Abstract
Metastatic breast cancer (MBC) remains an incurable disease, with the goals of care aimed at maximizing the patient’s duration and quality of life. Treatment options for MBC have become more efficacious and numerous. In addition to endocrine and chemotherapy agents, a number of targeted agents, including trastuzumab and bevacizumab, have further enhanced the landscape of therapeutic options. Eribulin mesylate (E7389) is a nontaxane microtubule dynamics inhibitor, and a structurally simplified synthetic analog of the natural marine product, halichondrin B, with a novel mechanism of action that has shown antitumor activity in pretreated MBC. Eribulin has shown a manageable tolerability profile in Phase I–II clinical trials and an improvement in overall survival compared with treatment of physician’s choice, without relevant toxicities in a recently published Phase III trial. This review will focus on eribulin as a new active agent for MBC and its role in the management of breast disease.
Introduction
Breast cancer is the most frequently diagnosed cancer worldwide and is also the second leading cause of death in women in the United States.Citation1 The diagnosis of breast cancer metastases without a history of early-stage disease is rare, and approximately 20% of patients with early breast cancer develop distant metastases within 5 years of the initial diagnosis.Citation1,Citation2 Despite improvements in the numerous chemotherapeutic agents that have been developed for the treatment of metastatic breast cancer (MBC), there is no standard of care for patients who have experienced failure with initial treatment.
Optimal treatment for patients with MBC is dependent upon the risks and benefits associated with each treatment option, as well as with the stage of disease and performance status of each patient. Anthracyclines and taxanes are increasingly used as (neo) adjuvant therapy, and therefore the number of patients previously exposed to these agents by the time they develop MBC is rising.Citation3 Current chemotherapeutic options for third-line or later treatment of MBC include the vinca alkaloids,Citation4,Citation5 gemcitabine,Citation6–Citation8 capecitabine,Citation9–Citation11 and ixabepilone,Citation12–Citation15 as well as new formulations of older drugs, such as liposomal anthracyclinesCitation16,Citation17 and nanoparticle albumin-bound paclitaxel.Citation18 Despite the large number of treatment options, the only approved monotherapies for late-line treatment of MBC are capecitabine and ixabepilone. Capecitabine has been approved in the United States and Europe for patients who are resistant to both taxane and anthracycline regimens, and for patients who experience taxane resistance or in whom anthracycline therapy is not indicated. On the other hand, ixabepilone is currently approved in the United States for use in combination with capecitabine in patients who do not respond to anthracyclines and taxanes, or as a single agent for patients who have failed on anthracyclines, taxanes, and capecitabine.Citation19–Citation22
The management of MBC is complex due to the absence of clear evidence-based guidelines for clinicians and the large number of clinical studies developed with several compounds. Moreover, because consecutive diverse therapeutic regimens are administered, there is an increased risk of different cumulative toxicities and development of drug resistance, limiting the current treatment options available. Despite these risks, overall survival in patients with MBC is increasing, and many patients with MBC still benefit from three or more lines of treatment.Citation23 Moreover, additional treatment options are needed for heavily pretreated MBC patients. Eribulin mesylate has emerged as a new option in the late-line setting. This review will focus on the current data for this new drug.
Antimicrotubule agents
Microtubules are polymers made from proteins called α- and β-tubulin and are part of the cytoskeleton within the cytoplasm of the cell. In addition to providing structural support, microtubules take part in many other cellular processes. During the early stages of mitosis, many microtubules increase in length by attachment of more tubulin dimers to one end, and grow out from the spindle for long distances (10 μm) into the cell, searching for an unattached chromosome. If none is found, the microtubule loses dimers and shrinks again. This expansion and retraction is repeated many times until eventually it meets and becomes chemically attached to a chromosome. When every chromosome has been captured by a microtubule, they are collected into the correct order and are then separated into two halves to divide the cell in two parts.Citation24,Citation25 With this division, apoptosis is induced.
The central role of antimicrotubular agents in the treatment of common epithelial cancers is further highlighted by their ability to induce remission in patients with classic drug-resistant epithelial cancers.Citation26 Taxanes, vinca alkaloids, and epothilones are all microtubule-targeted agents which bind to tubulin with varying affinities and target different binding sites, with subsequent disruption of microtubule dynamics. This disruption occurs during mitosis with the induction of G2/M phase cell-cycle arrest that eventually leads to cell death by apoptosis.Citation27,Citation28 Among these agents, there are microtubule-stabilizing (paclitaxel, nab- paclitaxel, docetaxel, and the epothilones, eg, ixabepilone) and microtubule-destabilizing drugs (vinca alkaloids, eg, vincristine, vinblastine, and vinorelbine).Citation29 However, current microtubule-targeted treatment is often limited by the development of drug resistance and common side effects,Citation27,Citation30 frequently based on high incidences of chronic peripheral sensory and motor neuropathy, with some studies reporting up to 20%–30% for patients experiencing grade 3/4 neuropathic symptoms.Citation31 Other common adverse events which impact upon quality of life in patients who receive these treatments are neutropenia and fatigue, and often result in dose modification or discontinuation of treatment.Citation31,Citation32
Eribulin
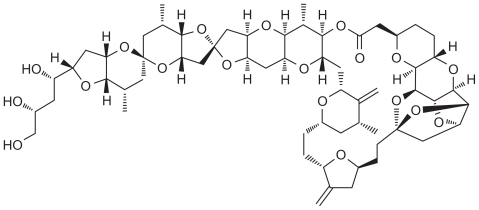
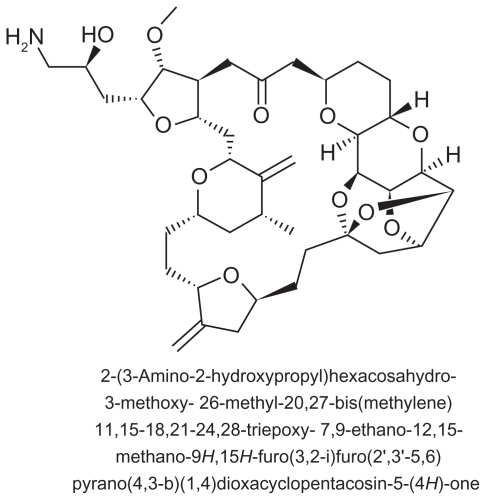
Eribulin mesylate (E7389) is a structurally simplified synthetic analog of the natural marine product, halichondrin B, a nontaxane microtubule dynamics inhibitor extracted from the marine sponge Halichondria okadai ( and ) which inhibits structures called microtubules via a novel mechanism of action. Eribulin works by binding to microtubule polymerization, without affecting depolymerization, and with the additional sequestration of tubulin into nonfunctional aggregates.Citation33 By inhibiting mitotic spindle formation, eribulin causes irreversible mitotic block, which leads to cell cycle arrest in the G2/M phase and apoptosis.Citation34,Citation35 Moreover, eribulin binds to a limited number of high affinity sites at the plus ends of the microtubules, and there is some evidence against its binding to interdimer interfaces in pre-existing polymers. This property distinguishes eribulin mechanistically from other antimicrotubule agents, such as paclitaxel, ixabepilone, and vinblastine.Citation34,Citation36,Citation37
Eribulin, which retains the potency of halichondrin B against human cancer cell lines, has a mean terminal half-life of 40 hours, and minimal renal excretion have been shown in preclinical studies.Citation38 Although it has been noted that this compound is metabolized by cytochrome P450 (CYP) 3A4, preclinical research established that it does not affect the metabolism of other therapeutic agents, such as diazepam, paclitaxel, midazolam, or tamoxifen, which are also metabolized by this system.Citation39 Eribulin has shown antiproliferative effects against a broad range of human cancer cell lines, including breast, prostate, melanoma, and colorectal cancer,Citation40 has been associated with tumor regression and elimination in a variety of well established human tumor xenograft models,Citation41 and has demonstrated activity against paclitaxel-resistant cell lines, including those with mutations in β-tubulin.Citation36,Citation42
Based on its novel mechanism of action, which is distinct from that of other known classes of tubulin-targeted agents, and its encouraging preclinical activity, eribulin was selected for evaluation in clinical trials. A comparison between eribulin and other antimicrotubule inhibitors is made in .
Table 1 Comparison between eribulin and other antimicrotubule agents active in MBC
Clinical efficacy and activity
Phase I trials
Four Phase I clinical trials have evaluated eribulin mesylate in various dose regimens in patients with different types of advanced solid tumors.Citation38,Citation43–Citation45 Briefly, in the weekly regimen studies, the maximum tolerated dose of eribulin was reported to be 1.4 mg/m2 and 1 mg/m2. Eribulin was administered on days 1, 8, and 15 of a 28-day cycle.Citation44,Citation45 On the other hand, a maximum tolerated dose of 2 mg/m2 was established on day 1 of a 21-day cycle schedule, and finally, dosing on days 1 and 8 of a 21-day cycle led to a maximum tolerated dose of 1.4 mg/m2.Citation38,Citation43
Interestingly, some activity was observed in these trials. In the study by Goel et al, a partial response was observed in one patient (3.1%) and stable disease was observed in 10 patients (31.3%).Citation44 Synold et al reported two partial responses (5.3%) and 12 patients (31.6%) experienced stable disease.Citation45 In addition, 12 patients (57.1%) showed stable disease in the study reported by Tan et al,Citation38 and Minami et al reported three patients with a partial response (20%) and four patients who achieved stable disease (26.7%).Citation43
The most commonly reported dose-limiting toxicity in all four Phase I trials for eribulin was neutropenia. Two dose-limiting toxicities were reported at 2.0 mg/m2 (grade III febrile neutropenia in one patient, and grade IV neutropenia in another patient). Other serious nonhematologic toxicities included hypoglycemia, hypophosphatemia, and fatigue. In the study by Goel et al, grade III fatigue was observed in one patient at 0.5 mg/m2 which led to the expansion of that cohort. At 1.4 mg/m2, three patients developed grade III/IV neutropenia, which was considered to be a dose-limiting toxicity based on the protocol criteria; febrile neutropenia developed in all three patients at 4.0 mg/m2, and two developed neutropenia at 2.8 mg/m2, which contributed to dose-limiting toxicity at these different doses.Citation44 In the study by Minami et al, dose-limiting grade IV neutropenia occurred in two of 15 patients (at 1.4 mg/m2 and 2.0 mg/m2, respectively), and grade III neutropenia occurred in four of 15 patients on the same dosing regimens.Citation43
A further Phase IB combination study of eribulin and carboplatin in patients with advanced solid tumors determined the maximum tolerated dose of eribulin to be 1.1 mg/m2 in combination with carboplatin (area under the curve 6 mg/dL/minute). The study reported a partial response in two patients (3.8%) and one complete response (1.9%).Citation46 Encouraging tumor response data from these four Phase I trials led to the initiation of Phase II studies in breast cancer patients, as well as in other types of solid tumors.
Phase II trials
Three Phase II studies of eribulin in patients with advanced breast cancer or MBC have already been completed. Patients who participated in these trials had received extensive pretreatments with a median of three or four (range 1–11) prior chemotherapy regimens.Citation47–Citation49 The first study was published by Vahdat et alCitation50 who investigated the efficacy and safety of eribulin in 87 evaluable patients with MBC who had received prior treatment with an anthracycline and taxanes. Based on the results of the previous Phase I study,Citation45 eribulin mesylate 1.4 mg/m2 was initially administered as a 2–5-minute intravenous infusion on days 1, 8, and 15 of a 28-day cycle. However, many patients experienced severe neutropenia on day 15 of the cycle (66% grade 3/4 in a 28-day cohort) and therefore the schedule was amended; eribulin mesylate was finally administered on days 1 and 8 of a 21-day cycle. In this study, eribulin demonstrated an objective response rate of 11.5% (95% confidence interval [CI], 5.7–20.1, all partial responses) and had a clinical benefit rate (partial response + stable disease for at least 6 months) of 17.2% (95% CI, 10.0–26.8).Citation49 The median duration of response, median progression-free survival, and median overall survival were 171 days (5.6 months; range 1.4–11.9), 79 days (2.6 months; range 0.03–14.9), and 275 days (9.0 months; range 0.5–27.2), respectively.Citation49 The most common drug-related grade 3/4 toxicities were neutropenia (64%), leucopenia (18%), and fatigue (5%).
In the second Phase II study, reported by Cortes et al, the patient population was based on 269 patients with locally advanced disease or MBC who had received prior treatment with anthracyclines, taxanes, and capecitabine. The patients received eribulin mesylate 1.4 mg/m2 as a 2–5-minute intravenous infusion on days 1 and 8 of a 21-day cycle. The primary endpoint of objective response rate by independent reviewer was 9.3% (95% CI, 6.1–13.4, all partial responses), the stable disease rate was 46.5%, and the clinical benefit rate (complete response + partial response + stable disease for at least 6 months) was 17.1%; the investigator-reported objective response rate for this study was 14.1% (95% CI, 10.2–18.9). The median duration of response was 4.2 months, with median reported progression-free survival and overall survival times of 2.6 months and 10.4 months, respectively. The most common treatment-related grade 3/4 toxicities were neutropenia (54%), leucopenia (14%), and asthenia/fatigue (10%, no grade 4 reported). Grade 3 peripheral neuropathy occurred in 5.5% of patients (no grade 4 was reported).Citation47
Finally, in the third Phase II trial, reported by Iwata et al,Citation49 the safety and efficacy of eribulin was investigated in 81 Japanese patients with advanced breast cancer who had previously been treated with an anthracycline and a taxane. This population study was less heavily pretreated than in the other two Phase II studies, with a median of only three prior treatments compared with four for the previously discussed two studies. The study implemented the same dosing regimen and mode of administration as that of the study by Cortés et alCitation51 due to the schedule amendment needed in the study of Vahdat et al.Citation50 The objective response rate by independent reviewer was 21.3% (all partial responses; 95% CI, 12.9–31.8) and the stable disease and clinical benefit rates were 37.5% and 27.5% (95% CI, 12.9–31.8), respectively. The median duration of response was 119 days (95% CI, 85–148 days), the progression-free survival was 112 days (95% CI, 61–133 days), and overall survival was 331 days (95% CI, 234, no upper limit determined due to shortage of events), respectively.Citation48
In all three Phase II studies, eribulin showed a manageable tolerability profile, with most of the common drug-related adverse events being neutropenia, fatigue, alopecia, nausea, and anemia ().Citation47–Citation49 Eribulin was also associated with a low incidence of peripheral neuropathy overall and severe peripheral neuropathy, which was limited to grade 3 only.Citation47–Citation49
Table 2 Summary of most common grade 3/4 treatment-related adverse events from Phase II studies of eribulin
Phase II trials
Following the encouraging pharmacokinetic and pharmacodynamic results observed in the Phase I trials and the response rates without severe adverse events observed in the Phase II trials in patients with extensively pretreated locally advanced or MBC, a Phase III trial lead to the approval of eribulin in the United States for the treatment of MBC in patients who have received at least two previous chemotherapeutic regimens, including an anthracycline and a taxane. EMBRACE (Eisai Metastatic Breast Cancer Study Assessing Physician’s Choice Versus E7389) randomized patients with locally recurrent disease or MBC previously treated with 2–5 prior chemotherapy regimens (including anthracyclines and taxanes) to eribulin or treatment of physicians’ choice (TPC).Citation50 Based on data obtained from the Phase II trials, eribulin mesylate was administered at a dose of 1.4 mg/m2 as a 2–5-minute intravenous infusion on days 1 and 8 of a 21-day cycle and compared with TPC, defined as a single-agent chemotherapy, hormonal therapy, or biological therapy approved for the treatment of cancer and administered according to local practice, radiotherapy, or symptomatic treatment alone. Treatment continued until disease progression, unacceptable toxic effects, a patient or physician request to discontinue, or serious protocol noncompliance. The primary endpoint of the study was to compare overall survival between the two treatment groups; secondary objectives were to compare progression-free survival, objective response rate, and duration of response. Of the patients who received TPC (254 of a total of 762 included in the study), 96% received chemotherapy and 4% received hormonal therapy, with no patients receiving biological therapy or best supportive care. Baseline demographic characteristics were well balanced across the treatment groups, as shown in . Most of the patients included in the study were heavily pretreated with a median of four previous chemotherapy regimens. The median duration of eribulin treatment was 3.9 months, and 295 patients (59%) received five or more eribulin cycles. The study reached its primary objective, with a statistically significant increase in overall survival (hazard ratio 0.81; 95% CI, 0.66–0.99; P = 0.004) in the eribulin group (13.1 months) compared with TPC group (10.6 months).Citation51 Median progression-free survival was 3.7 and 2.2 months (hazard ratio 0.87; 95% CI, 0.71–1.05; P = 0.14), for the eribulin and TPC groups, respectively. The objective response rate was 12% in the eribulin group and 5% in the TPC group (P = 0.005). Finally, median duration of response for eribulin was 4.2 months (95% CI, 3.8–5.0) and for TPC was 6.7 months (95% CI, 6.7–7.0; P = 0.159). Adverse events occurred in 497 (99%) of 503 patients receiving eribulin and 230 (93%) of 247 patients given TPC. Grade 3/4 adverse events associated with eribulin were asthenia/fatigue (8.2% grade 3; 0.6% grade 4), neutropenia, and peripheral neuropathy, demonstrating a manageable tolerability profile for this agent when given as monotherapyCitation51 (). Globally, neutropenia was the most common clinical grade 3 or 4 adverse event with eribulin (21.1% grade 3; 24.1% grade 4); neutropenia also occurred in the TPC subgroups treated with vinorelbine (30% grade 3; 10% grade 4), taxanes (13% grade 3; 16% grade 4), or gemcitabine (20% grade 3; 7% grade 4). It was managed with dose delays, dose reductions, and granulocyte colony-stimulating factor (given to 18% of patients in the eribulin group and 8% in the TPC group). The overall incidence of peripheral neuropathy on eribulin was 35% (7.8% grade 3; 0.4% grade 4), and was similar to that observed in the taxane subgroup (45% overall, 5% grade 3, no grade 4). Moreover, it was the most common adverse event leading to discontinuation of eribulin in 5% of patients, but in those patients with grade 3 or 4 peripheral neuropathy who discontinued treatment, neuropathy improved to grade 2 or lower in later cycles after delays and dose reductions.Citation50
Table 3 Patient baseline characteristics in EMBRACE
Table 4 Main grade 3/4 toxicities of eribulin in EMBRACE
Taken together, EMBRACE has shown a statistically significant improvement in its primary endpoint of overall survival by a median of 2.5 months with eribulin compared with TPC, and has also demonstrated a manageable tolerability profile in patients with heavily pretreated MBC. This survival benefit for eribulin over standard therapy in this setting is remarkable and contrasts with the failure of other agents to improve overall survival when added to chemotherapy in other clinical trials. Moreover, the design of EMBRACE clearly reflects real practice, with the second arm of the study based on TPC as the comparator, the possibility of which allows choice of best therapy based on a combination of patient-related and tumor-related characteristics.
A second Phase III study is underway to compare the efficacy and safety of eribulin mesylate (1.4 mg/m2 as a 2–5-minute intravenous infusion on days 1 and 8 of a 21-day cycle) with capecitabine. In this trial, 1100 patients have been randomized to receive eribulin or oral capecitabine on a 2500 mg/m2/day schedule in two divided doses on days 1–14 of a 21-day cycle.Citation52 This study has two primary endpoints, ie, progression-free survival and overall survival, and in contrast with EMBRACE, it contains important quality of life and pharmacokinetic correlates. It will also use the same eribulin dosing schedule as EMBRACE and will also focus on those patients with disease progression despite receiving anthracyclines and taxanes. However, this study has more restrictive inclusion criteria and patients are not permitted to have received capecitabine for more than two previous chemotherapeutic regimens for MBC. The study has already finished recruitment and is currently investigating the effect of these drugs in combination in less extensively treated patients with locally advanced or MBC who have received up to three prior chemotherapy regimens, including anthracyclines and taxanes. Moreover, this will be the first study to provide a full analysis of the impact of eribulin upon quality of life.Citation52
Conclusion
Eribulin is a novel nontaxane microtubule dynamics inhibitor with a novel mechanism of action distinct from those of other tubulin-targeting agents. In Phase II and III trials, it has demonstrated therapeutic activity in patients with solid tumors, particularly in heavily pretreated patients with MBC. Moreover, in the Phase III EMBRACE study it was shown to prolong overall survival in heavily pretreated MBC patients who received eribulin as monotherapy with manageable toxicity and a modest incidence of neuropathy, which appears to be lower than with other microtubule agents. Overall, eribulin represents a promising new treatment option for single-agent chemotherapy in patients with locally advanced disease or MBC previously treated with an anthracycline and a taxane.
Future perspectives
MBC is generally an incurable disease, with survival ranging from months to several years depending on tumor and patient characteristics. A wide range of treatment choices have been developed and are currently available, but most of them have not demonstrated an impact on survival in patients with MBC. Although currently there is no clear standard of care for these patients, important but modest improvements in overall survival have been observed for women with MBC. For women with endocrine-responsive disease, hormonal therapy is the appropriate initial treatment of choice at the time of disease recurrence. However, initiation of systemic chemotherapy is appropriate for women with metastatic disease that is either hormone receptor-negative, refractory to endocrine therapy, or rapidly progressive, with important visceral involvement regardless of hormonal status.Citation53 The addition of an anti-HER2 agent to chemotherapy for women with HER2-positive breast cancer represents a clear standard of care for this population, with an impact on survival in this group of patients.Citation54 Eribulin represents a new option for patients with heavily pretreated MBC and, due to the results of the clinical trials, it is likely to be partnered with other chemotherapy agents, anti-HER2 agents, and other drugs targeting important biologic pathways.
Eribulin has also demonstrated efficacy in heavily pretreated patients with MBC and a statistically significant improvement in survival in this group of patients. This encouraging efficacy, coupled with a manageable tolerability profile, has led to its approval by the US Food and Drug Administration and the European Agency for the Evaluation of Medicinal Products for the treatment of MBC in patients who have previously received chemotherapy including an anthracycline and a taxane. In addition, there are clinical trials underway to assess the antitumor activity of eribulin in the preoperative setting and also the earlier use of eribulin in the course of metastatic disease. It is hoped that these studies will translate the important survival advantage seen in the heavily pretreated refractory setting of the EMBRACE study into corresponding benefits for those patients with early-stage breast cancer. Moreover, a randomized Phase II study is comparing neuropathy associated with eribulin and with ixabepilone,Citation55 and there are other ongoing studies of eribulin in multiple types of solid tumors, with some data showing activity in urothelial cancer, prostate cancer, and sarcoma.
In summary, eribulin is the only drug that has shown a survival advantage in late lines of therapy for patients with metastatic breast cancer. The benefit that eribulin has shown as a single agent in this setting suggests that this drug could become a new standard of care for these patients. Future studies should explore whether survival with late lines of therapy are indicative of a more effective drug used earlier and in the (neo)adjuvant setting, and should look to establish the optimal use of eribulin.
Disclosure
The authors report no conflicts of interest in this work.
References
- JemalASiegelRXuJWardECancer statisticsCA Cancer J Clin201060527730020610543
- ArriagadaRSpielmannMKoscielnySPatterns of failure in a randomized trial of adjuvant chemotherapy in postmenopausal patients with early breast cancer treated with tamoxifenAnn Oncol20021391378138612196363
- RiveraEManagement of metastatic breast cancer: monotherapy options for patients resistant to anthracyclines and taxanesAm J Clin Oncol201033217618519675449
- FumoleauPDelgadoFMDelozierTPhase II trial of weekly intravenous vinorelbine in first-line advanced breast cancer chemotherapyJ Clin Oncol1993117124512528315421
- LivingstonRBEllisGKGralowJRDose-intensive vinorelbine with concurrent granulocyte colony-stimulating factor support in paclitaxel-refractory metastatic breast cancerJ Clin Oncol1997154139514009193331
- SmorenburgCHBontenbalMSeynaeveCPhase II study of weekly gemcitabine in patients with metastatic breast cancer relapsing or failing both an anthracycline and a taxaneBreast Cancer Res Treat2001661838711368414
- BlacksteinMVogelCLAmbinderRGemcitabine as first-line therapy in patients with metastatic breast cancer: a phase II trialOncology20026212811810037
- ModiSCurrieVESeidmanADA phase II trial of gemcitabine in patients with metastatic breast cancer previously treated with an anthracycline and taxaneClin Breast Cancer200561556015899073
- BlumJLJonesSEBuzdarAUMulticenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancerJ Clin Oncol199917248549310080589
- BlumJLDierasVLo RussoPMMulticenter, Phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patientsCancer20019271759176811745247
- OshaughnessyJABlumJMoiseyenkoVRandomized, open-label, phase II trial of oral capecitabine (Xeloda) vs a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancerAnn Oncol20011291247125411697835
- LowJAWedamDBLeeJJPhase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in metastatic and locally advanced breast cancerJ Clin Oncol200523122726273415837987
- ThomasETaberneroJFornierMPhase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in patients with taxane-resistant metastatic breast cancerJ Clin Oncol200725233399340617606975
- PerezEALerzoGPivotXEfficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabineJ Clin Oncol200725233407341417606974
- RocheHYelleLCognettiFPhase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, as first-line therapy in patients with metastatic breast cancer previously treated with anthracycline chemotherapyJ Clin Oncol200725233415342017606972
- BatistGHarrisLAzarniaNLeeLWDaza-RamirexPImproved anti-tumor response rate with decreased cardiotoxicity of non-pegylated liposomal doxorubicin compared with conventional doxorubicin in first-line treatment of metastatic breast cancer in patients who had received prior adjuvant doxorubicin: results of a retrospective analysisAnticancer Drugs200617558759516702817
- Al-BatranSEGuntnerMPauligkCAnthracycline rechallenge using pegylated liposomal doxorubicin in patients with metastatic breast cancer: a pooled analysis using individual data from four prospective trialsBr J Cancer2010103101518152320978502
- GradisharWJTjulandinSDavidsonNPhase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancerJ Clin Oncol200523317794780316172456
- CardosoFSenkus-KonefkaEFallowfieldLCostaACastiglioneMESMO Guidelines Working GroupLocally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-upAnn Oncol201021Suppl 5v15v1920555067
- HoJZhangLTodorovaLWhillansFCorey-LislePYuanYBudget impact analysis of ixabepilone used according to FDA approved labeling in treatment-resistant metastatic breast cancerJ Manag Care Pharm200915646747519610679
- SparanoJAVrdoljakERixeORandomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxaneJ Clin Oncol201028203256326320530276
- ThomasESGomezHLLiRKIxabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatmentJ Clin Oncol200725335210521717968020
- DufresneAPivotXTournigandCImpact of chemotherapy beyond the first line in patients with metastatic breast cancerBreast Cancer Res Treat2008107227527917380382
- JordanMAKamathKMannaTThe primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growthMol Cancer Ther2005471086109516020666
- JordanMAWalkerDde ArrudaMSuppression of microtubule dynamics by binding of cemadotin to tubulin: possible mechanism for its antitumor actionBiochemistry1998375017571175789860873
- MillerRKMatheosDRoseMDThe cortical localization of the microtubule orientation protein, Kar9p, is dependent upon actin and proteins required for polarizationJ Cell Biol1999144596397510085294
- PasquierEKavallarisMMicrotubules: a dynamic target in cancer therapyIUBMB Life200860316517018380008
- JordanMAKamathKHow do microtubule-targeted drugs work? An overviewCurr Cancer Drug Targets20077873074218220533
- HigaGMThe microtubule as a breast cancer targetBreast Cancer201118210311920862571
- KavallarisMAnnereauJPBarretJMPotential mechanisms of resistance to microtubule inhibitorsSemin Oncol2008353 Suppl 3S22S2718538175
- SwainSMArezzoJCNeuropathy associated with microtubule inhibitors: diagnosis, incidence, and managementClin Adv Hematol Oncol20086645546718567992
- LeeJJSwainSMPeripheral neuropathy induced by microtubule-stabilizing agentsJ Clin Oncol200624101633164216575015
- JimenoAEribulin: rediscovering tubulin as an anticancer targetClin Cancer Res200915123903390519509144
- DabydeenDABurnettJCBaiRComparison of the activities of the truncated halichondrin B analog NSC 707389 (E7389) with those of the parent compound and a proposed binding site on tubulinMol Pharmacol20067061866187516940412
- SmithJAWilsonJAzarenkoOEribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instabilityBiochemistry20104961331133720030375
- OkounevaTAzarenkoOWilsonLInhibition of centromere dynamics by eribulin (E7389) during mitotic metaphaseMol Cancer Ther2008772003201118645010
- HuyckTKGradisharWManuguidFKirkpatrickPEribulin mesylateNat rev Drug Discov201110317317421358731
- TanARRubinEHWaltonDCPhase I study of eribulin mesylate administered once every 21 days in patients with advanced solid tumorsClin Cancer Res200915124213421919509146
- ZhangZYKingBMPelletierRDWongYNDelineation of the interactions between the chemotherapeutic agent eribulin mesylate (E7389) and human CYP3A4Cancer Chemother Pharmacol200862470771618431572
- HamelENatural products which interact with tubulin in the vinca domain: maytansine, rhizoxin, phomopsin A, dolastatins 10 and 15 and halichondrin BPharmacol Ther199255131511287674
- TowleMJSalvatoKABudrowJIn vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin BCancer Res20016131013102111221827
- NewmanSEribulin, a simplified ketone analog of the tubulin inhibitor halichondrin B, for the potential treatment of cancerCurr Opin Investig Drugs200781210571066
- MinamiHMNagaiSMukaiHNamikiMA phase I study of eribulin mesylate (E7389) in patients with refractory cancersEur J Cancer2008Suppl 6140 abstract 446
- GoelSMitaACMitaMA phase I study of eribulin mesylate (E7389), a mechanistically novel inhibitor of microtubule dynamics, in patients with advanced solid malignanciesClin Cancer Res200915124207421219509177
- SynoldTWMorganRJNewmanEMA phase I pharmacokinetic and target validation study of the novel anti-tubulin agent E7389: a California Cancer consortium trialJ Clin Oncol20052316S3036
- SwamiUPetrylakDPRaftopoulosHPhase IB study of eribuin mesylate in combination with carboplatin inpatients with advanced solid tumorsJ Clin Oncol20102815s
- CortesJVahdatLBlumJLPhase II study of the halichondrin B analog eribulin mesylate in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline, a taxane, and capecitabineJ Clin Oncol201028253922392820679609
- IwataHAogiKMasudaNEfficacy and safety of eribulin in Japanese patients (pts) with advanced breast cancerJ Clin Oncol201028151081
- VahdatLTPruittBFabianCJPhase II study of eribulin mesylate, a halichondrin B analog, in patients with metastatic breast cancer previously treated with an anthracycline and a taxaneJ Clin Oncol200927182954296119349550
- CortesJO’ShaughnessyJLoeschDEribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised studyLancet2011377976991492321376385
- TwelvesCLoeschDBlumJLA phase III study (EMBRACE) of eribulin mesylate versus treatment of physicians’ choice in patients with locally recurrent or metastatic breast cancer previously treated with an anthracycline and a taxaneJ Clin Oncol20102818s
- TwelvesCCortesJVahdatLTPhase III trials of eribulin mesylate (E7389) in extensively pretreated patients with locally recurrent or metastatic breast cancerClin Breast Cancer201010216016320299316
- MorrisPGMcArthurHLHudisCATherapeutic options for metastatic breast cancerExpert Opin Pharmacother200910696798119351274
- MurphyCGFornierMHER2-positive breast cancer: beyond trastuzumabOncology (Williston Park)201024541041520480738
- Study Comparig Eribulin Mesylate and Ixabepilone in Causing or Exacerbating Neuropathy in Patients With Advanced Breast Cancer. NCT00879086 Available from: http://clinicaltrials.gov/ct2/show/NCT00879086Accessed October 11, 2010