Abstract
Background
The global Phase III LUX-Lung 8 trial (ClinicalTrials.gov: NCT1523587) identified significant improvements in progression-free survival (PFS), overall survival (OS), and patient-reported outcomes (PROs) with second-line afatinib vs erlotinib in patients with advanced squamous cell carcinoma (SCC) of the lung.
Materials and methods
We conducted a post hoc analysis of data for patients in LUX-Lung 8 from mainland China (n=67). Compared with erlotinib, afatinib reduced the risk of disease progression or death (PFS) in the Chinese subgroup by 30% (HR=0.70; 95% CI: 0.38–1.27).
Results
The risk of death was reduced by 31% (HR=0.69; 95% CI: 0.39–1.21). The proportion of Chinese patients with improvements in PROs also favored afatinib vs erlotinib (global health status/quality of life [QoL], 52.8% vs 29.6%, P=0.072; dyspnea, 47% vs 26%, P=0.091; “dyspnea walked”, 44% vs 15%, P=0.017; QoL rate, 53% vs 26%, P=0.037).
Discussion
While this analysis was not powered to demonstrate differences compared to the overall trial population (OTP), and there were some differences in baseline characteristics (eg, the proportion of patients aged ≥65 years old), the benefits of afatinib treatment in Chinese patients with SCC of the lung appeared to be at least comparable to that observed in LUX-Lung 8. As with the OTP, the most common adverse events (AEs) with afatinib in the Chinese subgroup were diarrhea and rash/acne, and the incidence and type of the most frequently occurring AEs were similar.
Conclusion
The results suggest that afatinib represents a feasible treatment option for Chinese patients with advanced SCC of the lung following progression on platinum-based chemotherapy.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Lung cancer is the most frequently diagnosed malignancy and leading cause of cancer death in China.Citation1 Incidence and mortality rates are increasing steadily,Citation2 partly due to the dramatic increase in the prevalence of cigarette smoking in China over the past 30 years, which replicated the US trends of ~40 years earlier.Citation3–Citation7 As a result, further increases in the prevalence of lung cancer and related deaths are predicted.Citation4,Citation8,Citation9 In particular, as squamous cell carcinoma (SCC) accounts for a third of lung cancers in China,Citation9 increases in the number of cases are anticipated. Chinese patients with non-small-cell lung cancer (NSCLC) differ from Western patients in many ways, including differences in driver mutations, etiologies, and tolerances to treatment.Citation10 Despite the growing burden and the need for additional treatment options, recently approved drugs such as the immune checkpoint inhibitors nivolumab,Citation11 pembrolizumab,Citation12 and atezolizumab,Citation13,Citation14 and the anti-VEGFR-2 antibody ramucirumab (in combination with docetaxel),Citation15 have yet to be investigated in patients from Asia or China. Treatment options in China for stage IV SCC of the lung following progression on platinum-based chemotherapy are limited to single-agent therapy with docetaxel, gemcitabine, vinorelbine, or ifosfamide.
Given that ~60%–80% of SCC tumors express EGFR,Citation16,Citation17 and other ErbB family members such as HER2/ErbB2, and ErbB3 are implicated in the pathogenesis of SCC,Citation18 there is a clear biological rationale for assessing agents that target ErbB signaling in patients with advanced SCC of the lung. The global LUX-Lung 8 trial evaluated outcomes of second-line treatment with afatinib, an irreversible ErbB family blocker, vs erlotinib, a reversible EGFR tyrosine-kinase inhibitor (TKI), in this setting, following progression after platinum-based chemotherapy.Citation19 Significant improvements in progression-free survival (PFS), overall survival (OS), and patient-reported outcomes (PROs) were demonstrated with afatinib vs erlotinib; the safety profile of afatinib was predictable and manageable.Citation19,Citation20 On the basis of these findings, afatinib gained global regulatory approval for treatment of locally advanced or metastatic NSCLC of squamous histology and progression during/after platinum-based chemotherapy, and was recently approved in China for the same indication.
Currently, there is a paucity of data on outcomes of afatinib treatment in Chinese patients with advanced SCC of the lung. We conducted a post hoc analysis of patients’ data from Chinese study centers in LUX-Lung 8, to evaluate whether treatment outcomes (efficacy and PROs) and the safety of afatinib in Chinese patients were comparable to the overall study population.
Materials and methods
Patients and study design
LUX-Lung 8 (ClinicalTrials.gov NCT1523587) was a randomized, controlled, Phase III trial, conducted globally in 183 cancer centers, nine of which were in China. The study design was published previously.Citation19 Briefly, eligible patients had a confirmed diagnosis of stage IIIB or IV SCC of the lung, measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, Eastern Cooperative Oncology Group performance status of 0 or 1, and disease progression after ≥4 cycles of platinum-based chemotherapy (Supplementary slides).
Patients were randomized (1:1) to afatinib (40 mg) or erlotinib (150 mg), orally once daily; randomization was stratified by eastern Asian vs non-eastern Asian ethnic origin. The dose of afatinib was individualized according to tolerability. If patients had any grade ≥3 drug-related adverse events (AEs), or grade ≥2 diarrhea lasting 2 days or more, or nausea or vomiting for 7 consecutive days or more despite best supportive care, then afatinib was suspended for ≤14 days. After interruption of treatment and recovery to grade ≤1 or the baseline grade, afatinib was resumed at a lower dose (reduced by 10 mg decrements to a minimum of 20 mg/day). Treatment was permanently discontinued in patients who did not recover to grade ≤1 or the baseline grade. Dose reductions for erlotinib were also permitted. In both arms, treatment was continued until disease progression, unacceptable AEs preventing continuation, or any other reason necessitating withdrawal.
The study was conducted in accordance with the Declaration of Helsinki and guidelines on Good Clinical Practice, and the protocol was approved by local ethics committees at each participating center (Table S1). All patients provided written informed consent for trial participation.
Outcomes and assessments
The primary endpoint of LUX-Lung 8Citation19 was PFS, assessed by a blinded central independent review committee, according to RECIST (version 1.1), and the key secondary endpoint was OS. Other secondary endpoints included objective response rate (ORR) and disease control rate (DCR). Patients who received afatinib for 12 months or more were identified (post hoc) as long-term responders (LTRs).
Given the major impact of NSCLC symptoms on quality of life (QoL),Citation21 PROs were also evaluated, in particular, global health status (GHS). PROs were assessed at the first visit of each treatment course. The following were evaluated using the European Organisation for the Research and Treatment of Cancer (EORTC) Core Quality of Life Questionnaire-30 (QLQ-C30) and the Quality of Life Questionnaire-lung cancer-specific module (QLQ-LC13): improvements in GHS/QoL (QLQ-C30 questions 29–30); time to deterioration (TTD); and changes in pre-specified lung cancer symptoms over time, ie, cough (QLQ-LC13 question 1), dyspnea (QLQ-LC13 questions 3–5), and pain (QLQ-C30 questions 9 and 19).Citation22–Citation24 PRO responses were converted to a 0–100 scale and analyzed according to EORTC scoring algorithms.Citation22 An increase in functional scale or decrease in symptom scale of at least 10 points from baseline for each symptom or category was defined as an improvement, while TTD was defined as the time to a 10-point worsening from the baseline score. AEs were graded using the Common Terminology Criteria for Adverse Events (version 3.0).
All efficacy analyses were undertaken in the randomized intention-to-treat population; descriptive safety analyses included all patients who received ≥1 dose of study medication. The subgroup analysis was conducted to assess whether outcomes in Chinese patients and the overall population were similar, although the trial was not powered to detect significant differences in outcomes between subgroups of patients in the afatinib and erlotinib arms.
A Cox proportional-hazards model (stratified by ethnic origin) was used to estimate HRs and 95% CIs for survival. Treatment groups were compared using a log-rank test. Kaplan–Meier estimates and 95% CIs were calculated using Greenwood’s standard error estimate. Logistic regression models were used to compare proportions of patients with a response, or disease control, between subgroups. Statistical analyses were conducted using SAS version 9.2 or later (SAS Institute Inc., Cary, NC, USA).
Results
Patients
Of 795 patients randomized to treatment in LUX-Lung 8, 67 (8.4%) were from mainland China; 36 received afatinib and 31 erlotinib. Baseline demographic and clinical characteristics of the Chinese subgroup and the overall LUX-Lung 8 trial population (OTP) are shown in . Generally, the baseline characteristics of the Chinese subgroup were similar to the OTP, although fewer Chinese patients were ≥65 years old. In the Chinese subgroup, a greater proportion of females was randomized to afatinib (13.9%) than to erlotinib (6.5%); the same was true for patients aged ≥65 years (36.1% vs 22.6%).
Table 1 Patient demographics and baseline characteristics in the Chinese subgroup and the overall LUX-Lung 8 population
Efficacy outcomes
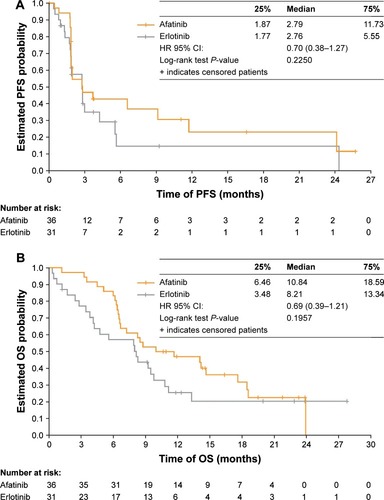
At the time of data cut-off, the mean duration of treatment in the Chinese patients was 5.3 months for afatinib (range: 0.5–27.6 months) and 3.5 months for erlotinib (range: 0.3–20.4 months). At the time of primary analysis of survival for the Chinese subgroup (March 2, 2015) median PFS was 2.8 months in both groups (HR=0.70; 95% CI: 0.38–1.27; ; ). Median OS was 10.8 months with afatinib and 8.2 months with erlotinib (HR=0.69; 95% CI: 0.39–1.21; ; ). The HRs for both PFS and OS favored afatinib.
Table 2 PFS by independent review, OS, and best overall tumor response by independent review, in the Chinese subgroup and the overall LUX-Lung 8 population
Figure 1 (A) PFS (independent review) and (B) OS for the Chinese subgroup.
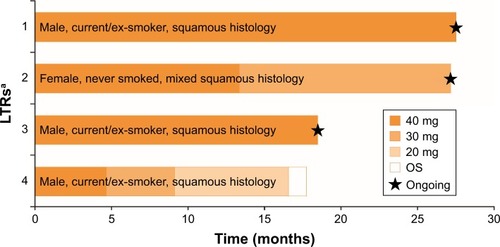
In the OTP, post hoc analysis was undertaken with the aim of identifying possible clinical and biomarkers indicative of long-term response to afatinib. Of 21 LTRs (5% of the OTP), four (11%) were from the Chinese subgroup. The median duration of treatment in these patients was 22.7 months (range: 16.6–27.6 months). At data cut-off, three of the four Chinese LTRs (8%) were alive and still on treatment (their OS was 17.5 months, 26.6 months, and 26.8 months; and Supplementary slides), while the fourth had disease progression (OS 17.6 months; and Supplementary slides). Two of the four LTRs had a confirmed objective response (OR); two had partial responses (including one female never-smoker with mixed squamous histology) and two had stable non-target disease in the absence of baseline target disease (non-complete response/non-progressive disease [NN]; and Supplementary slides). One Chinese patient randomized to erlotinib had been treated for 20.3 months, with a best response of NN and OS of 24.3 months.
Figure 2 Long-term benefit of afatinib in LTRs (Chinese patients).
Abbreviations: LTRs, long-term responders; OS, overall survival.
ORRs in the Chinese subgroup were 8.3% for afatinib and 6.5% for erlotinib (P=0.772; ). DCRs were 55.6% and 41.9% respectively (P=0.271; ). Changes in target lesion sum of diameters from baseline are shown in Figure S1 and Supplementary slides.
PROs
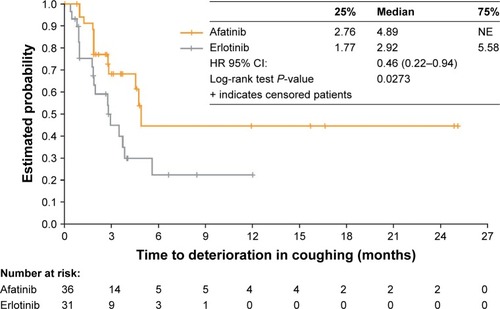
Among the Chinese subgroup, 52.8% of patients had an improvement in GHS/QoL with afatinib, vs 29.6% with erlotinib (P=0.072; Table S1). Improvements in individual PROs also occurred, including dyspnea (in 47% of patients taking afatinib vs 26% taking erlotinib; P=0.091), with significant differences in favor of afatinib in: “dyspnea walked” (afatinib, 44%; erlotinib, 15%; P=0.017); and QoL rate (afatinib, 53%; erlotinib, 26%; P=0.037; Table S1). TTD of cough was significantly longer for afatinib (median 4.9 months) than for erlotinib (2.9 months; HR=0.46; 95% CI: 0.22–0.94; P=0.03; ).
Safety outcomes
The most frequently occurring AEs (any grade, and grade 3) in the Chinese subgroup and the overall study population are shown in ; the most common AEs with afatinib in both the overall study population and the Chinese subgroup were diarrhea and rash/acne. The incidences of most AEs were similar between the overall population and the Chinese subgroup; notable discrepancies (of >10 percentage points) were for fatigue (34% and 19%, respectively), decreased appetite (25% and 6%), nausea (21% and 8%), and vomiting (13% and 0%). Overall, grade 3 AEs occurred in 22% of patients in the Chinese afatinib group (8/36; ), and serious AEs (SAEs) occurred in 22% (8/36; Table S2). The most frequently occurring AEs with erlotinib in both the Chinese subgroup and the overall study population were rash/acne, fatigue, and diarrhea, each of which was more frequent in the overall population than the Chinese subgroup (rash/acne, 70% and 52%; fatigue, 30% and 19%; diarrhea, 41% and 13%; ). Grade 3 AEs occurred in 19% of the Chinese erlotinib group (6/31; ) and SAEs occurred in 36% (11/31; Table S2).
Table 3 AETable Footnoteas in the Chinese subgroup and the overall safety population
Five Chinese patients (14%), including two LTRs, required a dose reduction due to AEs while taking afatinib (within 1 month of initiating treatment in three of the five patients), and four (11%) discontinued due to an AE (Table S3). Five Chinese patients in the afatinib arm died during the study (14%). The causes were lung infection (one patient), progression of malignant neoplasm (three patients), and multi-organ failure (one patient); none was considered related to treatment by the investigators.
Discussion
In LUX-Lung 8, afatinib significantly improved PFS, OS, and PROs (vs erlotinib) in patients with advanced SCC of the lung after failure of platinum-based therapy, while the safety profile of afatinib was predictable and manageable. In this post hoc analysis, we demonstrated trends toward improved PFS and OS with afatinib vs erlotinib, and improvements in PROs, in Chinese patients. This analysis was not powered to demonstrate differences compared with the OTP and there were several differences in baseline characteristics between the Chinese subset and the OTP (eg, more afatinib-treated patients in the OTP were ≥65 years old than in the Chinese subset). Nevertheless, the results of the current analysis indicated that the outcomes of afatinib treatment in Chinese patients were consistent with LUX-Lung 8 ().Citation19 Although the outcomes were consistent with the OTP, the CIs for the PFS and OS HRs overlap unity, due to the small size of the Chinese subgroup.
Four of the 36 Chinese patients (11%) were LTRs to afatinib (only one of 31 Chinese patients randomized to erlotinib was an LTR). Two of the four Chinese LTRs to afatinib had a confirmed OR; the median duration of treatment was 22.7 months and 17.6 months, respectively. Three of the Chinese LTRs were still receiving treatment at the time of data cut-off. Although not directly comparable, these data appear to be consistent with the OTP. Twenty-one patients (5%) treated with afatinib in the OTP were LTRs, seven of whom had a confirmed OR.Citation25
Impact on patients’ health-related QoL is a major consideration when considering the evidence for different oral anticancer drugs. In a survey of 83 patients with advanced lung cancer, one third rated QoL as more important than length of life, and more than half rated the two as equally important.Citation26 In the current analysis, 53% of Chinese patients treated with afatinib had an improvement in GHS/QoL compared with 30% of erlotinib-treated patients. These data are at least comparable to the OTP (36% vs 28%, respectively; P=0.041),Citation20 but did not achieve statistical significance (P=0.072), probably due to the relatively small sample size. In the Chinese subgroup, PROs that improved with afatinib included GHS/QoL, “dyspnea walked”, and TTD of cough. Lung cancer symptoms (dyspnea, pain, and fatigue) can interfere with activities of daily life, even when less severe.Citation21 In the Chinese subgroup, afatinib improved PROs related to dyspnea and cough, and improved GHS/QoL and QoL rate.
While EGFR TKIs are generally better tolerated than chemotherapy, some AEs (particularly gastrointestinal and cutaneous AEs) occur relatively frequently.Citation27 Indeed, in LUX-Lung 8, diarrhea of any grade occurred in 75% of the afatinib group, and rash/acne in 70%; incidences in the Chinese subgroup were 75% and 64%, respectively. However, none of the Chinese patients discontinued afatinib due to diarrhea or rash/acne, suggesting that clinicians in China were successful in managing the impact of AEs, possibly by means of tolerability-guided afatinib dose adjustments. The incidence and type of the most frequently occurring AEs were otherwise similar between the Chinese subgroup and the OTP ().
There were no instances of grade ≥3 diarrhea or discontinuations due to diarrhea in the Chinese subgroup treated with afatinib; the frequency of SAEs was 22%. The frequency of dose reductions and dose discontinuations due to AEs in Chinese patients treated with afatinib were 14% and 11%, respectively.
Given that EGFR mutations in SCC of the lung are rare,Citation28,Citation29 EGFR mutation testing was not mandated, and consequently, EGFR mutation data were not available for the Chinese subgroup, although next-generation sequencing was conducted in 238 patients (~30% of the overall LUX-Lung 8 population), retrospectively selected according to clinical benefit with afatinib or erlotinib (PFS >2 months, n=144; PFS ≤2 months, n=94). The proportion of patients with EGFR mutation was low overall (n=14 [6%]),Citation19 and it is therefore unlikely that the improved survival outcomes detected with afatinib were driven by molecular aberrations of EGFR.Citation19 Given the role of HER2 and ErbB3 in the pathogenesis of SCC (overexpression occurs in 20%–30% of squamous tumors),Citation18 and the involvement of several signaling molecules downstream of the ErbB receptors, broader irreversible ErbB blockade with afatinib may inactivate aberrant ErbB-dependent signaling cascades, which may underlie its efficacy in SCC of the lung.Citation19
In LUX-Lung 8, compared with erlotinib, afatinib significantly improved PFS, OS, and some PROs relating to symptoms commonly associated with NSCLC. The benefit of afatinib was also apparent in the Chinese subgroup. The availability of multiple tablet strengths of afatinib facilitates simple and effective tolerability-guided dose adjustment in the outpatient setting.Citation30–Citation32
Conclusion
Although this exploratory post hoc subgroup analysis was not powered to demonstrate significant differences between the Chinese subgroup and overall study population, given the need for new approved and convenient treatment options, the results suggest that afatinib represents a suitable treatment option for Chinese patients with advanced SCC of the lung, following progression during or after platinum-based chemotherapy.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This study was funded by Boehringer Ingelheim. Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Michael Simpson PhD, CMPP of GeoMed, an Ashfield company, part of UDG Healthcare plc, during the preparation of this manuscript.
We thank the patients, their families, and all the investigators who participated in this study. We acknowledge the support of Claudia Buehnemann of Boehringer Ingelheim Pharma GmbH & Co. KG in generating the EGFR data.
Disclosure
CZ reports lecture fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Roche, and Sanofi. AC, BP, NG, and EE report employment by Boehringer Ingelheim. The authors report no other conflicts of interest in this work.
References
- ChenWZhengRZengHZhangSThe incidence and mortality of major cancers in China, 2012Chin J Cancer20163517327484217
- HongQYWuGMQianGSPrevention and management of lung cancer in ChinaCancer2015121Suppl 173080308826331814
- LiQHsiaJYangGPrevalence of smoking in China in 2010N Engl J Med2011364252469247021696322
- MolinaJRYangPCassiviSDSchildSEAdjeiAANon-small cell lung cancer: epidemiology, risk factors, treatment, and survivorshipMayo Clin Proc200883558459418452692
- YangLParkinDMFerlayJLiLChenYEstimates of cancer incidence in China for 2000 and projections for 2005Cancer Epidemiol Biomarkers Prev200514124325015668501
- YouldenDRCrambSMBaadePDThe International Epidemiology of Lung Cancer: geographical distribution and secular trendsJ Thorac Oncol20083881983118670299
- ZhangHCaiBThe impact of tobacco on lung health in ChinaRespirology200381172112856737
- ChenWZhengRZengHZhangSEpidemiology of lung cancer in ChinaThorac Cancer20156220921526273360
- GaoYZhangJFLiQCThe clinicopathological and prognostic features of Chinese and Japanese inpatients with lung cancerOncotarget2016741674256743427608841
- ZhouQWuYLDeveloping CSCO lung cancer practice guidelines stratified by resource availability and treatment valueJ Glob Oncol20163428528828831436
- BrahmerJReckampKLBaasPNivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancerN Engl J Med2015373212313526028407
- HerbstRSBaasPKimDWPembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trialLancet2016387100271540155026712084
- FehrenbacherLSpiraABallingerMAtezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trialLancet2016387100301837184626970723
- RittmeyerABarlesiFWaterkampDAtezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trialLancet20173891006625526527979383
- GaronEBCiuleanuTEArrietaORamucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trialLancet2014384994466567324933332
- HirschFRVarella-GarciaMBunnPAJrEpidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosisJ Clin Oncol200321203798380712953099
- ZugazagoitiaJPonceSPaz-AresLNecitumumab for first-line treatment of advanced, squamous, non-small-cell lung cancer: a relevant step forward?Transl Lung Cancer Res201651959726958500
- HirshVNew developments in the treatment of advanced squamous cell lung cancer: focus on afatinibOnco Targets Ther2017102513252628546756
- SoriaJCFelipECoboMAfatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trialLancet Oncol201516889790726156651
- FelipEHirshVPopatSSymptom and quality of life improvement in LUX-Lung 8, an open-label Phase III study of second-line afatinib versus erlotinib in patients with advanced squamous cell carcinoma of the lung after first-line platinum-based chemotherapyClin Lung Cancer201819174.e1183.e1128729180
- TanakaKAkechiTOkuyamaTNishiwakiYUchitomiYImpact of dyspnea, pain, and fatigue on daily life activities in ambulatory patients with advanced lung cancerJ Pain Symptom Manage200223541742312007759
- AaronsonNKAhmedzaiSBergmanBThe European Organisation for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncologyJ Natl Cancer Inst19938553653768433390
- BergmanBAaronsonNKAhmedzaiSKaasaSSullivanMThe EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of LifeEur J Cancer199430A56356428080679
- European Organisation for Research and Treatment of CancerThe EORTC QLQ-C30 scoring manualEuropean Organisation for Research and Treatment of Cancer2001 Available from: http://www.eortc.be/qol/files/SCManualQLQ-c30.pdfAccessed June 4, 2018
- GadgeelSMSoriaJCFelipESecond-line afatinib vs erlotinib for patients with squamous cell carcinoma (SCC) of the lung (LUX-Lung 8 [LL8]): analysis of tumour and serum biomarkers and long-term respondersEur J Cancer2017721S185
- MeropolNJEglestonBLBuzagloJSCancer patient preferences for quality and length of lifeCancer2008113123459346618988231
- YangJCReguartNBarinoffJDiarrhea associated with afatinib: an oral ErbB family blockerExpert Rev Anticancer Ther201313672973623506556
- DeardenSStevensJWuYLBlowersDMutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap)Ann Oncol20132492371237623723294
- Cancer Genome Atlas Research NetworkComprehensive genomic characterization of squamous cell lung cancersNature2012489741751952522960745
- Boehringer Ingelheim International GmbHGilotrif US Prescribing Information2016 Available from: http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Gilotrif/Gilotrif.pdf?DMW_FORMAT=pdfGiotrifAccessed February 13, 2017
- GIOTRIF® (afatinib) tablets, for oral use [prescribing information]Boehringer Ingelheim International GmbH2016
- YangJCSequistLVZhouCEffect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: post hoc analyses of the randomized LUX-Lung 3 and 6 trialsAnn Oncol201627112103211027601237