Abstract
Introduction
We evaluated the expression of galectin-1 (Gal-1) and vasculogenic mimicry (VM) in gastric cancer (GC) and investigated their relationships with the clinicopathological factors and prognostic significance in GC.
Materials and methods
Immunohistochemical (IHC) staining and CD34–periodic acid-Schiff double stain were used to investigate Gal-1 expression and VM in paraffin-embedded sections from 127 patients with GC of all tumor stages. The relationships between Gal-1 expression and VM, clinicopathological variables, and survival were analyzed. P < 0.05 was considered statistically significant.
Results
Among the 127 cases, 86 (67.7%) were positive for Gal-1; VM was detected in 29 cases (22.8%). There was a significant association between VM and the Gal-1 IHC staining; all cases with VM were positive for Gal-1 staining. Gal-1 expression and VM in primary GC tissue were associated with tumor size, differentiation, depth of tumor invasion, stage, lymph node metastases, and tumor emboli in microvessels (all, P < 0.05). Kaplan–Meier analysis revealed that the overall survival time was 52.56 ± 2.44 months (95% confidence interval [CI]: 47.77–57.35) for patients with Gal-1-negative and VM-negative primary GC tissue, 43.83 ± 2.17 months (95% CI: 39.58–48.08) for patients with Gal-1-positive but VM-negative primary GC tissue, and 23.97 ± 2.44 months (95% CI: 19.18–28.76) for patients with Gal-1-positive and VM-positive primary GC tissue (χ2 = 60.21, P < 0.01). Gal-1 expression was positively associated with VM in primary GC tissue.
Conclusion
Both Gal-1 expression and VM in primary GC tissue are indicators of poor prognosis for GC after gastrectomy, and Gal-1 may be a novel target for treating VM in GC.
Introduction
As one of the most common cancers worldwide, gastric cancer (GC) is the second leading cause of cancer-related mortality after lung cancer.Citation1 Although a variety of treatments such as neoadjuvant chemotherapy and advanced surgical methods have improved the survival rate gradually over the past 30 years, the overall 5-year survival rate for resectable GC remains poor, especially that of the more advanced stages.Citation2–Citation4 Recently, treatment with ramucirumab, a monoclonal antibody that antagonizes VEGFR2, has become an important approach for treating GC.Citation5 However, drugs targeting VEGF signaling have failed to improve the survival of patients in Phase II and III clinical trials.Citation6 The presence of vasculogenic mimicry (VM) may represent a mechanism of resistance against angiostatic compounds.Citation7,Citation8
VM was first found in melanoma in 1999,Citation9 occurring when endothelium-dependent vessel growth is insufficient to support the rapid proliferation of tumor tissues, following which non-endothelial vascular networks provide oxygen and nutrients to tumors through a structure of channels,Citation10–Citation12 and it indicates that tumor cells can directly generate vascular channels that facilitate tumor perfusion independent of tumor angiogenesis by vascular endothelial cells.Citation9,Citation12 Maniotis et al defined this structure of channels composed of tumor basement membrane and tumor cells lacking blood vessel endothelium as VM.Citation9 Based on these features, VM can be distinguished using immunohistochemical (IHC) staining and histochemical double staining. While VM is CD31- or CD34-negative (endothelial markers) and periodic acid-Schiff (PAS)-positive, classic blood vessels are nevertheless double positive for endothelial and PAS markers.Citation10,Citation11 VM has been observed in many malignant tumors, including lung cancer,Citation13 breast cancer,Citation14 pancreatic cancer,Citation15 glioblastoma,Citation16 colorectal cancer,Citation17 ovarian cancer,Citation18 hepatocellular cancer,Citation19 prostate cancer,Citation20 esophageal cancer,Citation21 and GC,Citation22 and recent studies have shown that VM is associated with poor prognosis in human tumors.Citation14,Citation16,Citation22 VM can promote tumor growth and metastasis and is closely related to tumor neovascularization and cancer stem-like cells, which are associated with tumor invasion and drug resistance.Citation14 However, few studies have explored VM as a potential prognostic marker and therapeutic target in GC, and the underlying molecular mechanisms of VM remain largely unknown.
Encoded by the LGALS1 gene, galectin-1 (Gal-1) is a 14-kDa homodimer and prototype member of the galectin superfamily, which is characterized by high-affinity binding to β-galactosides through a well-conserved carbohydrate recognition domain.Citation23 Increasing clinical evidence has confirmed that Gal-1 is involved in a variety of biological processes, including selective deletion of specific thymocytes during T-cell development, T-cell homeostasis,Citation24 inflammatory responses,Citation25 and fetomaternal tolerance.Citation26 In addition, Gal-1 participates in tumor progression by evoking immunosuppression through the induction of activated T-cell apoptosis, transformation, angiogenesis, and metastasis and is associated with poor prognosis in many malignant tumors.Citation27–Citation30 A few studies have shown that Gal-1 overexpression in GC is associated with poor prognosis.Citation30,Citation31 Our previous studies have found that Gal-1 promotes epithelial–mesenchymal transition (EMT) in GC cells,Citation32 and other studies have found that EMT is an important step in VM.Citation33,Citation34 However, no study has clarified the correlation between Gal-1 and VM in GC. In this study, we performed IHC staining to examine Gal-1, and IHC and histochemical double staining to examine VM in GC tissues. We aimed to determine whether Gal-1 expression levels and the presence of VM are correlated with each other and with GC clinicopathological features and prognosis, including survival.
Materials and methods
Patient information
We enrolled 127 patients with gastric adenocarcinoma and complete clinicopathological and follow-up data in our study from July 2012 to May 2013. The patients were treated at the Department of Gastrointestinal Surgery, Taizhou People’s Hospital, Taizhou, Jiangsu, People’s Republic of China. shows the patients’ detailed clinicopathological data. All patients underwent radical gastrectomy for the primary tumor and D2 lymphadenectomy; no patient received chemotherapy or radiotherapy prior to surgery, had distant metastases prior to surgery, or other synchronous malignancies or serious diseases.
Table 1 Chi-square assessment of the associations between Gal-1 IHC staining and VM and the clinicopathological features of 127 patients with GC
Immunohistochemistry
IHC staining of all specimens was performed on formalin-fixed, paraffin-embedded tissue. Sections were cut at 4-μm thickness. The sections were deparaffinized in xylene and rehydrated in gradient ethanol. Endogenous peroxidases were blocked with 3% hydrogen peroxide in methanol for 10 min. The sections were washed with phosphate-buffered saline, and then pretreated with citrate buffer (pH 6.0) for 20 min at 95°C in a microwave oven for antigen retrieval. The slides were incubated with primary antibodies against Gal-1 (1:200; Cell Signaling Technology, Danvers, MA, USA) overnight at 4°C, followed by incubation with biotin-conjugated secondary antibodies, and then horseradish peroxidase-conjugated streptavidin. The sections were stained with diaminobenzidine (DAB), counterstained with hematoxylin, dehydrated, cleared, mounted, and coverslipped.
CD34–PAS dual staining
IHC staining was used to perform staining for CD34 (1:100; Abcam, Cambridge, UK). The procedure was the same as that described in the previous section. PAS staining was performed using a PAS staining kit (SN:DG0005; Leagene Biotechnology Co., Ltd., Beijing, People’s Republic of China). After DAB reaction, the sections were treated with 0.5% periodic acid solution for 10 min, and rinsed with distilled water three times for 3 min, followed by staining with Schiff solution for 20 min away from light. After rinsing with distilled water, the sections were counterstained with hematoxylin, dehydrated, cleared, mounted, and coverslipped.
Evaluation of IHC staining and CD34–PAS dual staining
Three sections per specimens were stained for each antibody. Two independent pathologists blinded to the patients’ clinical status assessed the results. Negative controls, from which primary antibodies had been omitted, were treated identically; positive controls were provided by the kit supplier. To quantify Gal-1 immunostaining, the slides were imaged digitally with equal light exposure and evaluated with Image-Pro Plus, a digitalized IHC scoring program (Media Cybernetics, Rockville, MD, USA). The immunostaining was scored based on the product of the percentage of immunopositive cells (0–100) multiplied by staining intensity score (0, 1, 2, and 3) to yield scores of 0–300. Receiver operating curve (ROC) statistics was employed to estimate cutoff points of the IHC score to distinguish high and low expression of Gal-1 in 127 GC samples. VM was defined as positive or negative. In CD34–PAS double staining, VM-positive status appeared as channel-like structures with negative CD34 and positive PAS staining, and containing red cells.
Follow-up
Patients underwent continuous follow-up up to July 30, 2017. As patients without complete clinicopathological and follow-up data were excluded, no patient was lost to follow-up. The median follow-up duration after surgery was 39.6 months (range: 3.1–60.9 months).
Ethics
The protocol of this trial was designed in accordance with the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of Taizhou People’s Hospital (TZRY-EC-12-068). All patients were provided details on the assessment procedure, and all provided informed written consent.
Statistical analysis
Statistical analysis was conducted with SPSS 16.0 (SPSS, Chicago, IL, USA). Continuous variables are expressed as the means ± SE and were compared between groups using Student’s t-test. The correlations between Gal-1 expression and VM and the clinicopathological features were analyzed using the chi-square test. The Kaplan–Meier method was used for survival analysis; intergroup survival differences were assessed with log-rank testing. In all analyses, P < 0.05 was considered statistically significant.
Results
Gal-1 expression and VM in GC tissues
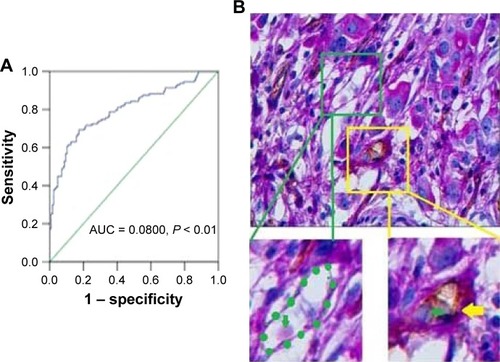
In the GC tissues, the median Gal-1 IHC scores were 78.29 (9.51–186.24). IHC staining of Gal-1 expression in GC tissues is shown in . ROC statistics were used to estimate the IHC cutoff scores to distinguish positive and negative Gal-1 expression in the 127 cases. Scores ≥ 56.80 were considered to indicate positive expression (). In accordance with this standard, Gal-1 expression was positive in 86 of 127 GC cases (67.7%) and negative in the remaining 41 cases (32.3%). As shows, CD34–PAS staining showed endogenous cell-dependent vessels (yellow arrow) and VM (green dotted line) in one GC specimen red blood cells are shown by green arrow in VM and endogenous cell-dependent vessels. CD34–PAS double staining revealed VM in 29 cases (22.8%); the remaining 98 cases were VM-negative (77.2%).
Figure 1 Immunohistochemical staining of Gal-1 expression in GC tissues.
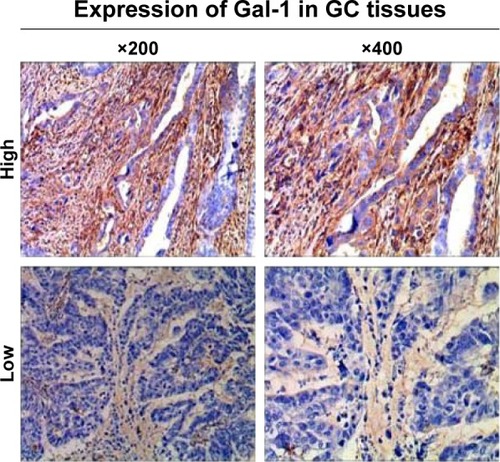
Figure 2 Gal-1 expression and VM in GC tissues. (A) ROC statistics were used to estimate the Gal-1 IHC cutoff score in human GC tissue. (B) CD34–PAS staining showing endogenous cell-dependent vessels (yellow arrow) and VM (green dotted line) in one GC specimen; red blood cells are shown by green arrow in VM and endogenous cell-dependent vessels. Original magnification: ×400.
Correlation between Gal-1 expression and VM and clinicopathological features
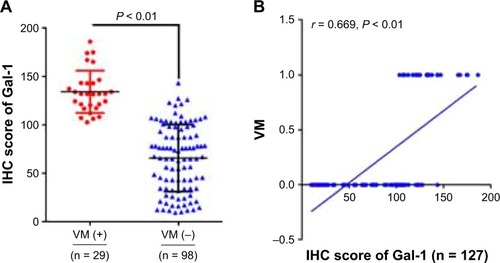
The Gal-1 IHC scores in primary tumors with VM were significantly different from the Gal-1 score in primary tumors without VM (P < 0.01; ), and there was a significant association between the Gal-1 IHC scores and VM (r = 0.669, P < 0.01; ); all VM-positive samples were positive for Gal-1. Gal-1 expression and VM in primary GC tissue were associated with tumor size, differentiation, depth of tumor invasion, stage, lymph node metastases, and tumor emboli in the microvessels (all P < 0.05), but were not correlated with age, sex, and tumor location (all P > 0.05; ).
Figure 3 Correlation between Gal-1 expression and VM and clinicopathological features. (A) The Gal-1 IHC scores in primary tumors with VM were significantly different from Gal-1 scores in primary tumors without VM (P < 0.01). (B) Spearman correlation analysis showed significant correlation between Gal-1 expression and VM (r = 0.669, P < 0.001). The Y-axis values indicate VM status: 0, VM-negative; 1, VM-positive.
Correlation between Gal-1 expression and VM and survival
The final follow-up date was July 30, 2017. The median follow-up duration after surgery was 39.6 months (range: 3.1–60.9 months). A total of 68 patients (53.54%) had tumor-related deaths. Kaplan–Meier analysis revealed that the overall survival (OS) time for Gal-1-positive and Gal-1-negative patients was 52.56 ± 2.44 months (95% confidence interval [CI]: 47.77–57.35) and 37.17 ± 1.95 months (95% CI: 33.36–40.99), respectively. The OS rate of patients relative to Gal-1 expression status in GC tissue samples is shown in . Gal-1 overexpression was significantly associated with poor survival (χ2 = 22.32, P < 0.01). The OS time for VM-positive and VM-negative patients was 47.71 ± 1.71 months (95% CI: 44.37–51.01) and 23.97 ± 2.44 months (95% CI: 19.18–28.76), respectively. Additionally, the VM-positive patients had shorter OS time than the VM-negative patients. The OS rate of patients relative to VM status in GC tissue samples is shown in . The presence of VM was significantly associated with poor survival (χ2 = 46.07, P < 0.01).
Figure 4 OS rate of patients. (A) The OS rate of patients relative to Gal-1 expression status in GC tissue samples. Gal-1 overexpression was significantly associated with poor survival (P < 0.01). (B) The OS rate of patients relative to VM status in GC tissue samples. The presence of VM was significantly associated with poor survival (P < 0.01). (C) The OS rates of groups with Gal-1-negative and VM-negative primary GC tissue, Gal-1-positive but VM-negative primary GC tissue, and Gal-1-positive and VM-positive primary GC tissue. Both Gal-1 overexpression and presence of VM were significantly associated with poor survival (P < 0.01).
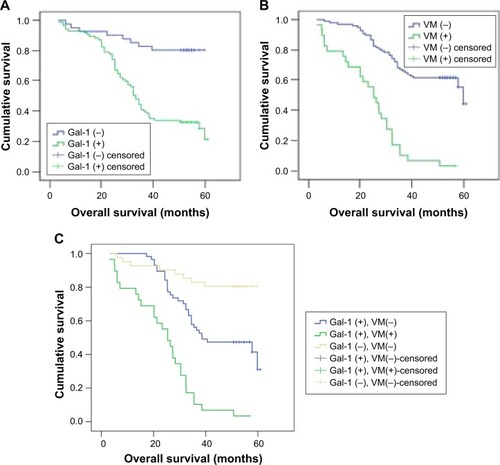
To evaluate the combined effect of Gal-1 expression and VM on GC prognosis, we classified the patients into three subgroups according to Gal-1 expression and VM in the primary GC tissue: Gal-1-negative and VM-negative, Gal-1-positive but VM-negative, and Gal-1-positive and VM-positive. Kaplan–Meier analysis revealed that OS time was 52.56 ± 2.44 months (95% CI: 47.77–57.35) for Gal-1-negative and VM-negative patients, 43.83 ± 2.17 months (95% CI: 39.58–48.08) for Gal-1-positive but VM-negative patients, and 23.97 ± 2.44 months (95% CI: 19.18–28.76) for Gal-1-positive and VM-positive patients. The OS rates of groups with Gal-1-negative and VM-negative primary GC tissue, Gal-1-positive but VM-negative primary GC tissue, and Gal-1-positive and VM-positive primary GC tissue are shown in . Both Gal-1 overexpression and the presence of VM were significantly associated with poor survival (χ2 = 60.21, P < 0.01).
Discussion
Although efforts have been made regarding prevention, early diagnosis, and improved therapeutic strategies, the mortality rates for patients with advanced-stage GC remain high.Citation1–Citation4 Once patients develop resistance to chemotherapeutic regimens, antiangiogenesis therapy becomes an important approach for treating GC; endothelial-lined vessels are inhibited by some antiangiogenic agents initially,Citation35 but the outcome of antiangiogenesis therapy is unsatisfactory, where VM and metastasis persistently increase thereafter.Citation7 The occurrence of VM is thought to be a crucial step in GC progression and metastasis,Citation22 suggesting that VM is a potential therapeutic target in GC and that VM-related molecules are potential targets for novel anti-GC therapies, and it is necessary to evaluate these new molecular targets for their potential roles as prognostic markers for patients with GC.
Gal-1 was the first protein discovered within the galectin family; it can form homodimers via non-covalent binding, which confers the ability to cross-link specific glycoconjugates. Intracellularly, Gal-1 is involved in pre-mRNA splicing; it interacts with oncogenic H-RAS and promotes cell migration, proving that it plays a key role in driving tumor transformation proteins to influence tumor progression, invasion, and angiogenesis.Citation23 The literature largely reports that high Gal-1 levels correlate with tumor aggressiveness and the acquisition of a metastatic phenotype.Citation27–Citation30 LGALS1 amplification and overexpression have been demonstrated in thyroid, head and neck, colon, ovary, and prostate carcinoma,Citation36 and high Gal-1 expression is associated with poor prognosis.Citation27,Citation28 However, as few studies have evaluated the correlation between Gal-1 overexpression and survival in GC,Citation30 the prevalence of Gal-1 in GC and its relationship with prognosis remain largely unknown. In the present study, we examined 127 GC samples for expression of the Gal-1 oncoprotein by immunohistochemistry. The median Gal-1 IHC score was 78.29 (9.51–186.24); in accordance with ROC statistics, 86 of 127 GC cases had positive Gal-1 expression (67.7%). Gal-1 expression was related to tumor size, differentiation, depth of tumor invasion, stage, lymph node metastases, and tumor emboli in the microvessels. Kaplan–Meier survival analysis confirmed a significant prognostic value of Gal-1 in GC. Gal-1-positive patients had significantly poorer outcome than Gal-1-negative patients. Thus, detecting Gal-1 expression in GC tissues might be helpful for prognosis in GC.
VM is found and considered a poor prognostic marker in many tumors;Citation13–Citation22 VM provides essential nutrients to rapidly growing tumors to promote tumor growth, and based on the structure of VM less of endothelium, VM increases tumor perfusion by leaky vessels, and VM tubes may even connect with the endothelial-lined vasculature,Citation10 which promotes tumor cell entry into the blood circulation, leading to metastasis. Drugs targeting endothelial signaling molecules such as bevacizumab, sunitinib, and sorafenib have been used clinically to treat various cancers, including GC, but their efficacy is limited.Citation37 Some studies have found that targeted endothelial therapy may even contribute to VM.Citation38 However, the clinical impact of VM on OS and prognosis in GC remains controversial. A study with insufficient clinical research indicated that VM is not closely associated with the prognosis of patients with GC.Citation39 Here, we examined 127 GC cases for VM, where 29 (22.8%) were VM-positive, and the incidence of VM increased with GC progression. VM was related to tumor size, differentiation, depth of tumor invasion, stage, lymph node metastases, and tumor emboli in the microvessels. We also confirmed a significant association between VM and poor survival.
The molecular mechanisms of VM are not fully understood; we show that Gal-1 IHC scores were significantly associated with VM in primary GC tissue, where all VM-positive samples were positive for Gal-1 staining. Gal-1 and VM had a concordant correlation with the clinicopathological features of GC, which suggests a connection between them. Our results also indicate that Gal-1 overexpression and the presence of VM, especially simultaneous Gal-1 overexpression and VM, are significantly correlated with poor survival in GC. Therefore, positivity for both Gal-1 and VM predicts a worse clinical outcome for patients with GC, and detecting Gal-1 expression and the presence of VM in primary GC tissue might be a novel prognostic marker.
Gal-1 is a direct target of HIF1, a key heterodimeric transcriptional factor for the cellular response to hypoxia.Citation40 Gal-1 interacts with oncogenic H-RAS intracellularly, and RAS signaling can induce or enhance SHH expression, which can activate Hh signaling and promote the expression of Gli-1 and promote EMT of GC.Citation32,Citation41 EMT participates in VM formation, and as a main EMT-mediated process regulator, TWIST reportedly promotes the upregulation of VE-cadherin and VEGFR1 expression and contributes to VM formation.Citation10 Therefore, we hypothesize that Gal-1 promotes VM in GC by regulating the RAS-Hh/Gli-1-TWIST signaling pathway. If this hypothesis is correct, Gal-1 and the pathway are potential therapeutic targets for GC. To date, the Gal-1 regulatory mechanism of VM in GC has not been well explored and requires further study.
Conclusion
Gal-1 expression is positively associated with VM in primary GC tissue. Both Gal-1 expression and the presence of VM in primary GC tissue are indicators of poor prognosis for GC after gastrectomy. Gal-1 may promote VM in GC by regulating the RAS-Hh/Gli-1-TWIST signaling pathway; Gal-1 and the pathway are potential therapeutic targets for GC.
Acknowledgments
This work was supported in part by China Postdoctoral Science Foundation (grant number 2018M632400), the National Natural Science Foundation of China (grant numbers 81172279 and 81572343). The authors would like to thank the native English-speaking scientists of Elixigen Company (Huntington Beach, CA, USA) for editing our manuscript.
Disclosure
The authors have no conflicts of interest to declare in this work.
References
- LinYUedaJKikuchiSComparative epidemiology of gastric cancer between Japan and ChinaWorld J Gastroenterol201117394421442822110269
- ZhengLWuCXiPThe survival and the long-term trends of patients with gastric cancer in Shanghai, ChinaBMC Cancer20141430024779704
- XuWBeeharryMKLiuWYanMZhuZPreoperative chemotherapy for gastric cancer: personal interventions and precision medicineBiomed Res Int20162016392358528105420
- SonTHyungWJLaparoscopic gastric cancer surgery: current evidence and future perspectivesWorld J Gastroenterol201622272773526811620
- JavleMSmythECChauIRamucirumab: successfully targeting angiogenesis in gastric cancerClin Cancer Res201420235875588125281695
- AllegraCJYothersGO’ConnellMJPhase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08J Clin Oncol2011291111620940184
- XuYLiQLiXYYangQYXuWWLiuGLShort-term anti-vascular endothelial growth factor treatment elicits vasculogenic mimicry formation of tumors to accelerate metastasisJ Exp Clin Cancer Res2012311622357313
- AngaraKBorinTFArbabASVascular mimicry: a novel neovascularization mechanism driving anti-angiogenic therapy (AAT) resistance in glioblastomaTransl Oncol201710465066028668763
- ManiotisAJFolbergRHessAVascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicryAm J Pathol1999155373975210487832
- QiaoLLiangNZhangJAdvanced research on vasculogenic mimicry in cancerJ Cell Mol Med201519231532625598425
- WangMZhaoXZhuDHIF-1α promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up-regulation in hypoxic tumor microenvironmentJ Exp Clin Cancer Res20173616028449718
- RacordonDValdiviaAMingoGStructural and functional identification of vasculogenic mimicry in vitroSci Rep20177698528765613
- LiYSunBZhaoXMMP-2 and MMP-13 affect vasculogenic mimicry formation in large cell lung cancerJ Cell Mol Med201721123741375128766880
- ShenYQuanJWangMTumor vasculogenic mimicry formation as an unfavorable prognostic indicator in patients with breast cancerOncotarget2017834564085641628915600
- YangJZhuDMZhouXGHIF-2α promotes the formation of vasculogenic mimicry in pancreatic cancer by regulating the binding of Twist1 to the VE-cadherin promoterOncotarget2017829478014781528599281
- HanGLiYCaoYOverexpression of leptin receptor in human glioblastoma: correlation with vasculogenic mimicry and poor prognosisOncotarget2017835581635817128938545
- LiWZongSShiQLiHXuJHouFHypoxia-induced vasculogenic mimicry formation in human colorectal cancer cells: involvement of HIF-1a, Claudin-4, and E-cadherin and VimentinSci Rep201663753427869227
- TangJWangJFanLcRGD inhibits vasculogenic mimicry formation by down-regulating uPA expression and reducing EMT in ovarian cancerOncotarget2016717240502406226992227
- ZhaoNSunBCZhaoXLRole of Bcl-2 and its associated miRNAs in vasculogenic mimicry of hepatocellular carcinomaInt J Clin Exp Pathol2015812157591576826884845
- WangHLinHPanJVasculogenic mimicry in prostate cancer: the roles of EphA2 and PI3KJ Cancer2016791114112427326255
- TangNNZhuHZhangHJHIF-1α induces VE-cadherin expression and modulates vasculogenic mimicry in esophageal carcinoma cellsWorld J Gastroenterol20142047178941790425548487
- GuoQYuanYJinZAssociation between tumor vasculogenic mimicry and the poor prognosis of gastric cancer in China: an updated systematic review and meta-analysisBiomed Res Int20162016240864527812528
- BarondesSHCastronovoVCooperDNGalectins: a family of animal beta-galactoside-binding lectinsCell19947645975988124704
- SotomayorCERabinovichGAGalectin-1 induces central and peripheral cell death: implications in T-cell physiopathologyDev Immunol200072–411712911097206
- Al-SalamSHashmiSGalectin-1 in early acute myocardial infarctionPLoS One201491e8699424498007
- ThanNGRomeroRErezOEmergence of hormonal and redox regulation of galectin-1 in placental mammals: implication in maternal–fetal immune toleranceProc NatI Acad Sci U S A2008105411581915824
- ChenLYaoYSunLClinical implication of the serum galectin-1 expression in epithelial ovarian cancer patientsJ Ovarian Res201587826589590
- SuYCDavuluriGVChenCHGalectin-1-induced autophagy facilitates cisplatin resistance of hepatocellular carcinomaPLoS One2016112e014840826859293
- JouveNDespoixNEspeliMThe involvement of CD146 and its novel ligand galectin-1 in apoptotic regulation of endothelial cellsJ Biol Chem201328842571257923223580
- HeXJTaoHQHuZMExpression of galectin-1 in carcinoma-associated fibroblasts promotes gastric cancer cell invasion through upregulation of integrin β1Cancer Sci2014105111402141025230369
- ChenJZhouSJZhangYClinicopathological and prognostic significance of galectin-1 and vascular endothelial growth factor expression in gastric cancerWorld J Gastroenterol201319132073207923599627
- ChongYTangDGaoJGalectin-1 induces invasion and the epithelial-mesenchymal transition in human gastric cancer cells via non-canonical activation of the hedgehog signaling pathwayOncotarget2016750836118362627835885
- LiuQQiaoLLiangNThe relationship between vasculogenic mimicry and epithelial-mesenchymal transitionsJ Cell Mol Med20162091761176927027258
- HanCSunBZhaoXPhosphorylation of STAT3 promotes vasculogenic mimicry by inducing epithelial-to-mesenchymal transition in colorectal cancerTechnol Cancer Res Treat20171661209121929333928
- ChenLTOhDYRyuMHAnti-angiogenic therapy in patients with advanced gastric and gastroesophageal junction cancer: a systematic reviewCancer Res Treat201749485186828052652
- CousinJMCloningerMJThe role of galectin-1 in cancer progression, and synthetic multivalent systems for the study of galectin-1Int J Mol Sci2016179 pii:E1566
- KreislTNKimLMooreKPhase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastomaJ Clin Oncol200927574074519114704
- EbosJMLeeCRCruz-MunozWBjarnasonGAChristensenJGKerbelRSAccelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesisCancer Cell200915323223919249681
- CaoZBaoMMieleLSarkarFHWangZZhouQTumour vasculogenic mimicry is associated with poor prognosis of human cancer patients: a systemic review and meta-analysisEur J Cancer201349183914392323992642
- ZhaoXYChenTTXiaLHypoxia inducible factor-1 mediates expression of galectin-1: the potential role in migration/invasion of colorectal cancer cellsCarcinogenesis20103181367137520525878
- BrechbielJMiller-MoslinKAdjeiAACrosstalk between hedgehog and other signaling pathways as a basis for combination therapies in cancerCancer Treat Rev201440675075924613036
