Abstract
Background
Alternative polyadenylation (APA) is an important post-transcriptional regulation in eukaryotic cells. It plays considerable roles in many biological processes and diseases, such as cell differentiation, proliferation and cancer. Colorectal cancer (CRC) is one of the most common malignancies worldwide, which is among the top five in incidence and mortality of all cancers in China. Although there have been some studies on the APA of CRC, the normal and carcinoma samples used for genome-wide profiling were not matched. The purpose of this study was to obtain genes with switched 3′-untranslated region (UTR) that may be associated with intracellular regulation of CRC by analyzing APA patterns of strict control groups from clinical patients.
Materials and methods
CRC and matched normal tissues were acquired from surgical specimens from three CRC patients. Their libraries of 3′-terminal fragments of mRNA with poly(A) tails were constructed by 3T-seq technology and sequenced by Illumina Hiseq X Ten. APA patterns of cancer and matched normal tissues were analyzed by bioinformatics analysis, and a representative gene, GPI, was verified by quantitative reverse transcription PCR.
Results
Overall, we identified 35,076 poly(A) sites in total. Compared to the matched normal tissues, we detected 350, 405 and 375 genes with significantly APA-mediated 3′-UTR alteration in cancer tissues of three patients, respectively. Forty-seven genes with switched 3′-UTR were shared in all three patients. In addition, most of these genes have shortened 3′-UTRs, some of which were associated with cancers, such as GPI.
Conclusion
Our studies found several genes with switched 3′-UTR in CRC patients, which may provide some important clues for more in-depth study of the cellular regulation in CRC from the perspective of post-transcriptional regulation. It may also help in the search for new biomarkers of CRC.
Introduction
Almost all eukaryotic pre-messenger RNAs (pre-mRNAs) and a portion of noncoding transcripts have poly(A) sites (PAS) and are polyadenylated.Citation1–Citation3 Recent discoveries have found that more than two-thirds of all genes have multiple PAS,Citation1 which means that alternative polyadenylation (APA) can take place. APA can be typically divided into the following two categories: untranslated region (UTR)-APA and coding region (CR)-APA.Citation4 APA is extensively used to regulate gene expression by producing transcript isoforms with diverse 3′-UTRs. Alternative 3′-UTR may affect stability, cellular localization and translation efficiency of transcript isoforms, even if the encoded proteins have the same sequences.Citation5,Citation6 Recent studies have shown that APA alterations are displayed in a series of biological processes, such as development, cell differentiation, proliferation, neuron activity and cancer.Citation7
A study has shown that ~70% of protein-coding genes have conserved microRNA (miRNA) target sites within their 3′-UTRs.Citation1 During transformation, the cell starts using the PAS most proximal to the open reading frame (ORF) to generate a short 3′-UTR, which makes the mRNA resistant to miRNA by eliminating miRNA-binding sites.Citation8 A recent study reported a widespread preferential usage of proximal PAS in cancers, such as breast, lung, liver and colorectal cancers (CRCs),Citation2 even if the role of APA in transformation and cancer is still not very clear.
CRC, which is one of the most common malignancies worldwide, remains among the top five in incidence and mortality of all cancers in China, even though it is not a high prevalence area in comparison with Western Europe and North America.Citation9 Although Morris et alCitation10 have studied APA in CRC patients, their normal and carcinoma samples in the high-throughput sequencing data used for analysis were not matched. The establishment of the control group in that study was not sufficiently rigorous, which may influence the accuracy of APA sites obtained through high-throughput sequencing.
In this study, we used a previous published method, the 3T-seq technology, to profile genome-wide APA sites in CRC patients to analyze the effects of this kind of post-transcriptional modification on CRC. Comparisons between cancer samples and the matched normal samples help to understand the role of APA in clinical patients.
Materials and methods
Collection of human tissue samples
Fresh tissue samples from three CRC patients were collected at the Anhui Medical University, First Affiliated Hospital, Anhui, China. This study plan was approved by the institutional review boards of Anhui Medical University and was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). All patients had signed an informed consent form. All samples were examined by one experienced pathologist, and clinical information of all individuals are listed in . After being collected, the samples were put in liquid nitrogen quickly and preserved in low temperature environment. TRIzol (Thermo Fisher Scientific, Waltham, MA, USA) was used to isolate total RNA from tissue samples following the manufacturer’s protocol. Total RNA quantity was determined with Nanodrop 2000 (Thermo Fisher Scientific), and quality was assessed by running a 1% agarose gel electrophoresis, stained with 4S Red Plus Nucleic Acid Stain (Sango, Shanghai, China).
Table 1 Pathology information of clinical samples
3T-seq library preparation
The libraries were prepared following the previous published method.Citation11 In general, after being extracted, ~50 µg of total RNA was incubated with Dynabeads™ M-280 streptavidin (Thermo Fisher Scientific) with biotin-modified reverse transcription GsuI-Oligo (dT)20 primers. Catalyzed by Super Script III (Thermo Fisher Scientific), the first strand was synthesized, in which 5-methylated-dCTP had replaced dCTP to prevent GsuI from cutting the new synthetic chain. When the second strand of cDNA chain was synthesized, dNTP mixture contained dUTP instead of dTTP. Then, cDNA was randomly fragmented to ~200–400 bp with Fragmentase (NEB, Ipswich, MA, USA). After that 3′-terminal fragments were released from beads by GsuI (Fermentas, Waltham, MA, USA) digestion. Next, Illumina p5/p7 adaptors were ligated to the released cDNA. Before PCR amplification, the second strand of cDNA which had dUTP was digested by USER (NEB) to achieve chain specificity sequencing. Finally, a series of 3T-seq libraries were sequenced by Illumina Hiseq X Ten. Raw sequence data have been submitted to the EMBL-EBI ArrayExpress (accession number: E-MTAB-6403).
Data analysis
The data analysis process is similar to our previous study.Citation11 Briefly, raw reads were filtered with custom C++ scripts to obtain usable and valid reads. The selected reads were then mapped to the UCSC human reference genome (hg19) with bowtie2.Citation12 The PAS were identified through iteratively clustering the neighbor sites. The linear trend test was employed to identify the genes with significantly switched 3′-UTR. The function annotation was performed with the online tool DAVID (https://david.ncifcrf.gov).
Quantitative reverse transcription PCR validation
One microgram of total RNA was reverse transcripted by using PrimeScript™ RT reagent kit (TAKARA, Kyoto, Japan). RT primer mixture used in reverse transcription was a mixture of oligo dT and random hexamer primers. qRT-PCR was performed with PowerUp SYBR Green Master Mix (Thermo Fisher Scientific). Reactions were run in triplicate and normalized against ACTB. Primers design and data analysis were based on those in a previous study.Citation13 Primer sequences are shown in .
Table 2 Primer sequence of qRT-PCR
Results
Identification of polyadenylation sites
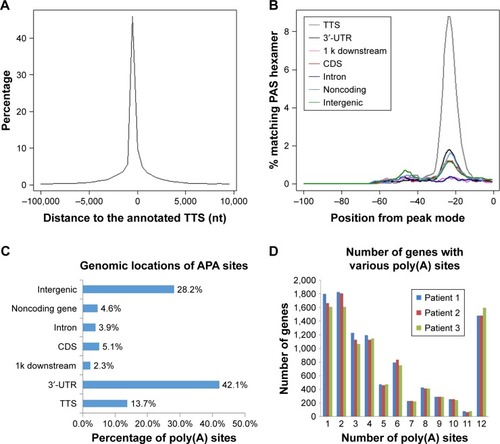
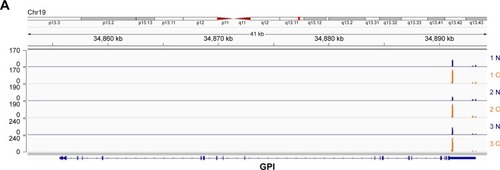
After total RNA was extracted, we constructed libraries for sequencing by using the 3T-seq technology.Citation11 The 3′-terminal fragments were subjected to deep sequencing by Illumina Hiseq X Ten. Data from high-throughput sequencing were exhibited on Integrative Genomics Viewer (IGV) software (). Totally, we generated ~107 million reads, of which ~52% are uniquely mappable (). Possible polyadenylation sites, whose downstream 20 nt bases included continuous 8 or 12 or more adenine ribonucleic acids, were identified as internal priming and filtered out.Citation14 Approximately 43 million reads passed internal priming filter, and most of them were concentrated near the annotated transcription termination sites (TTSs) (). Identification method of PAS was based on that in a previous study;Citation15 totally, we identified 34,982 PAS in patient 1, 34,187 PAS in patient 2 and 35,303 PAS in patient 3. We examined the presence of a hexamer motif such as AAUAAA within 20–30 nt upstream of the identified sites and reported that motifs could be found in this interval (). For example, among the 34,982 identified PAS in patient 1, 13.7% were mapped to UCSC TTS and 42.1% to 3′-UTR regions (). Among expressed genes detected in this study, an average of 64.9% genes had three or more PAS in three patients ().
Table 3 Summary statistics of sequencing data from Illumina Hiseq X Ten
Figure 1 A genomic view of APA sites defined by 3T-seq in IGV genome browser.
Abbreviations: APA, alternative polyadenylation; IGV, Integrative Genomics Viewer.
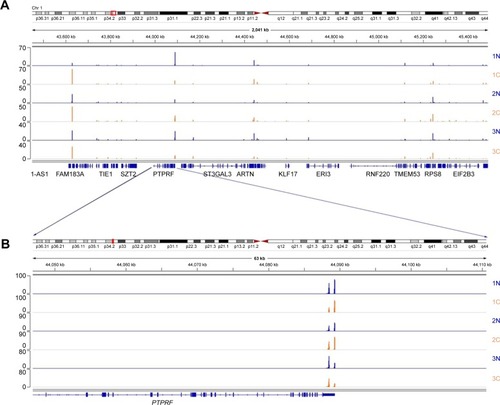
Figure 2 Characterizations and comparative analyses of APA sites in these clinical samples.
Abbreviations: APA, alternative polyadenylation; CULI, cancer 3′-UTR length index; PAS, poly(A) sites; TTS, transcription termination sites.
Variation analysis of APA between clinical samples
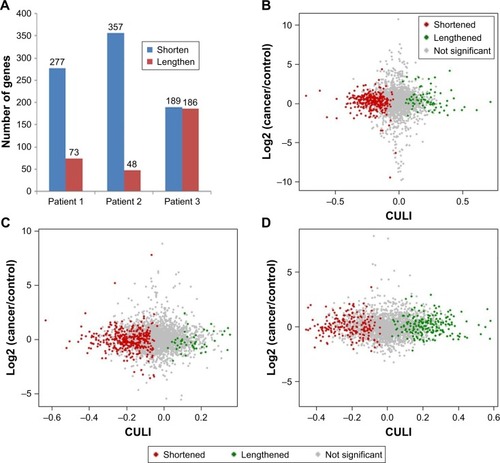
To facilitate comparison between samples, the cancer 3′-UTR length index (CULI) was adopted to quantitatively characterize the 3′-UTR alteration in CRC patients.Citation15 A positive CULI means that a gene harbors lengthened 3′-UTR in cancer tissues compared with their corresponding normal tissues, and a negative CULI suggests the shortened one (Table S1). With this standard, the number of genes was identified with a significant difference in 3′-UTR length between cancer tissues and their corresponding normal tissues. In patient 1, the number was 350, and in patients 2 and 3, the numbers were 405 and 375, respectively (). In patient 1, 79.1% genes in cancer tissues had shortened 3′-UTR, while in other patients, the percentage became 88.1% (in patient 2) and 50.4% (in patient 3). To obtain the relationship between APA alteration and gene expression, we compared the APA change with mRNA abundance, and the result showed that there was no linear correlation on transcriptome scale ().
Figure 3 APA-mediated 3′-UTR alteration and the transcriptional activity of the affected genes in CRC patients compared with normal counterparts.
Abbreviations: APA, alternative polyadenylation; CRC, colorectal cancer; CULI, cancer 3′-UTR length index; FDR, false discovery rate; UTR, untranslated region.
Functional enrichment of genes with switched APA sites
To understand the biological consequences of altered patterns in clinical CRC patients, genes with shortened 3′-UTR were analyzed by DAVID (https://david.cifcrf.gov). Analysis of 277 genes with 3′-UTR shortening in patient 1 yielded proliferation-related biological processes that were statistically overrepresented, including metabolic process, mRNA processing and RNA splicing ( and Table S2). Similar results were also observed in other two patients ( and Table S2).
Genes associated with cancer preferentially use proximal APA in cancer samples
Because shortened 3′-UTR increased the stability of the mRNA and meanwhile reduced cis elements that can interact with the transcription regulation of trans function factor (such as RNA binding protein) or miRNA interaction, these genes were more likely to escape gene silencing induced by miRNA, which then leads to higher expression level.Citation5 Based on this, genes that had switched APA patterns between cancer tissues and their corresponding normal tissues in three patients were further analyzed. Especially, some of them had a tendency to preferentially use proximal APA and higher expression level in cancer tissues.
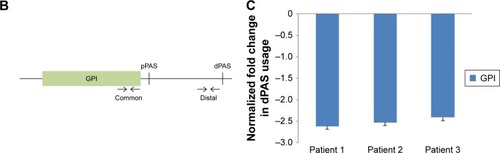
There were 35 genes with shortened 3′-UTR present in all three patients. Gene ontology analysis showed that most of them are related to the metabolic process (Table S3). GPI, which was one of them, showed increased expression in cancer tissues in all three patients (). qRT-PCR was used to prove the shortening of 3′-UTR. The design of primers was based on a previous research, common primers targeted the ORF, and distal primers were located just before the distal PAS ().Citation13 The relative use of the distal PAS was calculated, and genes preferentially used proximal APA in cancer tissues when the value was negative. Taking GPI as an example, qRT-PCR results showed that it tended to use proximal PAS in the cancer tissues of three patients ().
Figure 5 GPI preferentially use proximal APA in cancer samples.
Abbreviations: APA, alternative polyadenylation; CRC, colorectal cancer; GPI, glucose-6-phosphate isomerase; PAS, poly(A) sites; UTR, untranslated region.
Discussion
With an increasing number of studies reported, APA events are involved in various biological processes.Citation7 APA can affect mRNA stability, translation and localization. The shortening of the 3′-UTR can eliminate miRNA-binding sites, which can be found in longer 3′-UTR and usually result in the escape of miRNA-regulated programmed cell death.Citation22 The APA events in the whole genome, which may have a major impact on mechanisms of tumorigenesis and antitumor, can be investigated by means of genome-wide analyses. Through comparison of cancer tissue samples and their corresponding normal tissue samples, APA events in cancer tissue samples were found to have significant differences with APA patterns of normal tissue samples. There were 350 genes that have changed 3′-UTR in a cancer tissue sample of patient 1, and 79.1% of them had shortened 3′-UTR. The number of genes in the other two patients was 405 and 375, respectively, and 3′-UTR shortened genes accounted for 88.1% and 50.4%, respectively (). It was interesting to note that the proportion of genes with shortened 3′-UTR seemed to be consistent with the disease stage. However, cases were too few to draw from this conclusion.
3′-UTR shortening could increase gene expression level by eliminating miRNA-binding sites.Citation22 However, in our data, genes with shortened 3′-UTR did not always have higher expression level. In patient 1, 180 of 277 genes had shortened 3′-UTR and higher level of expression in the cancer tissue. In patient 2, the number became 168 of 357, and it became 83 of 189 in patient 3. This suggested that the length and expression of 3′-UTR were not simply negatively correlated. In addition, similar phenomenon has been found in a previous study.Citation15 The mechanism of the reduction of 3′-UTR to the expression level has not been fully understood.
Cancer tissues in three patients all preferentially used proximal APA of GPI. GPI, alternatively known as PGI or PHI, has been identified as the autocrine motility factor (AMF), which can regulate tumor cell growth and stimulate metastasis.Citation16–Citation18 Overexpression of AMF has been shown to induce epithelial-to-mesenchymal transition (EMT) in some cancers.Citation19,Citation20 Elevated serum GPI levels have been used as a prognostic biomarker for various cancers, including CRC.Citation21 Nevertheless, 3′-UTR-shortened genes only partially overlapped in three patients. This may be due to the heterogeneity of individuals. When the number of samples increases, the heterogeneity may be more obvious. In recent studies, Morris et alCitation10 also found a series of genes with changed PAS in CRC, some of which were overlapped with our results. However, 3T-seq was more focused on 3′-UTR PAS, and relative to 3′seq when paired-end sequencing was used; this method largely avoided sequencing desynchronization. More importantly, the normal control that Morris et alCitation10 used in high-throughput sequencing was not sufficiently rigorous.
Conclusion
Briefly, in this study we used a robust approach, 3T-seq, to profile global APA sites in three patients and observed that hundreds of genes exhibit shortened 3′-UTR, and some of them have been reported to play a key role in cancer. Comparative results provide some clues for more in-depth study of the cell regulation mechanism of CRC from post-transcriptional regulation.
Acknowledgments
This work was supported by the Development Program for Basic Research of China (2014YQ09070904), National Natural Science Foundation of China (31671299), Shanghai Science and Technology Committee Program (17JC1400804), Medical Engineering Cross Fund (YG2017ZD15 and YG2015QN35) and Laboratory Innovative Research Program of Shanghai Jiao Tong University (17SJ-18).
Disclosure
The authors report no conflicts of interest in this work.
References
- DertiAGarrett-EngelePMacisaacKDA quantitative atlas of polyadenylation in five mammalsGenome Res20122261173118322454233
- LinYLiZOzsolakFAn in-depth map of polyadenylation sites in cancerNucleic Acids Res201240178460847122753024
- ShepardPJChoiEALuJFlanaganLAHertelKJShiYComplex and dynamic landscape of RNA polyadenylation revealed by PAS-SeqRNA201117476177221343387
- RehfeldAPlassMKroghAFriis-HansenLAlterations in polyadenylation and its implications for endocrine diseaseFront Endocrinol2013453
- SandbergRNeilsonJRSarmaASharpPABurgeCBProliferating cells express mRNAs with shortened 3′ untranslated regions and fewer microRNA target sitesScience (New York, N. Y.)2008320588316431647
- JiZLeeJYPanZJiangBTianBProgressive lengthening of 3′ untranslated regions of mRNAs by alternative polyadenylation during mouse embryonic developmentProc Natl Acad Sci U S A2009106177028703319372383
- ElkonRUgaldeAPAgamiRAlternative cleavage and polyadenylation: extent, regulation and functionNat Rev Genet201314749650623774734
- MasamhaCPWagnerEJThe contribution of alternative polyadenylation to the cancer phenotypeCarcinogenesis2017391210
- ChenWZhengRBaadePDCancer statistics in China, 2015CA: a cancer journal for clinicians3201666211513226808342
- MorrisARBosADiosdadoBAlternative cleavage and polyadenylation during colorectal cancer developmentClin Cancer Res201218195256526622874640
- LaiDPTanSKangYNGenome-wide profiling of polyadenylation sites reveals a link between selective polyadenylation and cancer metastasisHum Mol Genet201524123410341725759468
- LangmeadBTrapnellCPopMSalzbergSLUltrafast and memory-efficient alignment of short DNA sequences to the human genomeGenome Biol2009103R2519261174
- MasamhaCPXiaZYangJCFIm25 links alternative polyadenylation to glioblastoma tumour suppressionNature2014510750541241624814343
- BeaudoingEFreierSWyattJRClaverieJMGautheretDPatterns of variant polyadenylation signal usage in human genesGenome Res20001071001101010899149
- FuYSunYLiYDifferential genome-wide profiling of tandem 3′ UTRs among human breast cancer and normal cells by high- throughput sequencingGenome Res201121574174721474764
- StokerMGherardiEPerrymanMGrayJScatter factor is a fibroblast-derived modulator of epithelial cell mobilityNature198732761192392422952888
- WatanabeHTakehanaKDateMShinozakiTRazATumor cell autocrine motility factor is the neuroleukin/phosphohexose isomerase polypeptideCancer Res19965613296029638674049
- SillettiSRazAAutocrine motility factor is a growth factorBiochem Biophys Res Commun199319414464578392842
- TsutsumiSHoganVNabiIRRazAOverexpression of the autocrine motility factor/phosphoglucose isomerase induces transformation and survival of NIH-3T3 fibroblastsCancer Res200363124224912517804
- LiYCheQBianYAutocrine motility factor promotes epithelial-mesenchymal transition in endometrial cancer via MAPK signaling pathwayInt J Oncol20154731017102426201353
- BaumannMBrandKMatthias BaumannKBPurification and characterization of phosphohexose isomerase from human gastrointestinal carcinoma and its potential relationship to neuroleukinCancer Res19884824 Pt 1701870213191476
- MayrCBartelDPWidespread shortening of 3′UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cellsCell2009138467368419703394