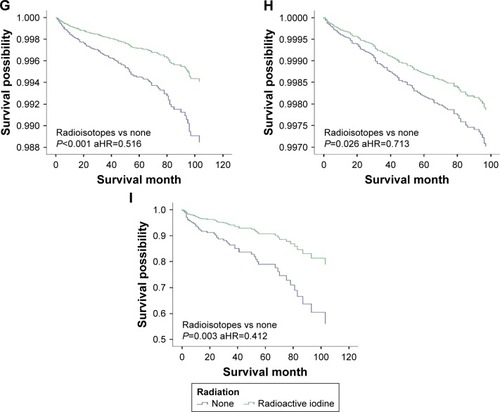
To further examine the associations between RAI therapy and DSS, patients were stratified (). RAI therapy did not improve the DSS in all tumor <2 cm groups, except for patients with distant metastasis. For patients with tumors >2 cm, DSS benefited from RAI therapy only for those involving gross extrathyroidal extension, age >45 years or with disease in the lymph nodes; DSS was improved in tumor >2 cm whether or not patients had distant metastasis ( and ).
Figure 1 Stratification of patients based on tumor size, tumor extension, age, lymph node and distant metastases.
Figure 2 DSS curves of multivariate Cox analysis in the group with tumors <2 cm. DSS is based on radioactive isotope in (A) patients with intrathyroidal extension; (B) patients with minimal extrathyroidal extension; (C) patients with gross extrathyroidal extension; (D) patients younger than 45 years; (E) patients older than 45 years; (F) patients without lymph node metastases; (G) patients with lymph node metastases; (H) patients without distant metastases; (I) patients with distant metastases.
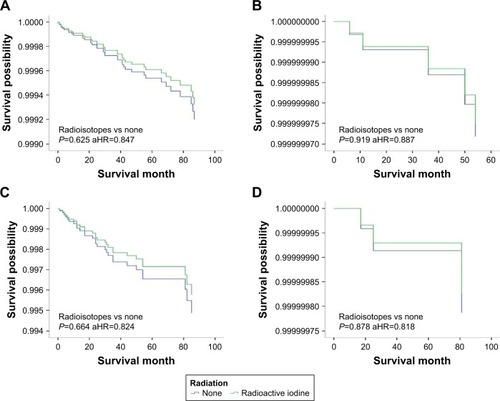
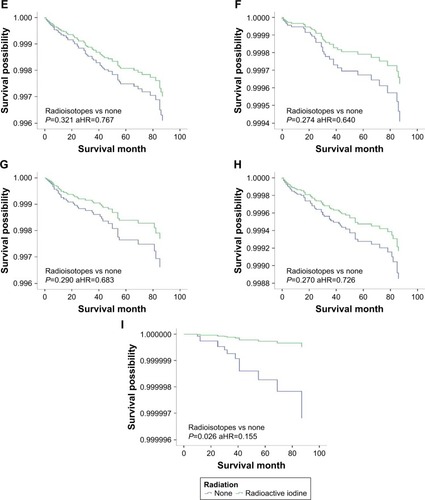
Figure 3 DSS curves of multivariate Cox analysis in the group with tumors >2 cm. DSS is based on radioactive isotope in (A) patients with intrathyroidal extension; (B) patients with minimal extrathyroidal extension; (C) patients with gross extrathyroidal extension; (D) patients younger than 45 years; (E) patients older than 45 years; (F) patients without lymph node metastases; (G) patients with lymph node metastases; (H) patients without distant metastases; (I) patients with distant metastases.
Discussion
Although postoperative RAI therapy is widely recommended for differentiated thyroid carcinoma,Citation15 the beneficial effects of RAI have been debated. In the current ATA Guidelines, RAI adjuvant therapy was not routinely recommended for low risk patients. It was considered after total thyroidectomy in ATA intermediate risk level differentiated thyroid cancer patients and high risk differentiated thyroid cancer patients.Citation7 The European Thyroid Association (ETA) guideline advised RAI for patients with T3/T4, N1 or M1 disease, while for young patients (<18 years) and patients with primary tumors between 1 and 2 cm without metastasis, it only gave a relative indication for RAI therapy.Citation16 The Society of Nuclear Medicine (SNM) Procedure Guideline recommended postoperative RAI for patients with tumor >1.5 cm, or tumor <1.5 cm with metastasis.Citation17 The European Association of Nuclear Medicine Guidelines considered RAI ablation as a standard procedure in patients with differentiated thyroid cancer after total or nearly total thyroidectomy, except for patients with unifocal PTC <1 cm without metastasis, thyroid capsule invasion, history of radiation exposure or unfavorable histology.Citation8 Due to the lack of long-term randomized trails and the use of different stratification systems, it is not easy to find the optimal treatment for patients.
In our study, we conducted a retrospective, population-based cohort analysis using the SEER database. Patients were stratified into different subgroups based on risk factors. DSS was improved by RAI only in patients with tumors >2 cm, gross extrathyroidal extension, older age (>45 years) and lymph node metastasis. Distant metastasis did not affect the outcomes of RAI due to the low percentage of patients with distant metastasis (0.7%). In patients with tumors >2 cm, RAI could improve the DSS for those with gross extrathyroidal extension, lymph node metastases, distant metastases or aged >45 years. Although patients without distant metastases in this group also benefited from RAI ablation, they should be further analyzed based on their characteristics (age, tumor extension, lymph nodes metastases), because the majority of patients did not have distant metastases. In patients with tumors <2 cm, DSS was not affected by the use of RAI regardless of age, extension, and lymph node metastasis, except for those with distant metastasis. Podnos et al suggested that post-surgical RAI therapy could only improve the survival in PTC patients older than 45 years with tumors >2 cm, involving lymph node and distant metastases.Citation9 Some studies stated that surgery alone for small tumors (,1.5 cm) had favorable prognosis and RAI was not required. In addition, RAI did not influence the recurrence rates in papillary thyroid micro-carcinoma even for those with disease in lymph nodes.Citation18–Citation20 Mazzaferri et al demonstrated that RAI therapy significantly reduced recurrence rate and cancer-related death in patients with tumors >1.5 cm.Citation10 Other studies revealed that RAI benefited patients with tumors >1 cm or residual and metastatic disease after surgery.Citation16,Citation21 These results were consistent with ours. For patients with tumors <2 cm and without distant metastasis, total or nearly total thyroidectomy had favorable prognosis, and RAI ablation could not improve it, except for those with distant metastasis. Surgery alone could successfully remove the remnant, even in patients with extrathyroidal extension or lymph node metastasis. A recent study demonstrated that minimal extrathyroidal extension did not affect the survival in most well differentiated thyroid cancer, and those patients should have a more aggressive surgical or RAI treatment.Citation22
In our study, RAI therapy did not show any benefits in patients with minimal extrathyroidal extension, but multivariate analysis demonstrated that minimal extrathyroidal extension was an independent risk factor for DSS. For patients with tumors >2 cm, completeness of surgical resection is hard to achieve, especially when the tumor has invaded into the trachea, larynx or esophagus. Gross extrathyroidal extension has been regarded as a poor prognostic feature for many years.Citation13 It is reported that many patients with extrathyroidal extension responded very well to RAI.Citation23 However, it remains controversial whether the presence of lymph node metastasis is a risk factor of mortality.Citation24 Previous studies showed that lymph node metastasis increased the recurrence rate and decreased the survival rate in patients older than 45 years.Citation25–Citation27 In our study, patients with lymph node metastasis had poor OS and DSS. Recent ATA guidelines also recommended prophylactic central-compartment neck dissection in patients with PTC clinically involved lateral neck nodes.Citation7 RAI ablation eliminated potential tumor nest remnants in the lymph nodes after surgery, thus improving the DSS. Another study also considered lymph node metastasis as an indication of RAI.Citation28 Age was another risk factor of recurrence and death.Citation26,Citation29 In our study, all patients older than 45 years benefited from RAI therapy.
Our goals with RAI therapy are to eradicate any normal residual thyroid tissue and locoregional or distant tumor deposits, thereby increasing the sensitivity and specificity of follow-up examinations of persistence or recurrence, such as thyroglobulin measurement and the whole-body radioiodine scan. However, treatment with 131I may have dose-dependent adverse effects: dysfunction of salivary gland, reduction of tear production, transient dysfunction of male/female gonad, and higher risk of developing other cancers. It is important to consider these adverse effects when choosing RAI therapy in patients with PTC.Citation30 The adverse effects of RAI were not further examined in our study due to the lack of records of them in the SEER database.
There are several limitations in our study. We did not stratify patients into low- or high-risk groups using the current risk stratification systems. To determine whether RAI therapy benefited the survival, we classified patients into different groups with specific features, and we found that tumor size, extension, age, lymph node and distant metastases should be considered in RAI therapy. This retrospective analysis was potentially biased by prejudices: those receiving RAI had generally more risk factors (larger tumor size, extrathyroidal extension, lymph nodes and distant metastases) and a more advanced stage of disease; they tend to have a poorer prognosis, thus the survival benefit of RAI therapy would be cancelled by the worse disease features. To decrease the bias caused by patients’ characteristics, we categorized patients into different groups (), and we found that only those who had relatively high risk factors benefited from RAI therapy. In addition, molecular markers were not included in SEER. It is reported that BRAF had clinical value in planning the treatment strategy. BRAF was associated with more aggressive clinical behaviors, thus it predicted higher necessity of postoperative RAI ablation.Citation31 The SEER database has recorded no information about recurrence. Due to the indolent nature of PTC, recurrence is more meaningful than death. Despite these limitations, SEER is still important in understanding the associations between treatment outcomes and specific risk factors. Long-term randomized trials are needed for further study.
Conclusion
RAI ablation should be recommended to patients with tumors <2 cm with distant metastasis or tumors >2 cm with one of the following factors: gross extrathyroidal extension, age >45 years, lymph node and distant metastases. To optimize the treatment strategy for PTC patients, it is very important to consider all the risk factors.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
We thank Qi Wu for assistance in improving the quality of language and revising the statistical methods.
Disclosure
The authors report no conflicts of interest in this work.
References
- LimHDevesaSSSosaJACheckDKitaharaCMTrends in thyroid cancer incidence and mortality in the United States, 1974–2013JAMA2017317131338134828362912
- CadyBSedgwickCEMeissnerWAWoolMSSalzmanFAWerberJRisk factor analysis in differentiated thyroid cancerCancer1979433810820427722
- TalaHTuttleRMContemporary post surgical management of differentiated thyroid carcinomaClin Oncol (R Coll Radiol)201022641942920605708
- GrigsbyPWReddyRMMoleyJFHallBLContralateral papillary thyroid cancer at completion thyroidectomy has no impact on recurrence or survival after radioiodine treatmentSurgery2006140610431047 discussion 1047–104917188155
- MsHHLFRACSKaiPWPostablation stimulated thyroglobulin level is an important predictor of biochemical complete remission after reoperative cervical neck dissection in persistent/recurrent papillary thyroid carcinomaAnn Surg Oncol201320265365922956067
- WartofskyLVan NostrandDRadioiodine treatment of well-differentiated thyroid cancerEndocrine201242350651322733393
- HaugenBRAlexanderEKBibleKC2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid CancerThyroid2016261113326462967
- LusterMClarkeSEDietleinMGuidelines for radioiodine therapy of differentiated thyroid cancerEur J Nucl Med Molecular Imaging2008351019411959
- PodnosYDSmithDDWagmanLDEllenhornJDSurvival in patients with papillary thyroid cancer is not affected by the use of radioactive isotopeJ Surg Oncol20079613717567872
- MazzaferriELJhiangSMLong-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancerAm J Med19949754184287977430
- JonklaasJSarlisNJLitofskyDOutcomes of patients with differentiated thyroid carcinoma following initial therapyThyroid200616121229124217199433
- GrebeSKHayIDFollicular cell-derived thyroid carcinomasCancer Treat Res19978989911409204190
- TsangRWBrierleyJDSimpsonWJPanzarellaTGospodarowiczMKSutcliffeSBThe effects of surgery, radioiodine, and external radiation therapy on the clinical outcome of patients with differentiated thyroid carcinomaCancer19988223753889445196
- TubianaMSchlumbergerMRougierPLong-term results and prognostic factors in patients with differentiated thyroid carcinomaCancer19855547948043967174
- LusterMReinersCRadioiodine therapy in differentiated thyroid cancerWorld J Endocr Surg200911712
- PaciniFSchlumbergerMDralleHEuropean consensus for the management of patients with differentiated thyroid carcinoma of the follicular epitheliumEur J Endocrinol2006154678780316728537
- SilbersteinEBAlaviABalonHRSociety of Nuclear Medicine Procedure Guideline for Therapy of Thyroid Disease with Iodine-131 (Sodium Iodide)Society of Nuclear Medicine Available from: https://www.researchgate.net/publication/240636680_Society_of_Nuclear_Medicine_Procedure_Guideline_for_Therapy_of_Thyroid_Disease_with_Iodine131_Sodium_IodideAccessed May 10, 2018
- BaudinETravagliJPRopersJMicrocarcinoma of the thyroid gland: the Gustave-Roussy Institute experienceCancer19988335535599690549
- HayIDGrantCSvan HeerdenJAGoellnerJREbersoldJRBergstralhEJPapillary thyroid microcarcinoma: a study of 535 cases observed in a 50-year periodSurgery1992112611391146 discussion 1146–11371455316
- PalimeriSGousisPVlassopoulouBIndications of radioactive iodine ablation in papillary thyroid cancerHell Cheirourgike20158715357
- American Thyroid Association Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid CancerCooperDSDohertyGMRevised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancerThyroid200919111167121419860577
- Al-QurayshiZShamaMARandolphGWKandilEMinimal extrathyroidal extension does not affect survival of well-differentiated thyroid cancerEndocr Relat Cancer201724522122628249964
- LeeNTuttleMThe role of external beam radiotherapy in the treatment of papillary thyroid cancerEndocr Relat Cancer200613497197717158749
- LeeYDSurgical strategy for papillary thyroid microcarcinomaJ Korean Thyroid Assoc2014714856
- SchneiderDFElfenbeinDLloydRVChenHSippelRSLymph node metastases do not impact survival in follicular variant papillary thyroid cancerAnn Surg Oncol201522115816325092163
- YuXMWanYSippelRSChenHShould all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 casesAnn Surg2011254465366021876434
- ZaydfudimVFeurerIDGriffinMRPhayJEThe impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinomaSurgery200814461070107819041020
- ChowSMLawSCMendenhallWMPapillary thyroid carcinoma: prognostic factors and the role of radioiodine and external radiotherapyInt J Radiat Oncol Biol Phys200252378479511849802
- MazuratATorroniAHendrickson-RebizantJBenningHNasonRWPathakKAThe age factor in survival of a population cohort of well-differentiated thyroid cancerEndocr Connect20132315416024008393
- ClementSCPeetersRPRonckersCMIntermediate and long-term adverse effects of radioiodine therapy for differentiated thyroid carcinoma – a systematic reviewCancer Treat Rev2015411092593426421813
- HanSAParkWSJangJHMinSYRyuJKSongJYBRAF mutation may predict higher necessity of postoperative radioactive iodine ablation in papillary thyroid cancerAnn Surg Treat Res201487417417925317411
- FritzAGInternational classification of diseases for oncology: ICD-OWorld Health Organization2000
