Abstract
Objective
To investigate the impact of postresection intra-arterial chemotherapy (IAC) on prognosis of stage 2–3 gallbladder cancer (GBC) after curative resection.
Methods
Between May 2010 and August 2014, 76 cases of GBC accepted curative surgery in our center and were pathologically staged as 2–3. After resection, 37 underwent 4 courses of intravenous chemotherapy (IVC) following 2 courses of IAC (ART group), and 39 received 6 courses of IVC (SYS group). Both the IAC and IVC regimens consisted of oxaliplatin (85 mg/m2) on day 1 and gemcitabine (800 mg/m2) on day 1 and day 8. Chemotherapy-related complications, disease-free survival (DFS), overall survival (OS), and hepatic metastases-free survival (HMFS) were retrospectively analyzed.
Results
Patient characteristics and chemotherapy complications did not differ between the two groups. There was no significant difference in 3-year DFS of the two groups (p=0.0822, HR=0.6270; 95% CI, 0.3627 to 1.0838). The ART group had significantly higher 3-year OS (p=0.0378, HR=0.5460; 95% CI, 0.3025 to 0.9856) and 3-year HMFS (p=0.0414, HR=0.5187; 95% CI, 0.2706 to 0.9940) than the SYS group.
Conclusions
IAC could effectively and safely decrease postresection hepatic metastases and improve 3-year HMFS and OS of stage 2–3 GBC.
Introduction
In spite of the advancement in chemotherapy and surgery, gallbladder cancer (GBC) remains a disease with an unfavorable survival and the 5-year overall survival (OS) ranges from 50% in stage 1 to 2% in metastatic disease.Citation1 Surgery offers a chance of cure, but most patients experience a recurrence despite curative resection.Citation2 Therefore, considerable interest exists in applying adjuvant therapy in resected GBC, although high-quality evidence supporting this practice is lacking and therapy largely relies on retrospective observational data.Citation3
Chemotherapy is still one of the most commonly applied postoperative adjuvant therapies for GBC, but most of the chemotherapy regimes cannot offer a favorable survival benefit and usually cause serious side effects.Citation4 Therefore, it is urgent to seek alternative adjuvant therapeutic methods. The liver is the most common site of recurrence after radical resection of GBC.Citation5 We previously found that intra-arterial chemotherapy (IAC) could significantly decrease metachronous liver metastases from colorectal cancer and improve long-term survival.Citation6 In this retrospective study, we aimed to figure out whether IAC could decrease hepatic recurrence and improve prognosis of stage 2–3 GBC after curative resection.
Method
Patients
Between May 2010 and August 2014, 76 patients with GBC underwent curative surgery in our center and were pathologically staged as 2–3 according to American Joint Committee on Cancer guidelines, 7th edition.Citation7 After resection, 37 underwent 4 courses of intravenous chemotherapy (IVC) following 2 courses of IAC (ART group), and 39 underwent 6 courses of IVC (SYS group). Criteria for enrollment were: histologically confirmed as stage 2–3 GBC, no previous anticancer treatment, Karnofsky performance score≥70, postoperative life span>6 months, sufficient liver, hematopoietic, and kidney function, and age from 18 to 75. According to the patients’ medical documents, the patient characteristics, chemotherapy-related complications, disease-free survival (DFS), OS, and hepatic metastases-free survival (HMFS) were retrospectively analyzed. Patients selected their chemotherapy regime and signed a consent form after reading the instruction about each chemotherapy regime. This study obtained approval from the ethics committee of First People’s Hospital affiliated to Huzhou Normal College.
Chemotherapy administration
Chemotherapy was initiated within 3 weeks after surgery and repeated every 28 days. Both the IAC and IVC regimens consisted of oxaliplatin (85 mg/m2) on day 1 and gemcitabine (800 mg/m2) on day 1 and day 8.
IAC was administered using a micropump through a catheter of which the end was placed into the common hepatic artery or proper hepatic artery.Citation8 At the end of the infusion, the catheter was removed. All patients were supervised and complications were appropriately treated. The severity of chemotherapy toxicities were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.Citation9 Treatment was ceased if unacceptable toxicities occurred or on the patient’s request.
Follow-up
All patients were followed up every month in the first postoperative year and every 3 months thereafter. Recurrence was diagnosed by enhanced computed tomography or magnetic resonance imaging and, if necessary, biopsy. Alternative chemotherapy regimens or palliative treatment were administered if recurrence was diagnosed.
Statistical analysis
All measurements were presented as mean±SD. Student’s t-test, chi-square test, or Fisher’s exact test was appropriately utilized to analyze the clinical data. Survival curves were calculated using the Kaplan–Meier method. The difference of the survival was evaluated using the log-rank test. All statistical analyses were carried out with statistical software package SPSS for Windows (version 17.0; SPSS Inc., Chicago, IL, USA). p<0.05 was considered significant.
Results
Patient characteristics
shows that patient characteristics did not differ between the 2 groups, such as age, tumor size, blood loss during surgery, level of carbohydrate antigen 19-9, gender, operating time, tumor stage, and tumor differentiation.
Table 1 Patient characteristics
Disease-free survival
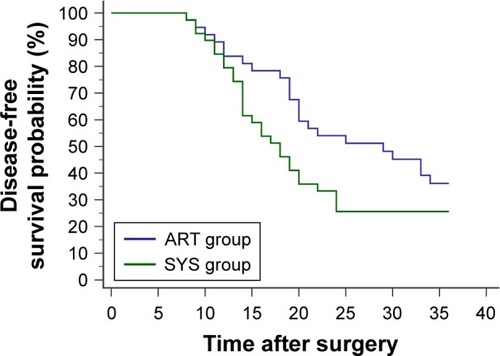
Within the first 3 postoperative years, recurrence was detected in 23 cases of the ART group and 29 of the SYS group. Details of recurrent sites are shown in . The 3-year DFS did not differ between the 2 groups (p=0.0822, hazard ratio [HR]=0.6270; 95% CI, 0.3627 to 1.0838) ().
Table 2 Sites of recurrence
Figure 1 Within the first 3 postoperative years, 23 patients from the ART group (4 courses of intravenous chemotherapy following 2 courses of intra-arterial chemotherapy) and 29 patients from the SYS group (6 courses of intravenous chemotherapy) developed relapse or metastasis. No significant difference was found in the 3-year disease-free survival probability between the 2 groups (p=0.0822, HR=0.6270; 95% CI, 0.3627 to 1.0838).
Overall survival
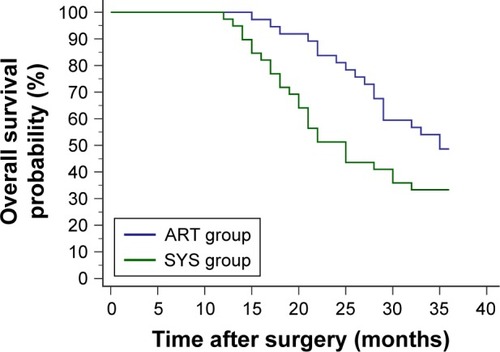
Within the first 3 postoperative years, 19 cases of the ART group and 26 of the SYS group died. The 3-year OS of the ART group was significantly higher than the SYS group (p=0.0378, HR=0.5460; 95% CI, 0.3025 to 0.9856) ().
Figure 2 Within the first 3 postoperative years, 19 patients from the ART group (4 courses of intravenous chemotherapy following 2 courses of intra-arterial chemotherapy) and 26 patients from the SYS group (6 courses of intravenous chemotherapy) died. The ART group had a significantly higher 3-year overall survival probability than the SYS group (p=0.0378, HR=0.5460; 95% CI, 0.3025 to 0.9856).
Hepatic metastases-free survival
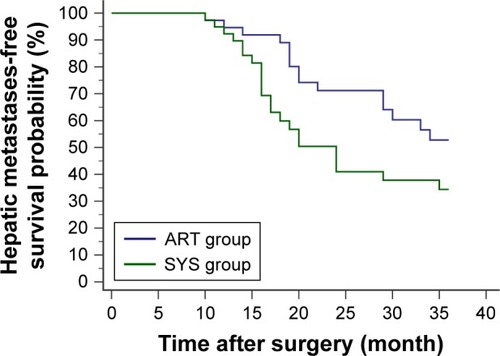
Within the first 3 postoperative years, 15 cases of the ART group and 22 of the SYS group developed hepatic metastases. The 3-year HMFS of the ART group was significantly higher than the SYS group (p=0.0414, HR=0.5187; 95% CI, 0.2706 to 0.9940) ().
Figure 3 Within the first 3 postoperative years, hepatic metastases were reported in 15 patients from the ART group (4 courses of intravenous chemotherapy following 2 courses of intra-arterial chemotherapy) and 22 patients from the SYS group (6 courses of intravenous chemotherapy). Survival analysis showed a significantly higher 3-year hepatic metastases-free survival probability in the ART group compared with the SYS group (p=0.0414, HR=0.5187; 95% CI, 0.2706 to 0.9940).
Complication and toxicity
lists the chemotherapy toxicities of the 2 groups and no difference was found. All patients underwent 6 courses of chemotherapy. Hematoma of the femoral artery puncture site was documented in 4 cases of the ART group and was controlled by pressure dressing. All complication and toxicity was managed by conservative treatment.
Table 3 Toxicities
Discussion
GBC is the most aggressive biliary tree cancer (BTC) and only radical resection can offer the chance for cure.Citation10 Nevertheless, incidence of postoperative relapse is still high, even in patients with resectable GBC (stage 2–3) who received curative resection, including extensive lymph node dissection and adjacent organ resection.Citation1 The liver is the most common site of recurrence after radical resection of GBC.Citation5 IAC is an effective treatment and prophylactic option for recurrence in the liver from primary carcinoma.Citation6 Several studies demonstrated that IAC could offer significant survival benefit both in unresectable and resected GBCs.Citation11–Citation13 Moreover, IAC might be a safer and more effective adjuvant therapy strategy because administration of chemotherapy drugs via the hepatic arterial circulation can reach higher regional drug concentrations with lighter systemic toxicities than intravenous injection.Citation14–Citation16
Clinical trials were performed to investigate the survival benefit of different postoperative chemotherapy regimens for GBC.Citation17,Citation18 It was demonstrated that the 5-fluorouracil-based adjuvant chemotherapy offered no survival benefit for resected GBC.Citation19 A chemotherapy regime consisting of gemcitabine and oxaliplatin could offer a modest antitumor activity and is well tolerated in patients with biliary cancer including GBC.Citation20 Due to the efficacy and safety of the gemcitabine and oxaliplatin chemotherapy regime, it was recognized as the first-line chemotherapy regime for BTCs.Citation21 In the present study, we included resectable (stage 2–3) GBC patients and both the IAC and IVC regimens consisted of gemcitabine and oxaliplatin.
In the current study, the ART group had significantly higher 3-year OS and HMFS than the SYS group. However, the 3-year DFS did not differ, implying that the survival benefit of IAC may result from the decrease of hepatic metastases.
This study further verified the safety of the IAC regime. The comparison of toxicities showed no difference between the ART group and the SYS group. All of the patients tolerated 6 courses of chemotherapy and the toxicities were controllable.
Conclusion
IAC could effectively and safely decrease postresection hepatic metastases and improve 3-year HMFS and OS of stage 2–3 GBC. However, because of the limitation of the retrospective characteristic and single-center experience, prospective and multicenter studies are required to verify the results of the current study.
Author contributions
Cheng Chen and Min Feng designed the study and wrote the manuscript. Wenming Feng, Ying Bao, and Yinyuan Zheng collected and analyzed the data. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- MantripragadaKCHamidFShafqatHOlszewskiAJAdjuvant therapy for resected gallbladder cancer: analysis of the National Cancer Data BaseJ Natl Cancer Inst20161092 pii: djw202
- WangJNarangAKSugarEAEvaluation of adjuvant radiation therapy for resected gallbladder carcinoma: a multi-institutional experienceAnn Surg Oncol201522Suppl 3S1100S110626224402
- AloiaTAJárufeNJavleMGallbladder cancer: expert consensus statementHPB (Oxford)201517868169026172135
- ShuklaSKSinghGShahiKSBhuvanPantPStaging, treatment, and future approaches of gallbladder carcinomaJ Gastrointest Cancer201849191529234972
- MargonisGAGaniFBuettnerSRates and patterns of recurrence after curative intent resection for gallbladder cancer: a multi-institution analysis from the US Extra-hepatic Biliary Malignancy ConsortiumHPB (Oxford)2016181187287827527802
- WangYSunXRFengWMBaoYZhengYYPostoperative prophylactic hepatic arterial infusion chemotherapy for stage III colorectal cancer: a retrospective studyOnco Targets Ther201695897590227713643
- EdgeSBComptonCCThe American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNMAnn Surg Oncol20101761471147420180029
- KasugaiHKojimaJTatsutaMTreatment of hepatocellular carcinoma by transcatheter arterial embolization combined with intraarterial infusion of a mixture of cisplatin and ethiodized oilGastroenterology19899749659712550311
- National Cancer InstituteCommon Terminology Criteria for Adverse Events (CTCAE) Version 4.0Rockville, MDNational Cancer Institute2009
- DuffyACapanuMAbou-AlfaGKGallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC)J Surg Oncol200898748548918802958
- NishimuraMA successful treatment by hepatic arterial infusion therapy for advanced, unresectable biliary tract cancerWorld J Hepatol20102519219721160995
- NogitaHUchidaSIshikawaHHisakaTHoriuchiHKinoshitaHShirouzuKA long-term survivor of advanced gallbladder carcinoma treated with curative operation and hepatic arterial infusionGan to Kagaku Ryoho201340810811083 Japanese23986056
- BodeMKPeräläJMäkeläJTLeinonenSIntra-arterial chemotherapy with mitomycin C in gallbladder cancer: a follow-up studyJ Surg Oncol200591210210616028283
- AckermanNBLienWMKondiESSilvermanNAThe blood supply of experimental liver metastases. I. The distribution of hepatic artery and portal vein blood to “small” and “large” tumorsSurgery1969666106710725402533
- ConwayJGPoppJAJiSThurmanRGEffect of size on portal circulation of hepatic nodules from carcinogen-treated ratsCancer Res1983437337433786850642
- ArcherSGGrayBNVascularization of small liver metastasesBr J Surg19897665455482474356
- ParkHSLimJYYoonDSOutcome of adjuvant therapy for gallbladder cancerOncology2010793–416817321212704
- ChoudharySAsthanaAKImpact of adjuvant therapy on survival in curatively resected gallbladder carcinomaJ Clin Diagn Res201599XC01XC04
- de AretxabalaXRoaIBerriosMChemoradiotherapy in gallbladder cancerJ Surg Oncol200693869970416724351
- JangJSLimHYHwangIGGemcitabine and oxaliplatin in patients with unresectable biliary cancer including gall bladder cancer: a Korean Cancer Study Group phase II trialCancer Chemother Pharmacol201065464164719652971
- VerderameFRussoADi LeoRGemcitabine and oxaliplatin combination chemotherapy in advanced biliary tract cancersAnn Oncol200617Suppl 7vii68vii7216760298
