Abstract
Background
A suppressive immune microenvironment and pathological angiogenesis are hallmarks of gastric cancer. Theoretically, immune checkpoint inhibitors (ICIs) stimulate pre-primed neoantigen-specific T cells, and antiangiogenic agents then facilitate their infiltration into the tumor niche by promoting vascular normalization. Currently, the interconnections of these two phenotypes and their relevance to the tumor microenvironment (TME) have not been fully characterized in gastric cancer.
Materials and methods
Transcriptome profiling data retrieved from The Cancer Genome Atlas (TCGA) database were used to deconvolute the feature of TME for gastric cancer (N = 375). Machine learning, correlation, and prognosis analysis were applied to elucidate the correlations between angiogenesis, cytotoxic T lymphocyte infiltration, and patient survival.
Results
Substantial heterogeneous infiltration of immune cell populations among cases was observed. Furthermore, among targetable pathways, angiogenesis was identified as the dominant factor in discriminating different infiltration statuses. Most importantly, the angiogenesis pathway was negatively correlated with the amount of activated CD8+ T cells only for patients with a higher infiltration, and the concomitance of low angiogenesis signaling and highly activated CD8+ T-cell infiltration was associated with a significant survival benefit.
Conclusion
Our findings demonstrated a negative correlation between angiogenesis signaling and cytotoxic function in gastric cancer patients with a highly infiltrated immune niche. These data provided a rationale for potential combination strategy and further clinical investigations of ICIs plus antiangiogenesis agents for patients with gastric cancer with an inflamed TME.
Introduction
Gastric cancer is the fifth most common malignant disease and the third leading cause of cancer-related mortality worldwide.Citation1 However, the clinical benefit of available treatment strategies is limited, emphasizing the need to develop novel strategies to improve disease outcomes. Cancer immunotherapy has made encouraging breakthroughs in a variety of other solid tumors,Citation2 which has also been evaluated for use as a treatment for gastric cancer recently. In Phase Ib KEYNOTE-012 trial, researchers found prolonged survival in advanced gastric cancer patients treated with pembrolizumab, an anti-programmed cell death-1 (PD-1) antibody,Citation3 and further identified upregulation of interferon-γ-related genes, including STAT1, HLA-DRA, IFNG, IDO1, and CXCL9/10, in the tumor microenvironment (TME) of responders.Citation3 This phenomenon suggests that successful stimulation of the immunological suppressive TME requires pre-primed and infiltrated neoantigen-specific T cells in the tumor niche to eliminate the mutated cells. Therefore, the prognostic value of these immune signatures provides a convincing rationale for exploring combination therapies to improve the objective response rate (ORR) by promoting immune cell infiltration.
Emerging evidence suggests that impaired infiltration of cytotoxic T cells (CTLs) in the TME may be related to the increased level of vascular endothelial growth factor A (VEGFA),Citation4 which facilitates vascularization without well-regulated expression of adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion-1 (VCAM-1). The AVAGAST trial demonstrated that the addition of bevacizumab, a VEGFA-directed monoclonal antibody, to the standard chemotherapy increased the percentage of patients achieving an ORR and slightly prolonged progression-free survival (PFS) and overall survival (OS).Citation5 Another antagonist targeting VEGFR-2, ramucirumab, was also shown to provide some clinical benefits as monotherapy or in combination with paclitaxel as a second-line regimen in REGARDCitation6 and RAINBOWCitation7 trials. Moreover, administration of apatinib, a VEGFR tyrosine kinase inhibitor, was associated with improved PFS and OS as third-line or later treatment.Citation8 The favorable effects of antiangiogenic agents in patients with metastatic gastric cancer are thought to be related to reduce blood supply to tumor cells rather than immune modulation. Elucidating the relationships between angiogenesis and cytotoxic functions would expand our understanding of the immune microenvironment of gastric cancer and provide a basis for further investigations of combination therapies.
In this study, we aimed to elucidate the immune landscape of gastric cancers by analyzing previous clinical cases reported in The Cancer Genome Atlas (TCGA) project. We identified several dominant immunological features involved in determining local immune cell infiltration status. Among these features, angiogenesis, the most important drug-targetable signaling pathway, was significantly correlated with the magnitude of the signature of cytotoxic function, particularly in patients with highly infiltrated TME. Therefore, our study provided a rationale for potential combination therapeutic strategies and further clinical investigations of combination therapies.
Materials and methods
Data sources
The RSEM-normalized RNA sequencing data for the stomach adenocarcinoma (STAD) cohorts were downloaded from TCGA Data Portal (https://gdc-portal.nci.nih.gov/). All gastric cancers analyzed in this study were untreated primary lesions (N = 375). Additional data sets were also downloaded from TCGA in May 2017. The Hallmark gene sets were downloaded from GSEA Molecular Signatures Database v6.0 (http://software.broadinstitute.org/gsea/msigdb/index.jsp).
Gene signatures and single-sample gene set enrichment analysis (ssGSEA) scores
The levels of immune infiltration were quantified using a previously validated computational method based on expression profiles of immunomodulatory factors from 28 immune cell types, comprising 782 genes in total.Citation9 The degree of immune infiltration was determined by the ssGSEA,Citation10 computed using R-package Gene Set Variation Analysis (GSVA). Cytotoxic (CYT) activity was computed as the geometric mean of expression levels of PRF1 and GZMA, as previously reported.Citation11 For antigen-presenting machinery (APM), we used a seven-gene signature composed of MHC class I genes and antigen-processing genes.Citation12 The IFN-γ signature was calculated as the mean of a series of gene profiles as described in KEYNOTE-059,Citation13 CheckMate-275,Citation14 KEYNOTE-012,Citation3 and POPLARCitation15 clinical trials. The angiogenesis and Wnt/β-catenin pathway were calculated using ssGSEA scores from HALLMARK_ANGIOGENESIS and HALLMARK_WNT_BETA_CATENIN_SIGNALING.
Identification of determinants of the tumor immune microenvironment
Cases of high infiltration and low infiltration groups were selected for examination. A random forest classifier, including 42 parameters from the Hallmark gene set, was trained to separate the group of cases with high infiltration levels from cases with low infiltration levels using the R package randomForest with 10,000 trees. Eight signaling pathways of Hallmark that directly characterized the inflammation were excluded (TNFA_SIGNALING_VIA_NFKB, IL6_JAK_STAT3_SIGNALING, INTERFERON_ALPHA_RESPONSE, INTERFERON_ GAMMA_RESPONSE, HALLMARK_COMPLEMENT, INFLAMMATORY_RESPONSE, IL2_STAT5_SIGNALING, and ALLOGRAFT_REJECTION). The mean decrease in Gini over all out-of-bag cross-validated predictions was applied to rank the predictors. The immunophenogram was visualized as previously described.Citation16 The aforementioned infiltration signatures of 28 immune cell gene profiles were selected for multidimensional scaling (MDS) analysis. Sample-wise Z-scores from gene expression data were calculated for cell types, and an average Z-score from the corresponding gene sets was calculated.
Statistical analysis
Sample sizes from available TCGA data were regarded as adequate according to power estimates performed previously using equivalent tests.Citation17 Two-sided Student’s t-test and one-way analysis of variance (ANOVA) were used for comparisons using the R package. Unsupervised clustering for tumor cases, immune cell types, and gene sets was performed with hierarchical clustering using Pearson’s correlation. Furthermore, Pearson’s correlation coefficients were also reported for ssGSEA scores in the angiogenesis pathway, Wnt/β-catenin signaling pathway, and immune signatures. For survival analysis, p-values in were obtained from Cox proportional-hazards regression models using the R package survival. Kaplan–Meier curves were generated using the survfit function from the survival package.
Ethical approval and informed consent
This article does not contain any studies with human participants or animals performed by any of the authors.
Results
Gastric cancers exhibited heterogeneous immune cell infiltration
To evaluate immune cell infiltration in the TME of gastric cancer, we analyzed the RNA-sequencing profiles retrieved from TCGA. ssGSEA was introduced to deconvolute the relative infiltration level into a normalized ssGSEA score.Citation10 Significant heterogeneous infiltration of diverse immune cell populations was identified by visualizing the calculated scores ( and Table S1). Based on their infiltration spectrum, all cases were classified into high, median, or low immune infiltration groups by an unsupervised classification method. The high infiltration group seemed to have more immune-active cells, containing activated T cells (TACTs), central memory T cells, effector memory T cells, natural killer T cells, type 1 T helper cells (Th1), T follicular helper cells, γδT cells, activated B cells, and activated dendritic cells (Figure S1A). With the higher levels of activated subtypes, immune-suppressive subtypes, such as myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs), were also enriched in the high infiltration group (Figure S1A), implying a negative feedback in modulating the cytotoxic response.
Figure 1 Heterogeneous infiltration landscape of gastric cancers.
Abbreviations: ssGSEA, single-sample gene set enrichment analysis; APM, antigen-presenting machinery; CYT, cytotoxic; IQR, interquartile range; ANOVA, analysis of variance.
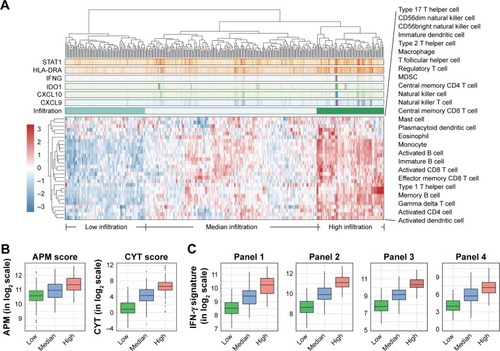
To thoroughly characterize the immunophenotypes of distinct groups, with a particular focus on cytotoxic function, we compared levels of several representative immunomodulatory and effector molecules, including APM, CYT scores,Citation11 and gene panels of IFN-γ signatures, including panels applied in KEYNOTE-059Citation13 (panel 1), CheckMate-275Citation14 (panel 2), KEYNOTE-012Citation3 (panel 3), and POPLARCitation15 (panel 4). We observed enhanced cytotoxic function in the high infiltration group, characterized by elevated APM, CYT score, and the IFN-γ signature of different standards ( and Table S2), strongly suggesting high cytotoxic potential in patients with gastric cancer exhibiting higher immune cell infiltration.
Additionally, we compared the aforementioned signatures among different pathologic subtypes and found no significant difference between any two distinct types (Figure S1B). This suggested that infiltration levels were not subject to pathologic type.
Machine learning identified angiogenesis as a predominant determinant of immune cell infiltration
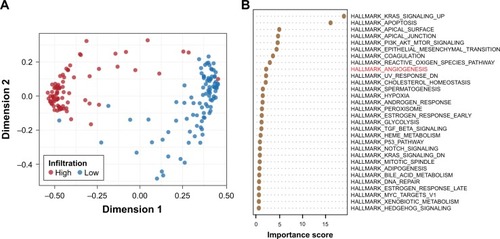
To identify the most dominant determinants of immune cell infiltration in gastric cancer, we introduced a machine learning approach (random forest), based on a multitude of decision trees.Citation18 A total of 42 different input variables from the Hallmark database were included to build the decision trees for discriminating the high infiltration versus low infiltration status. Eight signaling pathways that directly characterized the inflammation were excluded because they were usually indicators but not the original reason for immune signatures of TME. By MDS visualization, the similarities in immunophenotypes of every two cases were indicated as their spatial distance (). The most dominant variables were identified by sorting the importance of each pathway (). There was a huge gap between the first two elements (KRAS and apoptosis) and the remaing factors. As for KRAS, it has been reported that expression of a mutant KRAS oncogene could induce strong responses by activating cytokines and T cells and thereby potently influence the immune milieu.Citation19,Citation20 As for apoptosis, its positive correlations with host immunity have also been confirmed by several studies.Citation21,Citation22 Interestingly, angiogenesis pathway was identified as the most important variable among the drug-targetable signaling pathway (). However, the Wnt/β-catenin pathway, which is thought to play a critical role in hindering T-cell infiltration in patients with melanoma,Citation23 was not ranked high in the TME of gastric cancer. These findings suggested tumor-type-specific regulation of immune cell infiltration and highlighted the role of angiogenesis in forming the immune microenvironment in gastric cancer.
Figure 2 Determinants of immune characterization in gastric cancers.
Abbreviation: MDS, multidimensional scaling.
Angiogenesis, but not the Wnt/β-catenin signaling pathway, was associated with cytotoxic function in TMEs exhibiting high infiltration
Previous studies have found that VEGF/VEGFR inhibitors might repolarize immune cells to the Th1 phenotype, resulting in enhanced antitumor effects.Citation24 Here, we analyzed the association between angiogenesis and cytotoxic function in the TME of a large cohort of gastric cancers. CYT scores and the infiltration of CD8+ TACTs were examined as indicators of cytotoxic function due to their ultimate effective roles in tumor immunity. When the correlations were tested for the entire cohort, we found an almost irrelevant correlation between the angiogenesis pathway and cytotoxic function, which was not represented by the CYT score or the infiltration of CD8+ TACTs ( and Table S3). However, when comparisons for each infiltration status were applied separately, prominent negative associations between angiogenesis and CYT scores were found, particularly for highly infiltrated TME (). A similar tendency was also observed between angiogenesis and CD8+ TACTs ( and Table S3). Immune-inhibitory cell types, such as MDSCs and Tregs, showed positive correlations ( and Table S3), which is consistent with previous reports.Citation25,Citation26
Figure 3 Correlations between immune signatures and the angiogenesis pathway.
Abbreviations: ssGSEA, single-sample gene set enrichment analysis; CYT, cytotoxic; MDSC, myeloid-derived suppressor cell.
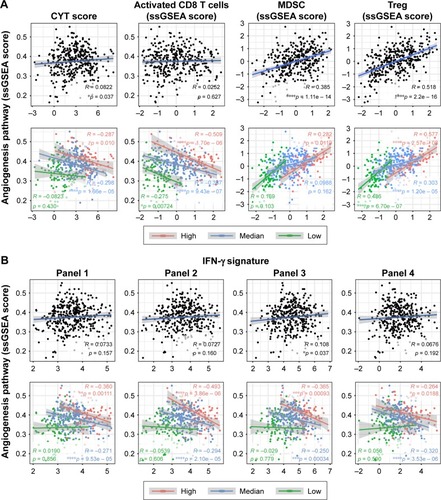
As mentioned earlier, the Wnt/β-catenin signaling pathway has been validated to regulate the development of CTLs, the activation of Tregs, and further influence antitumor immunity in malignant melanoma.Citation23,Citation27 However, we observed no significant correlations between Wnt/β-catenin signaling and infiltration characteristics in gastric cancer (Figure S2A and B and Table S4), which is consistent with the findings in and implied a tumor-type-dependent mechanism of tuning immune cell infiltration.
Based on these findings, we then analyzed the relationships between angiogenesis and IFN-γ signature in detail using several clinically tested gene panels. Our exploration revealed interesting results ( and Table S5): 1) there were almost no correlations between angiogenesis and IFN-γ signature when judged at the integral level; 2) in the low infiltration group, angiogenesis was irrelevant to IFN-γ signature; and 3) inverse correlations with angiogenesis were only observed in the high and medium infiltration groups. Considering the valuable significance of the IFN-γ signature in response prediction of immune checkpoint inhibitors (ICIs), these data indicated that the angiogenesis pathway, but not the Wnt/β-catenin signaling pathway, was inversely related to the responsiveness to immunotherapy, particularly with inflamed immune niche, supporting further clinical investigations of targeting angiogenesis to improve clinical outcomes of immunotherapy by enhancing immune cell infiltration and ameliorating immune suppression.
Favorable survival predicted by angiogenesis and infiltration of activated CD8+ T cells
Higher infiltration of immune-activating cells, especially CD8+ TACTs, is usually associated with improved responsiveness to immunotherapy and always results in better prognosis.Citation28 Therefore, we compared the clinical outcomes between two cohorts grouped by the infiltration of CD8+ TACTs (ActCD8Hi and ActCD8Lo). Unexpectedly, the ActCD8Hi group did not show significantly prolonged survival compared with the ActCD8Lo group ( and Table S6), indicating that CD8+ TACTs infiltration might not be an effective predictor of prognosis in gastric cancer. We then checked the correlation between angiogenesis and prognosis. Interestingly, better prognoses in the ANGIOLo group were observed in both high and median infiltration subpopulations, but not in the low infiltration subpopulation ( and Table S6). These data suggested that angiogenesis participated as a negative predictor of prognoses in patients with gastric cancer, particularly in those with inflamed immune characterization.
Figure 4 Prognosis predictions by angiogenesis and infiltration of activated CD8+ T cells.
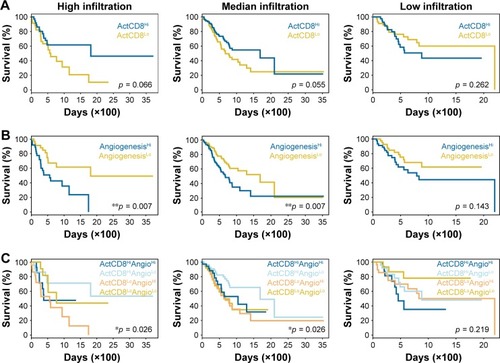
From findings, we hypothesized that lower angiogenesis and higher infiltration of CD8+ TACTs cells may be favorable for prolonging survival. To test this, cases were categorized into four groups (ActCD8HiAngioHi, ActCD8HiAngioLo, ActCD8LoAngioHi, and ActCD8LoAngioLo) based on the ssGSEA scores of CD8+ TACTs and angiogenesis. As for the high and median infiltration groups, a comparison of the prognoses of the four groups showed that ActCD8HiAngioLo patients exhibited better prognoses than patients in other groups ( and Table S6). On the contrary, in the low infiltration group, ActCD8HiAngioLo was not inclined to exhibit prolonged survival compared to other subpopulations and even showed inverse trends ( and Table S6). These data provided insights into further testing of immunotherapy/antiangiogenic combination therapy in the clinical setting.
Discussion
Major breakthroughs in immunotherapy have recently been made in various solid tumors, while several hurdles have been met in gastric cancer. Early clinical trials on tremelimumab only observed disappointing results for metastatic gastric adenocarcinomas.Citation29 Soon after, compelling data KEYNOTE-012,Citation3 KEYNOTE-028,Citation30 and KEYNOTE-059 studies,Citation13 with an ORR of 22%, 30.4%, and 13.3%, respectively. These exciting results made pembrolizumab the first third-line therapy for advanced gastric cancer approved by the US Food and Drug Administration (FDA). Another PD-1 antibody nivolumab also exhibited encouraging clinical activities.Citation31 Nevertheless, the results of monotherapy with ICIs in gastric cancer may not yet be as impressive as those seen in melanoma,Citation32 lung,Citation15 and kidney cancers.Citation14 The effective response to ICIs in patients with gastric cancer has been associated with high PD-L1 expression, microsatellite instability, and high IFN-γ signature score;Citation3,Citation13 these characteristics can only be found in a minor proportion of patients, which is a major limitation to providing clinical benefit to the entire population. Therefore, enhancing the efficacy of ICIs in gastric cancers has become an imperative issue.
The aforementioned characteristics are usually related to immune characterization in the TME, suggesting that ameliorating the TME may improve the ORR of ICIs. The constitution of TME is attributed to a multitude of tumor-intrinsic and tumor-extrinsic mechanisms, wherein angiogenesis plays an important role. Pathologic angiogenesis may influence antitumor immunity in the following ways: 1) impeding lymphocytic migration to tumor loci via hypoxia-induced downregulation of adhesion molecules;Citation33,Citation34 2) inducing immune tolerance and anergy by overexpression of immune-inhibitory molecules via endothelial cells in the tumor vasculature;Citation35,Citation36 3) directly regulating immune cells through VEGFA, HIF-1α, and other factors.Citation4,Citation37 In our study, the potent impact of angiogenesis on the TME of gastric cancers was validated by machine learning, using RNA sequencing data in a large cohort of gastric cancers from the TCGA database. The direct association between angiogenesis and immune infiltration was further demonstrated by correlation analysis. Our data highlighted the essential role of angiogenesis in determining TME immune characterization. Meanwhile, we also demonstrated the unessential role of Wnt/β-catenin signaling, different from discoveries in melanoma,Citation23 suggesting a tumor-type-dependent mechanism of tuning immune cell infiltration.
Clinical trials on antiangiogenic drugs, including ramucirumab and apatinib, have achieved encouraging outcomes.Citation6–Citation8 Considering the determinant role of angiogenesis in the immune niche, it is reasonable to believe that combination of immunotherapy and antiangiogenesis will result in a favorable prognosis. Such a treatment strategy is under investigation in other solid tumors and has verified the reinvigoration of immune infiltration, as well as impressively improved response.Citation38–Citation41 Our data also indicated the potential benefit of combination therapy for patients with gastric cancers, particularly for those with inflamed immune characterization.
Nevertheless, all conclusions herein were drawn at the sequencing level and thus are still theoretical; our observations require future validation by clinical investigations. In addition, prolonged survival of patients with low angiogenesis signaling and high CTL infiltration was not observed in the low infiltration group, implying that there may be other targets for patients with extremely impaired antitumor immunity. If potential mechanisms defining the prognoses of immune-inhibitory population are resolved clinically, there will be additional clinical benefits available for a greater number of patients.
Conclusion
Our study identified the distinct infiltration landscapes of gastric cancers, which were significantly associated with angiogenesis, the most targetable signaling pathway. The relationship between angiogenesis and the tumor immune microenvironment suggested that targeting angiogenesis may ameliorate the immune niche and provide a rationale for combination therapy and subsequent clinical investigations in gastric cancer.
Author contributions
QJ, YF, and BZ conceived and designed the experiments. YD, J-NC, and CS collected public data and directed the cohort studies. ZG, XZ, LZ, and YF wrote scripts for data analysis. YF and QJ analyzed and interpreted data. YF, QJ, and BZ wrote the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (grant number: 81222031).
Disclosure
The authors report no conflicts of interest in this work.
References
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365E359E38625220842
- GentzlerRHallRKunkPRBeyond melanoma: inhibiting the PD-1/PD-L1 pathway in solid tumorsImmunotherapy20168558360027140411
- MuroKChungHCShankaranVPembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trialLancet Oncol201617671772627157491
- VoronTColussiOMarcheteauEVEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumorsJ Exp Med2015212213914825601652
- OhtsuAShahMAVan CutsemEBevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III studyJ Clin Oncol201129303968397621844504
- FuchsCSTomasekJYongCJRamucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trialLancet20143839911313924094768
- WilkeHMuroKVan CutsemERamucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trialLancet Oncol201415111224123525240821
- LiJQinSXuJApatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trialJ Clin Oncol201331263219322523918952
- HanzelmannSCasteloRGuinneyJGSVA: gene set variation analysis for microarray and RNA-seq dataBMC Bioinformatics201314723323831
- BarbieDATamayoPBoehmJSSystematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1Nature2009462726910811219847166
- RooneyMSShuklaSAWuCJGetzGHacohenNMolecular and genetic properties of tumors associated with local immune cytolytic activityCell20151601–2486125594174
- MandalRSenbabaogluYDesrichardAThe head and neck cancer immune landscape and its immunotherapeutic implicationsJCI Insight2016117e8982927777979
- FuchsCSDoiTJangRW-JKEYNOTE-059 cohort 1: efficacy and safety of pembrolizumab (pembro) monotherapy in patients with previously treated advanced gastric cancerJ Clin Oncol20173515_suppl400329040031
- SharmaPRetzMSiefker-RadtkeANivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trialLancet Oncol201718331232228131785
- FehrenbacherLSpiraABallingerMAtezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trialLancet2016387100301837184626970723
- CharoentongPFinotelloFAngelovaMPan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockadeCell Rep201718124826228052254
- AngelovaMCharoentongPHacklHCharacterization of the immunophenotypes and antigenomes of colorectal cancers reveals distinct tumor escape mechanisms and novel targets for immunotherapyGenome Biol2015166425853550
- BreimanLRandom forestsMach Learn2001451532
- JiHHoughtonAMMarianiTJK-ras activation generates an inflammatory response in lung tumorsOncogene200625142105211216288213
- OkumuraTEricksenRETakaishiSK-ras mutation targeted to gastric tissue progenitor cells results in chronic inflammation, an altered microenvironment, and progression to intraepithelial neoplasiaCancer Res201070218435844520959488
- GaihaGDMcKimKJWoodsMDysfunctional HIV-specific CD8+ T cell proliferation is associated with increased caspase-8 activity and mediated by necroptosisImmunity20144161001101225526311
- Pereira-ManfroWFRibeiro-GomesFLFilardyAAInhibition of caspase-8 activity promotes protective Th1- and Th2-mediated immunity to Leishmania major infectionJ Leukoc Biol201495234735524072877
- SprangerSBaoRGajewskiTFMelanoma-intrinsic beta-catenin signalling prevents anti-tumour immunityNature2015523755923123525970248
- AllenEJabouilleARiveraLBCombined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formationSci Transl Med20179385eaak967928404866
- HendrySAFarnsworthRHSolomonBAchenMGStackerSAFoxSBThe role of the tumor vasculature in the host immune response: implications for therapeutic strategies targeting the tumor microenvironmentFront Immunol2016762128066431
- HorikawaNAbikoKMatsumuraNExpression of vascular endothelial growth factor in ovarian cancer inhibits tumor immunity through the accumulation of myeloid-derived suppressor cellsClin Cancer Res201723258759927401249
- StaalFJLuisTCTiemessenMMWNT signalling in the immune system: WNT is spreading its wingsNat Rev Immunol20088858159318617885
- HugoWZaretskyJMSunLGenomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanomaCell20161651354426997480
- RalphCElkordEBurtDJModulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinomaClin Cancer Res20101651662167220179239
- DoiTPiha-PaulSAJalalSIUpdated results for the advanced esophageal carcinoma cohort of the phase 1b KEYNOTE-028 study of pembrolizumabJ Clin Oncol20163415_suppl4046
- JanjigianYYBendellJCCalvoECheckMate-032: phase I/II, open-label study of safety and activity of nivolumab (nivo) alone or with ipilimumab (ipi) in advanced and metastatic (A/M) gastric cancer (GC)J Clin Oncol20163415_suppl4010
- RibasAHamidODaudAAssociation of pembrolizumab with tumor response and survival among patients with advanced melanomaJAMA2016315151600160927092830
- TrompSCoude EgbrinkMGDingsRPTumor angiogenesis factors reduce leukocyte adhesion in vivoInt Immunol200012567167610784613
- HorsmanMRVaupelPPathophysiological basis for the formation of the tumor microenvironmentFront Oncol201666627148472
- ChoiJEnisDRKohKPShiaoSLPoberJST lymphocyte-endothelial cell interactionsAnnu Rev Immunol20042268370915032593
- CarmanCVMartinelliRT lymphocyte-endothelial interactions: emerging understanding of trafficking and antigen-specific immunityFront Immunol2015660326635815
- FacciabeneAPengXHagemannISTumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cellsNature2011475735522623021753853
- HodiFSLawrenceDLezcanoCBevacizumab plus ipilimumab in patients with metastatic melanomaCancer Immunol Res20142763264224838938
- WuXGiobbie-HurderALiaoXVEGF neutralization plus CTLA-4 blockade alters soluble and cellular factors associated with enhancing lymphocyte infiltration and humoral recognition in melanomaCancer Immunol Res201641085886827549123
- WallinJJBendellJCFunkeRAtezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinomaNat Commun201671262427571927
- NakahamaKIsaSITamiyaAThe association between chemotherapy immediately before nivolumab and outcomes thereafterAnticancer Res201737105885589128982916
