Abstract
Background
Immunosenescence, the age-related decline of immunity, affects the immune responses of melanoma patients. Through immune responses, immune checkpoint inhibitors (ICIs) exert their antitumor robustness. In different ages of melanoma patients, especially the older patients, the effectiveness of ICIs remains unclear. It is still controversial whether ICIs should be used in treating older patients.
Materials and methods
The authors included clinical trials of ICIs in older and younger patients. The authors used hazard ratio (HR) and 95% CI of overall survival (OS).
Results
From four phase III randomized clinical trials 2,251 melanoma patients were included. We found that ICIs significantly prolonged the OS for melanoma patients in both younger (HR, 0.71; 95% CI, 0.60–0.82; P<0.001) and older groups (HR, 0.62; 95% CI, 0.41–0.83; P<0.001) compared with controls. Anti-programmed death-1 (PD-1) agents appeared to be more efficient in older melanoma patients (HR, 0.34; 95% CI, 0.14–0.53) versus younger patients (HR, 0.52; 95% CI, 0.26–0.78).
Conclusion
ICIs significantly prolonged the OS for melanoma patients in both younger and older groups than controls. Anti-PD-1 agents were more efficient in older melanoma patients versus younger patients. ICIs could be used for older melanoma patients.
Introduction
Immunosenescence is a phenomenon of gradual deterioration of the immune system with increasing age.Citation1 It involves both the host’s capacity to respond to infections and the development of long-term immune memory. It was observed that immune status altered in aged people,Citation1 and immunosenescence even remodel the immune system, hence inducing numbers of diseases.Citation2 Previous researches manifested that immunosenescence can change the adaptive immune response and induce neuroinflammatory diseases.Citation3 Aging of hematopoietic stem cell compartment, alteration of antigen-presenting cell functions, and reduced antitumor ability of T cells were all associated with increasing age.Citation4 Immunosenescence may cause tissue and cell damages and immunosurvey escape, thereby resulting in tumor development. However, in many fields, the impact of immunosenescence on the efficacy of some drugs still remains unclear.
Immune checkpoint inhibitors (ICIs), including programmed death-1 (PD-1)/PD-L1 inhibitors and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibitors, are novel antitumor drugs that have brought hope to cancer patients, especially the non-small cell lung cancer (NSCLC) patients and melanoma patients.Citation5,Citation6 The monotherapy and combination immunotherapy of ICIs have been applied in the clinical practice.Citation7 They were proved to be more efficient and generate less toxicity in cancer treatment, making them a very attractive therapeutic option.Citation8 It is currently well accepted by the clinicians that overall response rate, overall survival (OS), and progression-free survival (PFS) can be significantly improved in melanoma patients.Citation8 Several clinical trials have observed the impact of immunosenescence on the effectiveness of ICIs. Aged patients may benefit less from the PD-1 inhibitors and CTLA-4 inhibitors in certain cancers.Citation4
Two groups have reported that increasing age can influence the efficacy of PD-1 inhibitors in lung cancer treatment.Citation9 However, among melanoma patients belonging to different age groups, it still remains unclear whether CTLA-4 or PD-1 inhibitors are recommended. This topic is of importance considering the efficacy of ICI treatment and the increasing number of ICI application in daily clinic life. Differences in response to ICI in younger and older patients would have serious influence on clinical applicability, therapy recommendations, and treatment guidelines. We hypothesize that effectiveness of ICIs in melanoma patients may vary in different age groups. Hence, based on previous clinical trials, we conducted a meta-analysis that was particularly focused on melanoma patients belonging to different age groups.
Materials and methods
Study design and search strategy
This meta-analysis was based on data from randomized clinical trials (RCTs), which compared ICIs with controls in younger and older melanoma patients. We searched for trials on PubMed/Medline, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), and Google Scholar; January 2018 was the cutoff date. Keywords included ICI, age, and clinical trial. All the studies of potential interest were retrieved.
Selection criteria
Eligible studies should fulfill the following criteria: 1) trials that included patients with melanoma, 2) interventions of ICIs, 3) trials that compared ICIs and controls, 4) trials that reported hazard ratios (HRs) of OS or PFS and subgroup analysis by age, and 5) phase III randomized clinical trials. All the papers in non-English language were excluded.
Data extraction
Two reviewers (PL and LS) independently extracted data by using an extraction form. The two reviewers checked all the data carefully. All studies were identified by first author and the year of publication. Information from the included studies was extracted after review, including publication year, inclusion/exclusion criteria, patient numbers, treatment in the experimental and control cohort, and HR of OS in younger and older groups.
Risk of bias assessment
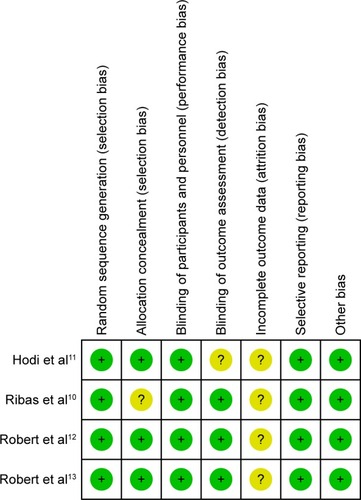
Two reviewers (PL and LS) independently assessed the risk of bias using the Cochrane Collaboration tool. All disagreements were resolved by the first author (PL). Details on the risk of bias in eight studies are illustrated in .
Statistical analysis
Statistical analysis, forest plots, and detection of publication bias were done with Stata SE (StataCorp, College Station, TX, USA). Risk of bias analysis was conducted using REVMAN (RevMan5.3; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). HRs were used for evaluation and 95% CIs were calculated for each estimate. A cutoff P-value of 0.05 was considered statistically significant. Heterogeneity was considered low, moderate, or high for ICitation2<25%, 25%–50%, and >50%, respectively. If the ICitation2 value was lower than 50%, a fixed model would be used; if the ICitation2 value was more than 50%, a random model would be used. Publication bias was assessed by both Begg’s test and Egger’s test.
Results
Patient characteristics
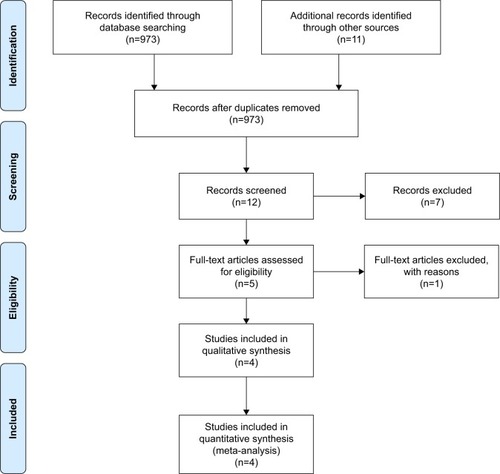
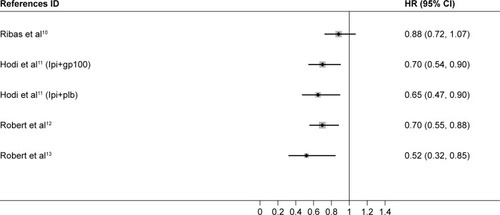
In all, 2,251 melanoma patients from four phase III RCTs were included in the analysis.Citation10–Citation13 The trial selection process is illustrated in . All the trials were phase III RCTs.Citation10–Citation13 In all, 764 older patients (aged ≥65 years) and 1,487 younger patients (aged <65 years) were included in the analysis. All the studies assessed the efficacy of ICIs including tremelimumab, ipilimumab, and nivolumab. The main characteristics of all the trials are available in .
Comparison of OS in younger and older patients
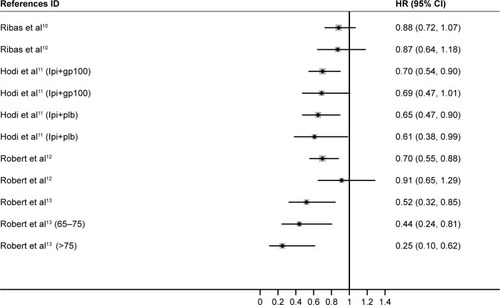
We first attempted to conduct the analysis of PFS; however, it was not further conducted due to the lack of available data. One trial,Citation14 which was initially included in the analysis, was excluded because HR of OS was not reported. We used Stata 14 SE to perform the statistical analysis, and the summary HR and 95% CI were calculated. A total of 2,251 melanoma patients from four trials were included in our analysis.Citation10–Citation13 We found that ICIs significantly prolonged the OS for melanoma patients in both younger (HR, 0.71; 95% CI, 0.60–0.82; P<0.001) and older groups (HR, 0.62; 95% CI, 0.41–0.83; P<0.001) compared with controls ( and ).
Subgroup analysis of OS
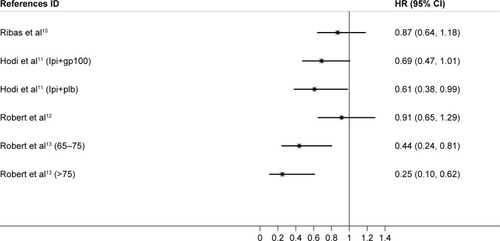
Depending on the heterogeneity, we used fixed-effects model and random-effects model in the younger (ICitation2=32.5%) and older group (ICitation2=68.9%), respectively. When treated with CTLA-4 inhibitors, both younger and older patients had significantly longer survival, and no significant difference was observed between the two groups (P=0.739) ( and ). Comparatively, anti-PD-1 agents appeared to be more efficient in older melanoma patients (HR, 0.34; 95% CI, 0.14–0.53) versus younger patients (HR, 0.52; 95% CI, 0.26–0.78), and this might result from a relatively small sample size. Notably, among older melanoma patients over 75 years treated with PD-1 inhibitors, a benefit of OS was significant (P=0.001). Compared with younger patients, patients older than 75 years benefit more from melanoma, which is totally different from the situation in NSCLC treatment. These data will require further evaluation in future clinical trials. The difference of effectiveness of PD-1 and CTLA-4 inhibitors in younger and older melanoma patients should get noticed in the future clinical practice.
Publication bias analysis, sensitivity analysis, and risk of bias assessment
A sensitivity analysis was conducted by recalculating the HR of different subgroups of studies based on relevant features, and no sensitivity analysis was positive (data not shown).
There was no publication bias based on the Begg’s test and Egger’s test for small-study effects. In Egger’s test, the P-value was 0.803. Likewise, in the Begg’s test, the continuity-corrected P-value was 0.788. This result indicated that no publication bias was detected in this study. The assessment of risk of bias is shown in .
Discussion
This meta-analysis was the first study investigating the association between ages and survival of melanoma patients in immune checkpoint blockade treatment. Recent clinical trials suggested the potential impact of age on the efficacy of ICIs. Nevertheless, there is no final conclusion partly because of the lack of statistical power. We conducted this study in order to verify our hypothesis, and we attempted to provide possible practical suggestions in melanoma treatments. We found that with a cutoff age of 65 years, ICIs significantly improved the OS and PFS in younger and older patients versus chemotherapy and gp100 vaccines. Interestingly, patients older than 75 years benefit more from melanoma compared with younger patients, which is totally different from the situation in NSCLC treatment. This observation was on the opposite with the possible impact of immunosenescence on the adaptive immune system. The observed results may not be entirely applicable to all patients treated in the community.
Immunosenescence refers to a physiological phenomenon causing alterations in the immune system with increasing age. In recent years, numbers of studies have focused on this field in order to elucidate the mechanism of immunosenescence, although a multitude of preclinical and clinical observations is lacking explanations. Immunosenescence will affect the function of dendritic cells, CD4+ T cells, CD8+ T cells, and regulatory T cells.Citation15 CTLA-4 is focused on secondary lymphoid organs, while PD-1 inhibitors function majorly in peripheral tissues and the tumor microenvironment. Aging contributes to decreased clonal expansion of CD8+ effector T cells, hence leading to decreased immunity.Citation16 This may have direct influence on the role of PD-1 inhibitors. EvidenceCitation17–Citation19 shows that aging results in the decline of the functions of DC and T cell and this may reduce the efficacy of ICIs, in particular CTLA-4 inhibitors.Citation15
Up-to-date trials indicated a potential efficacy-aging association in immune checkpoint blockade treatments. Scientists in CheckMate-0017 study found that the OS did not favor PD-1 inhibitors in patients aged over 75 years.Citation20 On the contrary, PD-1 inhibitor seems to favor melanoma patients aged over 75. Although this may be caused by the small sample size, our study, with a larger scope and more numbers of patients included, attempted to provide new findings. Another comprehensive review made a comparison of the efficacy of different ICIs in different tumor types generally.Citation21 Nevertheless, they did not provide a specific analysis and any specific suggestions for clinicians.
In the field of tumor immunology and immunotherapy, the impact of immunosenescence on ICI effectiveness varies in different cancers.Citation15 Interestingly, efficacy of PD-1 inhibitors is totally different in melanoma and NSCLC patients older than 75 years. Our observation indicated that melanoma patients older than 75 years benefit more from nivolumab. On the contrary, among NSCLC patients older than 75 years, there was even no significantly longer OS (HR: 1.02, 95% CI: 0.35–1.69, P=0.971) than controls. Theoretically, immunosenescence can reduce the efficacy of immune-based therapies. Future explorations are needed to give further explanations.
Our study did have some disadvantages. Patients with different types of melanoma were included with different etiologies. In order to minimize this disadvantage, we applied the randomized effect model when the value of heterogeneity was high. Also, comparatively small sample sizes were in the subgroups, and the subgroup findings required more assessments in future RCTs. We could not compare the toxicity of those drugs and evaluate the impact of immunosenescence on the safety of patients. Currently, there are no published data in relation to the immune-related adverse event in people belonging to different age groups, despite its significance in clinical practice. The biological role of immunosenescence-efficacy correlation should be investigated in the near future.
Conclusion
ICIs significantly prolonged the OS for melanoma patients in both younger and older groups than controls. Anti-PD-1 agents were more efficient in older melanoma patients versus younger patients. ICIs could be used for older melanoma patients. The difference of effectiveness of PD-1 and CTLA-4 inhibitors in younger and older melanoma patients should get noticed in the future clinical practice and guidelines.
Disclosure
The authors report no conflicts of interest in this work.
References
- PawelecGAge and immunity: what is “immunosenescence”?Exp Gerontol20181054929111233
- VenturaMTCasciaroMGangemiSBuquicchioRImmunosenescence in aging: between immune cells depletion and cytokines up-regulationClin Mol Allergy2017152129259496
- BoltonCSmithPAThe influence and impact of ageing and immunosenescence (ISC) on adaptive immunity during multiple sclerosis (MS) and the animal counterpart experimental autoimmune encephalomyelitis (EAE)Ageing Res Rev201841648129101043
- DasteADomblidesCGross-GoupilMImmune checkpoint inhibitors and elderly people: a reviewEur J Cancer20178215516628689093
- WuYJuQQianBZhangFShiHThe effectiveness of PD-1 inhibitors in non-small cell lung cancer (NSCLC) patients of different agesOncotarget2017987942794829487704
- SilvaIPLongGVSystemic therapy in advanced melanoma: integrating targeted therapy and immunotherapy into clinical practiceCurr Opin Oncol201729648449228914644
- WuYShiHJiangMThe clinical value of combination of immune checkpoint inhibitors in cancer patients: a meta-analysis of efficacy and safetyInt J Cancer2017141122562257028833119
- O’ReillyALarkinJThe safety of nivolumab for the treatment of metastatic melanomaExpert Opin Drug Saf201716895596128679287
- FerraraRMezquitaLAuclinEChaputNBesseBImmunosenescence and immunecheckpoint inhibitors in non-small cell lung cancer patients: does age really matter?Cancer Treat Rev201760606828889085
- RibasAKeffordRMarshallMAPhase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanomaJ Clin Oncol201331561662223295794
- HodiFSO’DaySJMcDermottDFImproved survival with ipilimumab in patients with metastatic melanomaN Engl J Med2010363871172320525992
- RobertCThomasLBondarenkoIIpilimumab plus dacarbazine for previously untreated metastatic melanomaN Engl J Med2011364262517252621639810
- RobertCLongGVBradyBNivolumab in previously untreated melanoma without BRAF mutationN Engl J Med2015372432033025399552
- RibasAPuzanovIDummerRPembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trialLancet Oncol201516890891826115796
- EliasRKarantanosTSiraEHartshornKLImmunotherapy comes of age: immune aging & checkpoint inhibitorsJ Geriatr Oncol20178322923528223073
- Czesnikiewicz-GuzikMLeeWWCuiDT cell subset-specific susceptibility to agingClin Immunol2008127110711818222733
- Grolleau-JuliusAHarningEKAbernathyLMYungRLImpaired dendritic cell function in aging leads to defective antitumor immunityCancer Res200868156341634918676859
- PlowdenJRenshaw-HoelscherMGangappaSEnglemanCKatzJMSambharaSImpaired antigen-induced CD8+ T cell clonal expansion in aging is due to defects in antigen presenting cell functionCell Immunol20042292869215474523
- GuoZTilburgsTWongBStromingerJLDysfunction of dendritic cells in aged C57BL/6 mice leads to failure of natural killer cell activation and of tumor eradicationProc Natl Acad Sci U S A201411139141991420425225399
- KeatingGMNivolumab: a review in advanced squamous non-small cell lung cancerDrugs201575161925193426514815
- NishijimaTFMussHBShacharSSMoschosSJComparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: a systematic review and meta-analysisCancer Treat Rev201645303726946217