Abstract
Introduction
Reactive oxygen species modulator-1 (Romo1) is a protein that modulates levels of reactive oxygen species (ROS) and has been reported to affect cancer cell invasion and proliferation via persistent inflammation. Several studies have demonstrated the clinical application of Romo1 as a prognostic marker in non-small cell lung cancer (NSCLC); however, there have been no studies investigating the mechanism by which Romo1 adversely affects the prognosis of these patients.
Methods
We examined Romo1, ROS, and vascular endothelial growth factor (VEGF) in tumor tissues immunohistochemically. We conducted survival analyses of patients who had curative resection (n=30) in accordance with clinical parameters including levels of Romo1 expression.
Results
Romo1 levels were associated with serologic inflammatory markers and high lymphatic metastatic tendencies. Significantly longer disease-free survival (68.7 vs 24.2 months, P=0.031) and overall survival (92.7 vs 51.6 months) were observed in the group with low Romo1 compared with high Romo1. Survival outcomes were also significantly associated with serologic inflammatory markers. Spearman’s correlation analyses demonstrated significant positive correlations of Romo1 expression with VEGF-C (P=0.008, R=0.478) and ROS (P=0.016, R=0.436) in tumor samples.
Conclusion
The current study demonstrates that Romo1 induces lymphatic metastasis of NSCLC by modulating persistent inflammation and oxidative stress (ROS)/VEGF signaling. Lymphatic metastasis associated with elevated Romo1 was shown to be a key reason for unfavorable survival rates.
Introduction
Lung cancer is the leading cause of cancer deaths, with an estimated 1.6 million new cases and 1.4 million deaths annually worldwide.Citation1 In the Republic of Korea, there are 23,000 new cases and ~16,000 deaths annually, which represents 22.6% of all cancer-related mortalities.Citation2 The World Health Organization classifies lung cancers into four groups: small cell lung cancer, non-small cell lung cancer (NSCLC) including adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Approximately half of all patients with NSCLC which account for about 80% of lung cancer diagnosesCitation3 are initially diagnosed as having metastatic disease which is not suitable for local treatment; thus, the role of systemic chemotherapy is crucial. Despite increasing use of targeted therapies for systemic treatment including epidermal growth factor receptor, tyrosine kinase inhibitor, and anaplastic lymphoma kinase inhibitor, 5-year survival rates of patients with NSCLC remain disappointingly low at <20%.Citation4 Recently, the introduction of immune checkpoint inhibitors is promising; however, at present, the only available biomarker for their use is programmed death-ligand 1 (PD-L1).Citation5 Continued research on appropriate biomarkers, and not just for immunotherapy, is required to develop new anticancer agents, expand indications for targeted agents or immune checkpoint inhibitors, and establish combination therapies required to maximize efficacy.
There is not a great deal of controversy about whether persistent inflammation is a trademark of malignancy.Citation6 Chronic inflammation is also thought to be one of the barriers against CD8+ T-cells which are major anticancer effector cells in the immuno-oncology field.Citation7 Recently, a novel protein called reactive oxygen species modulator-1 (Romo1), that induces mitochondrial production of reactive oxygen species (ROS), has been reported to affect cancer cell invasion and proliferation via persistent inflammation.Citation8,Citation9 It was first discovered in 2006 as a newly overexpressed gene in malignant tissues that had become resistant in the course of systemic chemotherapy.Citation10 A few studies have demonstrated the clinical application of Romo1 as a prognostic marker in NSCLC.Citation11,Citation12 In another study, upregulated Romo1 was shown to be associated with poorer survival rate of patients with hepatocellular carcinoma (HCC) and the authors suggested that cancer invasiveness induced by Romo1 was the reason for poor survival.Citation13 Romo1 was also shown to have potential as an adverse prognostic marker in patients with colorectal cancer (CRC), and was reported to be associated with high lymphatic metastatic tendency.Citation14
The aim of the current study was to demonstrate the value of Romo1 as a prognostic marker, to check the correlation between Romo1 and serologic biomarkers of persistent inflammation, and to investigate the mechanism by which Romo1 affects the prognosis of patients with NSCLC who had undergone curative resection.
Methods
Study patients and specimens
We collected formalin fixed, paraffin-embedded samples from patients with NSCLC who underwent curative resection in Korea University Guro Hospital from 2007 to 2010. All patients whose tissues were used in this research provided written informed consent for their use in future research. Patients who had died within 1 month of surgical resection, or received any treatment including chemotherapy or concurrent chemoradiotherapy before surgery, were excluded to reduce bias in the survival analysis. We also excluded three cases whose samples were not available for immunohistochemical (IHC) staining; thus, a total of 30 patients were enrolled. We collected the samples after surgery and verified pathologic staging according to the seventh edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual. We also collected relevant clinical data by reviewing medical records retrospectively. This study was performed with permission of the Institutional Review Board of Guro Hospital and written informed consent was obtained from all patients (KUGGR-2010-057).
Immunohistochemistry for Romo1, vascular endothelial growth factor (VEGF), and ROS
Tissue microarray (TMA) recipient blocks were made by using the collected paraffin blocks as donor blocks. In each donor block, morphologically ideal areas were designated and marked on their respective hematoxylin and eosin stained slides. Tissue cores of 0.6 mm diameter from all donor blocks were taken by a cylindrical tissue puncher (MicroDigital Co., Ltd. Seoul, Korea) and transferred into the hole on the recipient paraffin blocks.
Immunohistochemistry was used to demonstrate ROMO1, VEGF-C, and VEGF-D expression in primary tumor tissues. We deparaffinized the TMA slides with xylene and incubated them with peroxidase blocking reagent (3% H2O2 diluted with dextrose water) for 15 minutes to eliminate endogenous peroxidase activity. Subsequently, we retrieved antigens by steaming samples at 95°C for 20 minutes and freezing them for 10 minutes. Then, we blocked the TMA slides with blocking buffer, and incubated them with antibodies for Romo1 (OriGene, Rockville, MD, USA) at a 1:150 dilution, VEGF-D (Abcam, Cambridge, UK) at a 1:50 dilution, and VEGF-C (Abnova, Taipei City, Taiwan) at a 1:25 dilution. We then treated samples with 3,3′-diaminobenzidine chromogen solution for 7 minutes, followed by counterstaining with hematoxylin and dehydration. Negative control slides without the primary antibody were included.
For determination of ROS levels, the above-constructed TMA blocks were examined using a ROS detection kit (1:100, OxyIHC Oxidative Stress Detection Kit; Merck Millipore, Billerica, MA, USA) according to the manufacturer’s instructions.
All IHC staining was evaluated independently under a light microscope by two investigators (Baekhui Kim and Hong Jun Kim) who were unaware of the clinical information. When cytoplasmic staining was observed, the stained cells were recorded as positive. The staining intensity was graded from 0 to 3, and the staining percentage of positive cells was classified into six grades (percentage score 0–5). Results from the two evaluators werê80% identical. When the results were discordant between the two investigators, a third party conducted an inspection. IHC score was calculated by multiplying the percentage score by the intensity score (possible range 0–15).
Serologic inflammatory biomarkers
Evaluation of serologic inflammatory biomarkers, including white blood cell (WBC), neutrophil, lymphocyte, C-reactive protein (CRP), albumin, and platelet (PLT), was performed just before patients’ operations. The neutrophil–lymphocyte ratio (NLR) was calculated as the absolute neutrophil count divided by the absolute lymphocyte count; the platelet–lymphocyte ratio (PLR) was calculated as the PLT count divided by the absolute lymphocyte count. The CRP–albumin ratio was calculated as the serum CRP level (mg/L) divided by serum albumin level (g/dL).
Statistical analysis
We estimated the disease-free survival (DFS) and overall survival (OS) as clinical outcome variables. DFS was defined as the period from surgical resection until the time of the first detection of recurrence, or death from any cause. OS was defined as the period from surgical resection to death from any cause; we censored data for cases without cancer recurrence or death, at the last follow-up. Survival curves were constructed by the Kaplan–Meier method and analyzed by the log-rank test.
We categorized patients into two groups according to each clinical variable. For WBC, CRP, and PLT, patients were grouped by their upper normal values. For albumin level, patients were divided by the lower normal value. For those clinical data with no known normal range (Romo1, NLR, PLR, and CRP–albumin ratio), we performed receiver operating curve (ROC) analysis using 5-year survival rates to determine the optimal cutoff levels. We assessed the relationship between clinical parameters including serologic biomarkers with the Romo1 levels, by chi-square test. Using the Mann–Whitney U test, we measured the associations between Romo1 levels in two patient groups by clinical parameters. We considered a P-value <0.05 as statistically significant and used a Bonferroni corrected P-value (Pc) for multiple comparisons. All statistical analyses were performed using SPSS version 20.0 for Windows (IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics
Patient characteristics are shown in . Among 30 patients, eight (23.3%) were female and 22 (76.7%) were male. Their median age was 65.2 years (range 44–84 years). Eighteen patients (60%) were never smokers and 12 patients (40%) were current or former smokers. Nineteen patients (63.3%) had adenocarcinoma, nine had squamous cell carcinoma (30.0%), and two had pleomorphic carcinoma (6.7%). Eleven patients were stage IIA (36.7%) and 19 patients were stage IIIA (63.3%), meaning that all 30 patients showed postoperative pathologic findings of lymph node metastasis; 11 patients for N1 (36.7%) and 19 patients for N2 (63.3%). Only four patients (13.3%) had lymph node ratio (LNR) <0.1 between the metastatic and examined lymph nodes.
Table 1 The proportions of patients with low and high Romo1 expression according to clinicopathologic parameters
Romo1 expression and clinicopathologic parameters
Romo1 staining was typically observed in the cytoplasm of NSCLC cells. Romo1 expression levels were not normally distributed, and a representative example is shown in (average 7.6, range 0–15).
Figure 1 Representative IHC-stained tissue samples. Magnification, ×100; (A) Romo1, negative control, IHC score of 2, 10, and 15; (B) VEGF-C, negative control, IHC score of 2, 9, and 15; (C) VEGF-D, negative control, IHC score of 2, 8, and 15; (D) ROS, negative control, IHC score of 1, 7, and 15.
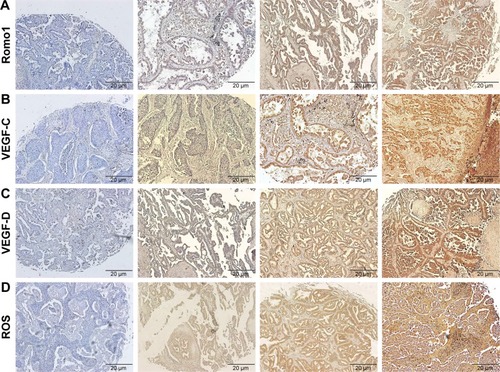
The process for determining the Romo1 cutoff level is described hereafter, and the cutoff was determined to be 8. With this cutoff, we classified 13 patients as being Romo1 low and 17 patients as Romo1 high. We evaluated the clinical parameters in these two groups, and NLR, CRP, CRP–albumin ratio, lymphatic invasion, LNR, and lymph node (N) stage were shown to be significantly associated with Romo1; P-values were 0.013, 0.04, 0.001, 0.024, 0.014, and 0.023, respectively (). These results were supported by the Mann–Whitney U test ().
Survival analyses
In the analyses described above, upregulated Romo1 was shown to be associated with high LNR, lymphatic invasion, and N2 stage rather than N1 stage, suggesting that Romo1 induces a high lymphatic metastatic tendency in patients with NSCLC. In addition, Romo1 was related to serologic biomarkers consistent with persistent inflammation which is believed to play an essential role in carcinogenesis and cancer progression. These findings suggest that Romo1 might augur an unfavorable prognosis for NSCLC patients. We evaluated the prognostic potential of Romo1, and several inflammatory markers in our cohort. The median follow-up time from curative resection was 38.8 months (range 1.4–115 months) and the median follow-up time among surviving patients was 50.6 months (range 5.4–115 months). Four patients out of 11 with stage IIA and 15 patients out of 19 with stage IIIA underwent vinorelbine plus cisplatin chemotherapy as postoperative adjuvant treatment. At the time of analysis, 14 patients had died (46.7%), and cancer had recurred in 20 patients (66.7%) after surgery. The median follow-up time from recurrence was 16.1 months (range 0.2–77.3 months). Eleven patients with recurrence showed local recurrence, 13 patients had distant metastasis, and four patients had both. Fifteen patients out of 20 who had had recurrent disease underwent palliative chemotherapy; the chemotherapy regimens included pemetrexed/cisplatin, gemcitabine/cisplatin, pemetrexed monotherapy, taxane monotherapy, etoposide/cisplatin, and gemcitabine/carboplatin. Ten patients had no recurring disease and were still alive.
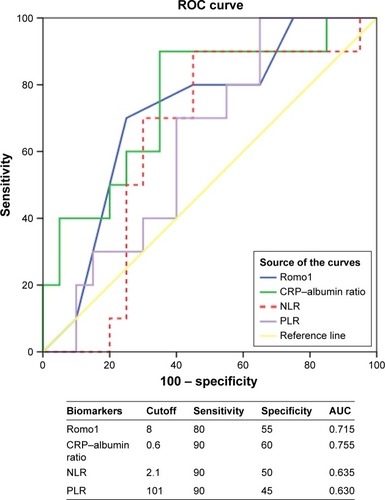
The median values for Romo1, NLR, PLR, and CRP–albumin ratio were 8.0 (range 0–15), 2.2 (range 0.7–5.9), 130.3 (range 55.9–351.6), and 0.87 (range 0.05–12.7), respectively. To examine the prognostic value of these markers, we chose 5-year survival as the stratifying point for ROC analysis, and the ROC curves of Romo1 and CRP–albumin ratio showed statistical utility being drawn above the reference line (). We used the cutoff value that made the sum of sensitivity and specificity the highest. The determined optimal cutoffs for Romo1, NLR, PLR, and CRP–albumin ratio were 8, 2.1, 101, and 0.6, with an area under the curve of 0.715, 0.635, 0.630, and 0.755, respectively.
Figure 2 ROC analysis to set best cutoff for Romo1 and serologic inflammatory biomarkers (NLR, PLR, and CRP–albumin ratio).
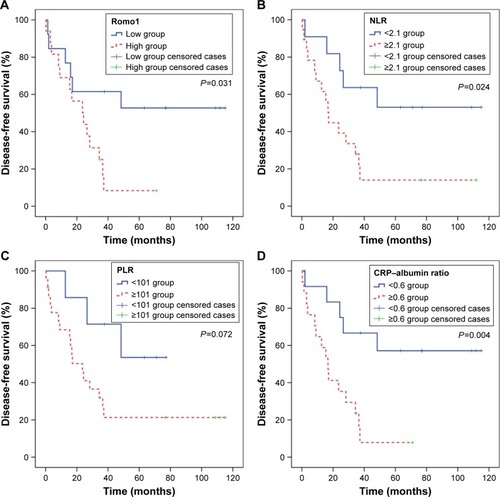
Analyses for DFS with respect to clinical parameters are shown in . The mean DFS was 47.4 months (95% CI 30.6–64.2 months). By the log-rank test, Romo1 ≥8 (P=0.031), NLR ≥2.1 (P=0.024), CRP ≥10.5 mg/L (P=0.007), and CRP–albumin ratio ≥0.6 (P=0.004) were significantly associated with shorter DFS ().
Figure 3 Kaplan–Meier curves of disease-free survival according to Romo1 (A), NLR (B), PLR (C), and CRP–albumin ratio (D).
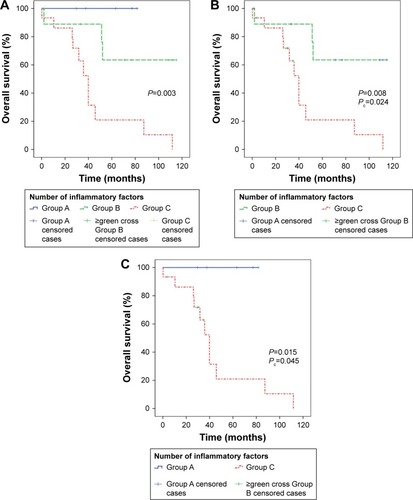
Analyses of OS with respect to clinical parameters are also summarized in . Mean OS was 70.0 months (95% CI 53.5–86.6 months). By the log-rank test, Romo1 ≥8 (P=0.011), NLR ≥2.1 (P=0.044), PLR ≥101 (P=0.026), and CRP–albumin ratio ≥0.6 (P=0.006) were significantly associated with shorter OS (). Based on the number of inflammatory markers identified as risk factors affecting OS, NLR, PLR, and CRP–albumin ratio, patients were divided into three groups (group A=0; group B=1 or 2; group C=3 risk factors), and the OS for each group was compared (). OS was significantly shorter in group C compared with group A (Pc=0.045) or B (Pc=0.024).
Figure 4 Kaplan–Meier curves of overall survival according to Romo1 (A), NLR (B), PLR (C), and CRP–albumin ratio (D).
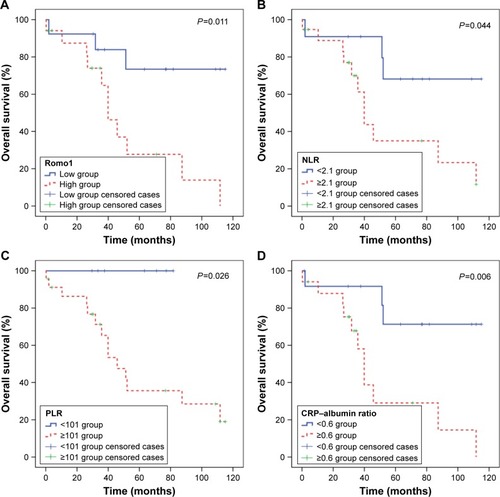
Figure 5 Kaplan-Meier curves of overall survival according to the number of inflammatory risk factors (A). Analyses using Bonferroni corrected P values (Pc) showed that overall survival was significantly shorter in group C compared with group B (B) or group A (C). Number of risk factors: group A =0; group B =1 or 2; group C =3.
Relationship between Romo1 and VEGF
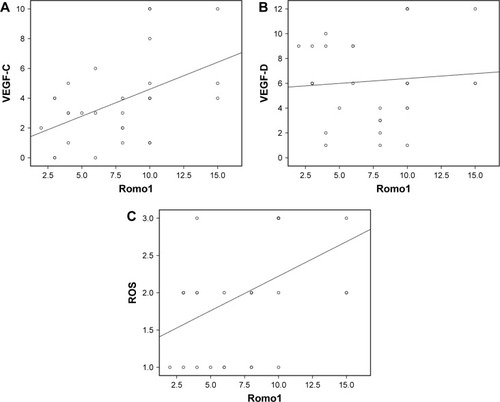
Romo1 regulates mitochondrial release of ROS, which induces angiogenesis/lymphangiogenesis via hypoxia-inducible factor/VEGF signaling.Citation15 Since Romo1 seems to influence a high lymphatic metastatic tendency, which is also associated with VEGF-C and VEGF-D, we investigated the correlation between Romo1 and VEGF-C, and VEGF-D in clinical samples by IHC staining (). To determine whether a relationship exists between Romo1 expression, VEGF-C, VEGF-D, and ROS, correlation analyses were performed in paired tissue samples. Spearman’s correlation analyses demonstrated significant positive correlations of Romo1 with VEGF-C (P=0.008, R=0.478) and ROS (P=0.016, R=0.436) ().
Figure 6 Spearman’s correlation analyses evaluating correlation among Romo1, VEGF-C, VEGF-D, and ROS; Romo1 expression levels showed significant positive correlation with VEGF-C (P=0.008, R=0.478) (A) and ROS (P=0.016, R=0.436) (C) in tumor samples. (B) There was no significant correlation between Romo1 expression and VEGF-D levels.
Discussion
In this study, Romo1 overexpression was shown to be significantly correlated with early recurrence and an unfavorable prognosis in NSCLC patients who underwent curative surgery. There were also significant associations of unfavorable clinical outcomes with novel inflammatory biomarkers including NLR, PLR, and CRP–albumin ratio. Furthermore, there was significant association of Romo1 with high LNR, lymphatic invasion, and N2 stage rather than N1 stage, demonstrating that the Romo1 expression level is related with lymphatic metastatic proclivity. In addition, Romo1 was associated with serum biomarkers of persistent inflammation, although this was likely predictable given that Romo1 controls oxidative stress. Considering both the oxidative stress/inflammation related with Romo1 and the lymphatic metastatic tendency associated with Romo1, we were able to speculate about a significant correlation between Romo1 and VEGF, and our subsequent analyses support it.
These results suggest that Romo1 might affect lymphatic metastasis of the primary tumor by regulating chronic inflammatory and oxidative stress (ROS)/VEGF signaling. Lymphatic metastasis related with Romo1 appears to be an essential correlate of the unfavorable clinical outcomes related to Romo1 overexpression. To our knowledge, this is the first study to provide a new hypothesis that lymphatic metastasis is the key reason for poor survival of NSCLC patients with elevated Romo1 and to propose persistent inflammation and oxidative stress/VEGF pathway as a possible mechanism by which Romo1 induces lymphatic metastasis of NSCLC.
ROS levels were shown to be increased in various malignant diseases and associated with carcinogenesis and cancer progression;Citation9 thus, studies on Romo1, a key regulator of mitochondrial ROS,Citation10 have mainly been performed in the oncology field, especially in regard to Romo1’s role in cancer cell invasiveness. In an earlier study, Romo1 knockdown induced downregulation of cell proliferation by blocking the extracellular signal-regulated kinases (ERK) pathway. Given that the ERK pathway is crucial for cell cycle entry, this finding suggests that Romo1 causes persistent oxidative stress via increasing ROS, resulting in carcinogenesis as well as cell proliferation.Citation8 In another study, nuclear factor kappa B (NF-kB) signaling pathway was downregulated by Romo1 knockdown, suggesting that Romo1 induces cancer cell invasion by upregulating the NF-kB pathway. Increasing invasiveness accelerated by Romo1 was interrupted by an inhibitor of kB kinase (IKK), demonstrating the cooperative relationship between the NF-kB pathway and Romo1.Citation16
Since Romo1 was first found a decade ago, there have been studies demonstrating Romo1 as a prognostic biomarker in HCC, CRC, and NSCLC,Citation11,Citation13,Citation14 but no study has investigated the mechanism by which Romo1 adversely affects prognosis of cancer patients. We have shown here that Romo1 expression is correlated with lymphatic invasion of the primary tumor, but not vascular or neural invasion; whether it is invasion of the primary tumor into lymphatics or vasculature currently depends on the lymphangiogenic or angiogenic vessels rather than the dormant vessels,Citation17 and the progression to lymphangiogenesis or angiogenesis depends on specific and different pro-angiogenic factors.Citation18 The VEGF gene family, which includes placental growth factor, VEGF-A, VEGF-B, VEGF-C, and VEGF-D,Citation19 has signal proteins that induce angiogenesis and lymphangio-genesis. Among them, VEGF-C and VEGF-D are reported to promote the growth of tumor-associated lymphatic vessels and enhance lymphatic metastasis.Citation20 Based on both the fact that Romo1 is an upstream regulator of ROS, which has a cooperative relationship with the VEGF family,Citation21 and the hypothesis that Romo1 may cause lymphangiogenesis, authors investigated the correlation of Romo1 with VEGF-C and VEGF-D. In the current study, Romo1 was significantly related only with VEGF-C, suggesting that Romo1 might cause lymphatic metastasis via oxidative stress/VEGF-C signaling.
Romo1 could be a druggable target. Upregulated Romo1 has been reported to be associated with cancer invasiveness in HCC patients,Citation13 and high lymphatic metastatic tendency in CRC patientsCitation14 as well as our study patients. Because the main driver of mortality in cancer is metastasis,Citation22 targeting Romo1 might improve survival rates. In fact, considering that chronic ROS stress is thought to be harmful in cancer progression, several antioxidants have been tested for their ability to prevent cancer invasion; however, all have shown disappointing results.Citation23 Rather than terminating ROS already released by mitochondria, regulating Romo1 may be a better option, making it a promising drug target.
In this study, Romo1 expression showed a significant correlation with novel inflammatory biomarkers, including NLR, PLR, and CRP–albumin ratio. Lately, these markers have been identified in several cancers as easily measurable prognostic indicators, and many studies suggest that systemic persistent inflammation has an essential role in cancer biology.Citation6,Citation24 Considering that Romo1 is a regulator of ROS and correlates with inflammatory biomarkers, we think that carcinogenic inflammation could be regulated by targeting Romo1.
Inflammation has both tumor suppressing and tumor promoting features; acute inflammation inhibits cancer progression, while chronic inflammation induces carcinogenesis.Citation7 This recognition guided clinical researchers into the immuno-oncology field in pursuing research that promotes the cytotoxic properties of antigen-specific T-cells and/or suppresses cancer promoting inflammation.Citation7 For over a decade, the immuno-oncology field has been in the spotlight, and admired for showing innovative practice changing successes.Citation25 However, given the absence of narrow patient selection based on a proper prediction of the therapeutic effect, immunotherapy has been considered as only stratified medicine rather than precision medicine like targeted agents. Microsatellite instability status and PD-L1 expression status have been studied as predictive markers in a few cancers including NSCLC and CRC.Citation26,Citation27 Romo1 also has potential as a proper predictive biomarker for immune checkpoint inhibitors, considering both the fact that Romo1 induces chronic harmful inflammations, and the fact that the tumor microenvironment corrupted by chronic inflammations is one of the barriers of major anticancer effector cells in immuno-oncology, CD8+ T-cells.Citation7,Citation28 Based on these, Romo1 is expected to have possible roles as not only a predictive biomarker but also a target of drugs, which could be combined with immune checkpoint blockade therapy, because regulating harmful inflammation by targeting Romo1 could be beneficial for avoiding the resistance of immunotherapeutic approaches.
Limitations
This study has several limitations. First, IHC was used for the quantification of protein levels including Romo1 and VEGF, although the IHC method possibly has variant results. However, there is no standard quantification method established for the analysis of Romo1 expression and IHC is a generally conducted method for measuring protein expression. Not only that, protein expression analyses with a TMA could cause sampling error, because of a small portion of the whole tumor sample. Therefore, caution is needed not to overinterpret results of this study. Second, the results shown represent only associations rather than causations among serum inflammatory markers, overexpression of biomarkers including Romo1, VEGF, and ROS, and clinical variables related with lymphatic metastasis. In order to demonstrate the hypothesis of the mechanism by which Romo1 induces lymphatic metastasis of NSCLC by modulating persistent inflammation and oxidative stress (ROS)/VEGF signaling, further properly designed in vitro studies are mandated. Third, the current study was conducted at a single center retrospectively with a relatively small sample size. Because our study cohort included patients with a relatively homogenous T stage, it is thought that possible bias in comparative analysis of Romo1 expression between the N1 group and N2 group (stage IIA and stage IIIA) was compromised. This might be the reason why the analyses were able to show statistical significance, even with a relatively small cohort. Our results should be validated by further prospective studies including many patients and using other quantification methods also.
Conclusion
The current study suggests a new hypothesis that Romo1 can induce lymphatic metastasis of NSCLC by modulating persistent inflammation/VEGF signaling. In this study, lymphatic metastasis related to Romo1 was shown to be a key reason of unfavorable survival rates. With further research, antibodies targeting Romo1 could be designed and used as novel targeted agents. Romo1 could also be used as an indicator of harmful inflammatory conditions, so that researchers could expect possible barriers of immune checkpoint inhibitors and overcome them by targeting Romo1.
Supplementary materials
Table S1 Association between Romo1 and clinicopathologic parameters
Table S2 Survival analyses for patients
Disclosure
The authors report no conflicts of interest in this work.
References
- JemalACenterMMDeSantisCWardEMGlobal patterns of cancer incidence and mortality rates and trendsCancer Epidemiol Biomarkers Prev20101981893190720647400
- JungKWWonYJKongHJCancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012Cancer Res Treat201547212714125761484
- FauciASLongoDLKasperDLJamesonJLLoscalzoJHauserSLHarrison’s Principles of Internal Medicine17th edNew York; LondonMcGraw-Hill Medical2008
- DeSantisCELinCCMariottoABCancer treatment and survivorship statistics, 2014CA Cancer J Clin201464425227124890451
- ReckMRodríguez-AbreuDRobinsonAGPembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancerN Engl J Med2016201637518231833
- BalkwillFCharlesKAMantovaniASmoldering and polarized inflammation in the initiation and promotion of malignant diseaseCancer Cell20057321121715766659
- CoussensLMZitvogelLPaluckaAKNeutralizing tumor-promoting chronic inflammation: a magic bullet?Science2013339611728629123329041
- NaARChungYMLeeSBParkSHLeeMSDo YooYA critical role for Romo1-derived ROS in cell proliferationBiochem Biophys Res Commun2008369267267818313394
- WuWSThe signaling mechanism of ROS in tumor progressionCancer Metastasis Rev200625469570517160708
- ChungYMKimJSYooYDA novel protein, Romo1, induces ROS production in the mitochondriaBiochem Biophys Res Commun2006347364965516842742
- LeeSHChoiSILeeJSReactive oxygen species modulator 1 (Romo1) predicts poor outcomes in advanced non-small cell lung cancer patients treated with platinum-based chemotherapyCancer Res Treat201749114114927188201
- LeeSHMinJWLeeJSReactive oxygen species modulator 1 (Romo1) overexpression is an independent predictor of poor survival in NSCLC patients who undergo surgical resectionLung Cancer2015871455225468147
- ChungJSParkSParkSHOverexpression of Romo1 promotes production of reactive oxygen species and invasiveness of hepatic tumor cellsGastroenterology201214341084109422749933
- KimHJJoMJKimBRReactive oxygen species modulator-1 (Romo1) predicts unfavorable prognosis in colorectal cancer patientsPLoS One2017125e017683428472059
- KimYWByzovaTVOxidative stress in angiogenesis and vascular diseaseBlood2014123562563124300855
- LeeSParkYHChungJSYooYDRomo1 and the NF-κB pathway are involved in oxidative stress-induced tumor cell invasionInt J Oncol20154652021202825673177
- DuaRGuiGIsackeCEndothelial adhesion molecules in breast cancer invasion into the vascular and lymphatic systemsEur J Surg Oncol200531882483216055299
- StackerSAAchenMGJussilaLBaldwinMEAlitaloKLymphangiogenesis and cancer metastasisNat Rev Cancer20022857358312154350
- FerraraNGerberHPLeCouterJThe biology of VEGF and its receptorsNat Med20039666967612778165
- KaramanSDetmarMMechanisms of lymphatic metastasisJ Clin Invest2014124392292824590277
- Ushio-FukaiMVEGF signaling through NADPH oxidase-derived ROSAntioxid Redox Signal20079673173917511588
- TamWLWeinbergRAThe epigenetics of epithelial-mesenchymal plasticity in cancerNat Med201319111438144924202396
- LadasEJJacobsonJSKennedyDDTeelKFleischauerAKellyKMAntioxidants and cancer therapy: a systematic reviewJ Clin Oncol200422351752814752075
- DiemSSchmidSKrapfMNeutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumabLung Cancer201711117618128838390
- BursteinHJKrilovLAragon-ChingJBClinical cancer advances 2017: annual report on progress against cancer from the American Society of Clinical OncologyJ Clin Oncol201735121341136728148207
- Van CutsemECervantesAAdamRESMO consensus guidelines for the management of patients with metastatic colorectal cancerAnn Oncol20162781386142227380959
- HerbstRSBaasPKimDWPembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trialLancet2016387100271540155026712084
- ElinavENowarskiRThaissCAHuBJinCFlavellRAInflammation-induced cancer: crosstalk between tumours, immune cells and microorganismsNat Rev Cancer2013131175924154716
