Abstract
Background
Pulsed electric field (PEF) has been considered as a cell permeability enhancing agent for cancer treatment. Nevertheless, application of PEF for conventional electrochemo-therapy is usually at high intensity, and contact or even invasive electrodes are typically used, which may cause unwanted side effects. In this study, a non-invasive way of applying low intensity, non-contact PEF was adopted to study its combination effect with herb, curcumin, against pancreatic cancer cells and the mechanism involved.
Methods
The pancreatic cancer PANC-1 cells were treated with curcumin and PEF alone or in combination, and MTT assay was used to determine the viability of PANC-1 cells. Apoptosis and uptake of curcumin were analyzed by microscopy and flow cytometry. Western blot was further performed to evaluate the expression of apoptotic proteins.
Results
Our results demonstrated that PEF synergized with curcumin to inhibit the proliferation of PANC-1 cells in a field strength- and dose-dependent manner and caused apoptotic death of PANC-1 cells. The apoptotic induction of combination treatment was characterized by an increase in Bax/Bcl-2 ratio, and cleavage of caspase-8, -9, and -3. Moreover, the increase of curcumin uptake via electro-endocytosis was clearly observed in the cells following the exposure of PEF.
Conclusion
We show for the first time that a non-contact approach using low intensity electric field in a pulsed waveform could enhance the anticancer effect of low-dose curcumin on PANC-1 cells through triggering both extrinsic and intrinsic pathways. The findings highlight the potential of this alternative treatment, non-invasive electric field and curcumin, to increase therapeutic efficacy with minimum cytotoxicity and side effects, which may provide a new aspect of cancer treatment in combination of PEF and other anticancer agents.
Introduction
Pancreatic cancer is one of the most common tumors and the fourth leading cause of cancer-related deaths in men and women.Citation1 It is usually diagnosed at the unresectable stage, and the majority of cases with advanced pancreatic cancer respond poorly to chemotherapy or radiotherapy. Despite improved therapeutic methods, the prognosis of pancreatic cancer still remains poor with a 5-year survival rate of only 2%–27%.Citation2,Citation3 Moreover, the incidence and death rates of pancreatic cancer have been increasing compared to those of most other cancers over the past few years.Citation1 Therefore, there is a continuing need to develop novel agents or alternative strategies to treat pancreatic cancer.
In recent years, natural products have attracted growing scientific attention because of their low toxicity and therapeutic potential against various cancer types.Citation4 Curcumin, a natural phenolic compound isolated from the rhizome of the herb Curcuma longa, has been widely used as a food and in traditional medicine for thousands of years. It has been shown to exhibit diverse pharmacologic properties such as antimicrobial, antioxidant, and anti-inflammatory activitiesCitation5 as well as anticancer activity against various cancer cells.Citation6–Citation9 Curcumin can inhibit cell proliferation and activate a multi-signal transduction pathway related to apoptosis, through the regulation of Bcl-2 family proteins, the release of cytochrome c, and the activation of caspases.Citation10–Citation12 Most of the studies that have demonstrated the anticancer effects of curcumin used concentrations ranging from 10 to 50 µM.Citation6,Citation7,Citation13–Citation17 However, it is important to note that a concentration that is cytotoxic to cancer cells could also be toxic to normal cells. For example, Balasubramanian and Eckert showed that curcumin (10–20 µM) induced the apoptosis in normal human keratinocytes.Citation18 Human retina endothelial cells and T cells have also been shown to undergo apoptosis when they were exposed to curcumin at concentrations of 10 and 25 µM, respectively.Citation19,Citation20 Thus, treatment of cancer cells with lower concentration of curcumin would be a favorable alternative. Unfortunately, there is a major obstacle that curcumin has poor bioavailability due to its water insolubility and instability.Citation21
Pulsed electric field (PEF) has long been investigated as a technique for cancer treatment, known as electrochemotherapy. The basis of electrochemotherapy is the combination of impermeant or poorly permeant anticancer agents and reversible membrane electroporation induced by short, high intensity PEF above hundreds of volts per centimeter to 1 kV/cm.Citation22 However, such a strong electric field may also cause undesirable side effects, which are mainly pain sensation and muscle contraction. Moreover, the high local current density could lead to edema and even local burns in some cases.Citation23 Some approaches have been proposed to reduce the required voltage for drug incorporation into cells. For example, Fulimoto et al have shown the enhancement of the therapeutic effects of drugs by using low intensity with longer duration.Citation24 Shankayi et al have developed the low intensity and higher repetition frequency electrochemotherapy.Citation25 In spite of that, in these studies, the PEF was delivered by the invasive insertion of the electrodes. Furthermore, the decreasing viability of cells has been reported as a cytotoxic effect of electrolysis, which was the expected result of an electric current passing through the samples due to direct contact of electrodes.Citation26
There have been recent studies of PEF using indirect contact of electrodes demonstrating the calculation of electric field effect and the induction of biological effects.Citation27–Citation29 Nevertheless, the electric fields of conducting these experiments are at high intensities (>1,000 V/cm) and near the verge of electric breakdown, which can be a danger of causing electric current to flow through the body. This paper presents the first demonstration of a combination of low-dose curcumin and non-invasive low intensity PEF with contactless electrodes in the cancer treatment of PANC-1 cells. Our results demonstrated that PEF synergized with curcumin to inhibit cancer cell proliferation, accompanied by an increase in Bax/Bcl-2 ratio, and cleavage of caspase-8, -9, and -3. The increased uptake of curcumin also unambiguously confirmed the enhanced cytotoxic effects of curcumin in PANC-1 cells under exposure to PEF. These results first indicate that non-invasive PEF could enable low-dose curcumin to achieve efficient therapeutic effects in the anticancer treatment of PANC-1 cells, which may provide a new aspect of cancer treatment in combination of PEF and other anticancer agents.
Materials and methods
Cell culture
Human pancreatic cancer cell line PANC-1 and human embryonic kidney (HEK293) cells were obtained from the Bioresource Collection and Research Center of the Food Industry Research and Development Institute (Hsinchu, Taiwan, Republic of China). Cells were plated in 75 cm3 cell culture flasks and grown in high-glucose Dulbecco’s Modified Eagle’s Medium (DMEM) (HyClone, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) (Hyclone) and 1% penicillin-streptomycin (Gibco Life Technologies, Thermo Fisher Scientific) in a humidified 5% CO2 incubator at 37°C.
PEF application
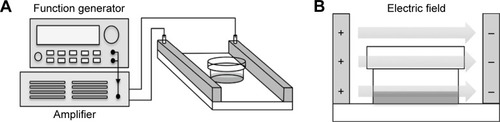
The externally applied PEF of various intensities (10, 30, 60, 90, and 120 V/cm) at 2 Hz with pulse width 2 ms was generated by a function generator (Agilent 33,220A; Agilent Technology, Palo Alto, CA, USA), which was connected to the input of a power amplifier (Trek PZD700; Trek Inc., Medina, NY, USA). The electric field device was constructed of two parallel copper plates separated by 40 mm and was connected with the output of the power amplifier (). The electric field was produced between two parallel plates, and the intensity was determined and equivalent to the applied voltage divided by the distance between two plates. The cells were cultured in a 35-mm Petri dish and continuously exposed to PEF for 24–72 hours. According to the dielectric properties, the field can penetrate the plastic of Petri dish, and thus, cells were under exposure to the fields (). Curcumin (Sigma-Aldrich, St Louis, MO, USA) was dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich) as a 10 mg/mL stock solution and stored at −20°C. Cells were divided into four groups with different treatments: 1) a Control group (Ctrl) was without any treatment; 2) a Curcumin group (Cur) was treated with 2 µg/mL curcumin; 3) a PEF group with a series of 2 Hz, 60 V/cm PEF (2 ms duration); and 4) a Combination group (Cur+ PEF) with 2 µg/mL curcumin and a series of 2 Hz, 60 V/cm PEF (2 ms duration).
Figure 1 Schematic diagram of the experimental setup.
Abbreviation: PEF, pulsed electric field.
MTT assay
Cell viability was accessed by 3-(4,5-dimethylthiazol-2-yl)–2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich) assay. Cells were seeded at 6×104 cells per 35 mm Petri dish and incubated overnight. After 48 hours treatment as described above, the cells were incubated in DMEM containing 0.5 mg/mL MTT for 4 hours at 37°C. Then, the medium was removed, and DMSO was added to dissolve the formazan crystals. The supernatant from each sample was transferred into a 96-well plate, and the absorbance was read at 570 nm using Multiskan GO microplate spectrophotometer (Thermo Fisher Scientific).
Hoechst 33342 staining
Hoechst 33342 (Thermo Fisher Scientific) staining was used to detect morphological characteristics of the nucleus. PANC-1 cells were cultured on glass coverslips in 35 mm Petri dishes. Following incubation with 48 hours treatment, cells were washed with phosphate buffered saline (PBS) (Hyclone) and fixed with 4% paraformaldehyde (PFA) (Sigma-Aldrich) for 10 minutes at room temperature. After washing with PBS, cells were stained with Hoechst 33342 for 10 minutes in the dark and then washed again with PBS. The cells were mounted using Fluoroshield mounting medium (Abcam, Cambridge, UK). The photograph of stained cells was taken under a fluorescent microscope (Axio Imager A1, ZEISS) at 40× magnification.
Cleaved caspase-3 immunofluorescence staining
For immunofluorescence staining, fixed cells were then washed with PBS, permeabilized with 0.1% Triton X-100 (BioShop Canada Inc, Burlington, Ontario, Canada) in PBS for 15 minutes. After that, cells were washed with PBS and blocked with 1% bovine serum albumin (BSA) (Bioshop Canada Inc) in PBS for 30 minutes at 37°C, followed by incubation with diluted primary antibodies against cleaved caspase-3 (Cell Signaling Technology, Danvers, MA, USA) overnight at 4°C. Next, cells were washed with PBS and incubated with Alexa647-conjugated donkey anti-rabbit secondary antibody (Jackson ImmunoResearch, West Grove, PA, USA) for 1 hour at 37°C in the dark. After a further wash with PBS, the cells were mounted using Fluoroshield mounting medium with DAPI (Abcam). The photograph was taken under a fluorescent microscope at 20× magnification.
Flow cytometric analysis of apoptosis
Apoptotic cells were examined by the Annexin V-FITC/PI detection kit (BD Biosciences, San Jose, CA, USA). Cells were harvested with trypsin-EDTA (Gibco) and collected after 48 hours treatment. Then, the cells were washed with cold PBS and resuspended in binding buffer containing Annexin V-FITC and PI. The cell suspensions were incubated for 15 minutes at room temperature in the dark and analyzed by FACSCantoTM II system (BD Biosciences).
Western blot analysis
Cells were collected and washed with cold PBS, and then lysed on ice for 30 minutes in lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.25% deoxycholic acid, 1% NP-40, 1.0% Triton X-100, 0.1% SDS, 1 mM EDTA, 1% phosphate, and protease inhibitor cocktail) (Millipore, Billerica, MA, USA). Equivalent amounts of protein were resolved by 10% SDS-PAGE and electrotransferred onto polyvinylidene fluoride membrane (PVDF) (Millipore) in transfer buffer (10 mM CAPS, pH 11.0, 10% methanol) (BioShop Canada Inc). The membranes were blocked with 5% nonfat dry milk/Tris-buffered saline, 0.1% Tween 20 (TBST; blocking buffer) for 1 hour at room temperature and then incubated overnight at 4°C with diluted primary antibodies in blocking buffer. The specific primary antibodies against Bcl-2, cleaved caspase-8, cleaved caspase-9, cleaved caspase-3 (Cell Signaling Technology), Bax (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and β-actin (GeneTex, Irvine, CA, USA) were used. After washing with TBST, the membranes were incubated with HRP-conjugated anti-goat (GeneTex) or anti-rabbit (Jackson Immunoresearch) secondary antibody. Chemiluminescence was detected using WesternBright ECL Western blotting reagent (Advansta Inc., Menlo Park, CA, USA). The intensities of bands were quantified by ImageJ software (NIH).
Cellular uptake of curcumin
After 48 hours treatment, cells were washed with PBS and fixed with 4% PFA for 10 minutes at room temperature. Finally, the cells were mounted using Fluoroshield mounting medium with DAPI for fluorescent imaging at 100× magnification. The absorption of curcumin was also quantified by flow cytometry. Cells were collected and washed with cold PBS. Afterward, curcumin was excited at 420 nm wavelength and detected at 550 nm by flow cytometry. Results were presented as percentage increase of the mean fluorescence intensity of the treated samples, compared to untreated controls.
Statistical analysis
The results were presented as mean ± SD. Statistical analysis using one-way analysis of variance (ANOVA) was performed with SigmaPlot software. The results were considered to be statistically significant when values of p were less than 0.05. Each experiment was done in triplicate.
Results
PEF enhances curcumin-induced inhibition of PANC-1 cell growth
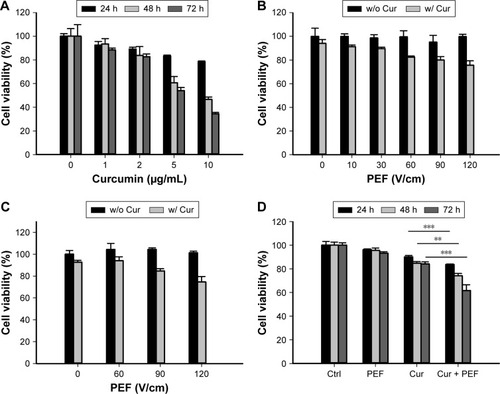
The inhibitory effect of PEF and curcumin alone or in combination on the growth of PANC-1 cells was examined by the MTT assay. In the combination treatment, the cells were continuously exposed to PEF for 24, 48, and 72 hours following the administration of curcumin. As shown in , treatment with curcumin induced a dose- and time-dependent decrease in the viability of PANC-1 cells. However, curcumin at the concentration of 5 and 10 µg/mL sharply decreased cell viability after 48 and 72 hours. Thus, we chose the concentration of 2 µg/mL for the following experiments. We performed the investigation of various PEF intensities ranging from 10 to 120 V/cm to examine its effect on curcumin activity in PANC-1 and non-cancer HEK293 cells. As shown in , our results showed that the efficacy of curcumin in PANC-1 cells was enhanced under the exposure of PEF in an intensity-dependent manner, except at the lower intensity of 10 and 30 V/cm. Although the combination of curcumin with PEF at either 90 or 120 V/cm cooperatively reduced the viability of PANC-1 cells, it also caused a decrease in the cell viability of non-cancer HEK293 cells (). Notably, curcumin in combination with 60 V/cm PEF showed the capability of inducing cytotoxicity in PANC-1 cells, but was found non-harmful toward non-cancer HEK293 cells. This indicates that non-cancer HEK293 cells treated with the co-treatment of curcumin and PEF shows less sensitivity as compared with pancreatic cancer PANC-1 cells. Based on these results, we studied the effect of curcumin in combination with 60 V/cm PEF on PANC-1 cells for the subsequent experiments. As shown in , the viability of PANC-1 cells in the curcumin group can be further reduced in a time-dependent manner when combined with 60 V/cm PEF. On the other hand, cell proliferation of the PEF group did not differ from that of the control group, indicating that PEF alone did not cause much damage to the cells. These results showed that the combination treatment significantly inhibited the cell viability of PANC-1 cells and that the PEF synergistically enhanced the antiproliferative effects of curcumin in PANC-1 cells.
Figure 2 Effects of curcumin and PEF alone or in combination on PANC-1 and HEK293 cell proliferation.
Abbreviation: PEF, pulsed electric field.
PEF enhances curcumin-induced apoptosis in PANC-1 cells
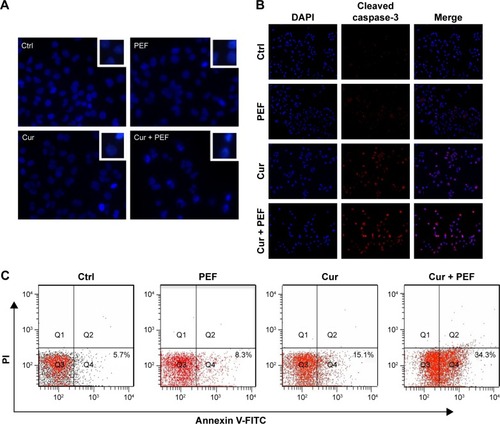
To confirm whether the reduction in cell viability was associated with induction of apoptosis, Hoechst 33342 staining and immunofluorescent detection were initially performed. As shown in , following treatment with PEF and curcumin alone or in combination for 48 hours, the morphological alterations, such as condensation of chromatin and nuclear fragmentation, were clearly observed. In addition, immunofluorescent staining also demonstrated the changes in expression of cleaved caspase-3 (). Both of these results indicate that the cells underwent apoptotic processes. In the further experiment, cells were analyzed by flow cytometry using Annexin V-FITC/PI staining to quantify the extent of apoptosis. Apoptotic rates were 7.6%±1.7%, 8.9%±2.3%, 15.5%±2.3%, and 31.6%±3.5% for control, PEF, curcumin, and curcumin combined with PEF, respectively (). These results suggest that combination treatment has a more prominent effect in inducing apoptosis than their respective individual treatments.
Figure 3 Combination treatment with PEF and curcumin induces apoptosis in PANC-1 cells.
Abbreviation: PEF, pulsed electric field.
Induction of apoptosis through alterations of Bcl-2 family proteins and caspases activation
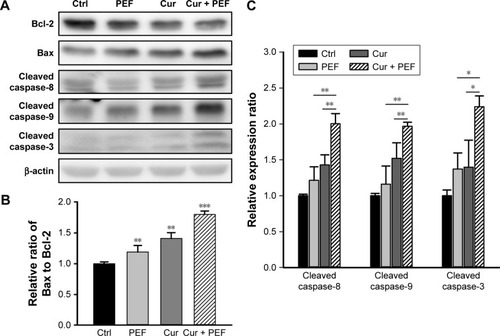
To further investigate the molecular mechanism of apoptosis induced by combination treatment with PEF and curcumin in PANC-1 cells, the expression levels of Bcl-2, Bax, and caspase-8, -9, and -3 were examined after 48 hours treatment. The Western blot analysis in shows that PEF co-treated with curcumin decreased Bcl-2 and increased Bax protein levels, which in turn led to a significant increase in the Bax to Bcl-2 ratio (). In addition, the expression levels of cleaved caspase-8, -9, and -3 were upregulated significantly after combination treatment (). These results suggest that upregulation of Bax and cleaved caspase-8, -9, and -3 expression, and downregulation of Bcl-2 expression could mediate apoptosis of PANC-1 cells induced by combination treatment with PEF and curcumin.
Figure 4 Combined effects of curcumin and PEF on expression of apoptosis-related proteins.
Abbreviation: PEF, pulsed electric field.
Cellular uptake of curcumin
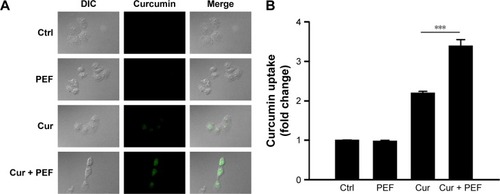
Based on the electrically enhanced effects on antiproliferation and pro-apoptosis mentioned above, the effect of PEF on cellular uptake of curcumin was studied. Here, fluorescence microscopy was used to visualize cellular uptake of cur-cumin, which is known to exhibit green fluorescence.Citation30 showed differential interference contrast (DIC) images, fluorescence images, and their merged images of cells after 48 hours treatment. There were no green fluorescence signals in control and PEF groups, while cells treated with curcumin showed clear signals of fluorescence. Particularly, fluorescence in cells treated with PEF and curcumin in combination was more evident. Furthermore, the uptake of curcumin into the PANC-1 cells was quantified by flow cytometry. The relative changes in intracellular curcumin content were detected as shown in . We found that the absorption of curcumin was further enhanced in combination treatment compared with curcumin alone. These results suggest the PEF enhancement of cellular uptake for curcumin is a potential mechanism for the increased anticancer ability.
Figure 5 Visualization and quantification of cellular uptake of curcumin.
Abbreviations: DIC, differential interference contrast; PEF, pulsed electric field; MFI, mean fluorescent intensity.
Discussion
Curcumin exerts anticancer effects in various cancer cells at concentrations generally higher than 10 µM, and such concentrations can cause cytotoxic effect to normal cells. Moreover, it is well known that curcumin suffers from low bioavailability because of its poor absorption. Previous reports have shown that the effectiveness of curcumin could be increased in human breast cancer MCF-7 cells and human leukemia HL-10 cells when electroporation was applied.Citation31,Citation32 However, such intense PEF with invasive electrodes have been reported to cause unwanted side effects. Therefore, research for finding adequate approaches that enable low concentration of curcumin to achieve maximum effect with minimum side effect is of vital importance. In this paper, low intensity PEF was administered non-invasively to investigate its synergistic effect on low-dose curcumin in PANC-1 cells. The concept of such a non-invasive strategy is somewhat similar to that of tumor treating fields (TTF), which is a few volts/centimeter field using attached electrodes.Citation33 However, the method we adopted in this study was not only different in terms of waveform and field strength but also a non-invasive treatment in a non-contact manner. There has been an in vivo rat study using contactless electrodes to investigate the biological effects under the exposure of high voltage (15–20 kV) alternating current electric field,Citation34 in which this concept was also proposed to apply to humans.Citation35 Nevertheless, in our study, we conducted a non-contact experiment using low voltage in a pulsed waveform and a herb, curcumin, to treat cancer for the first time.
Consistent with other reports,Citation36 we observed the time- and dose-dependent cytotoxic effect of curcumin on PANC-1 cells, and we chose curcumin at a low concentration of 2 µg/mL (5.4 µM) to examine the combination effects of PEF and curcumin. Our results showed that the exposure of PEF alone at various intensities did not have a significant change in the viability of PANC-1 and non-cancer HEK293 cells. Nevertheless, the anticancer activity of curcumin in PANC-1 cells can be enhanced under the exposure of PEF, especially at the field strength above 30 V/cm, and the PEF at intensity of 90 and 120 V/cm combined with curcumin was also found toxic to non-cancer HEK293 cells. It is worth mentioning that a reduction in the viability of PANC-1 cells in response to the combination treatment of curcumin and 60 V/cm PEF was not observed in non-cancer HEK293 cells, indicating that the 60 V/cm PEF has selectivity for PANC-1 cells. Besides, the survival rate from the time-course MTT assay demonstrated that the 60 V/cm PEF combined with curcumin exerted synergistic antiproliferative effect on PANC-1 cells in a time-dependent manner (). There was particularly a significant enhancement in the efficacy of curcumin under the continuous application of PEF for 72 hours. Many reports have shown that curcumin alone can be effective against pancreatic cancer. However, it is notable that the concentration of curcumin we used in this study is lower than those used in other research studies.Citation17,Citation37,Citation38 Furthermore, the enhanced cytotoxic efficacy of curcumin by non-invasive PEF demonstrated comparable result to the application of standard PEF on conventional drugs or curcumin.Citation31,Citation39,Citation40 Most importantly, the approach performed in this study is a non-invasive, gentle, and long-term continuous treatment using contactless electrodes. In view of the modifiability of the electric field to focus on a specific location and the non-cytotoxicity of the combination treatment in HEK293 cells, this approach presents a means to achieve a cancer-specific effect in a safe manner.
There are different forms of programmed cell death reported in recent studies, such as apoptosis, autophagy, and programmed necrosis.Citation41 Induction of apoptosis has been recognized as a major form of cell death and response to anticancer agents.Citation42 As we know, both the nuclear morphology alterations and the activation of caspase-3 are typical characteristics of cell apoptosis. Consistent with the results of the MTT assay, Hoechst and immunofluorescence analyses demonstrated that PANC-1 cells treated with curcumin alone exhibited mild apoptotic cell death, while combination treatment with PEF and curcumin was more effective in inducing apoptotic bodies along with activation of caspase-3. Consistently, a synergistic effect in increasing apoptosis rate was also observed by Annexin V-FITC/PI staining when cells were co-treated with PEF and curcumin. Our results suggest that apoptosis plays an important role in the enhanced anticancer effects of combination treatment with PEF and curcumin.
Apoptosis occurs through two main signaling pathways, the extrinsic death receptor-mediated pathway or intrinsic mitochondria-mediated pathway, which are activated by initiator caspase-8 and -9, respectively. A critical enzyme involved in both pathways is the effector caspase-3, which results in cleavage of a number of substrate proteins essential for cell growth.Citation43 Moreover, the Bcl-2 family proteins, including pro-apoptotic protein Bax and antiapoptotic protein Bcl-2, play a crucial role in the activation of caspases and the regulation of apoptosis. Studies have shown that the ratio of Bax to Bcl-2 determines the susceptibility of cancer cells to apoptosis.Citation44 Here, we found that the Bax/Bcl-2 ratio in curcumin-treated cells was further increased by PEF exposure. In addition, the levels of active caspase-8, -9 and the downstream of active caspase-3 were also markedly increased in combination treatment comparing to that of either treatment alone. This was in agreement with the results of immunofluorescence staining. These results suggest that PEF synergistically enhanced curcumin-induced apoptosis through both intrinsic and extrinsic pathways in PANC-1 cells. Furthermore, it has been reported that curcumin alone can trigger apoptosis through both pathways in pancreatic cancer,Citation17,Citation45 indicating that PEF might be an assisted agent for curcumin.
In view of earlier studies that demonstrated facilitated molecular uptake under PEF stimulation,Citation46 we examined the effect of PEF on curcumin uptake efficacy. In the combination treatment, the increased cellular uptake of curcumin under PEF exposure was clearly observed, and this provided direct evidence of enhanced anticancer effect. The exposure of cells to external electric field is known to result in a change in the transmembrane potential. When the transmembrane potential of cells exceeds its threshold value, the permeability of cell membrane is increased due to the formation of pores, a process called electroporation.Citation47 However, when the transmembrane potential is lower than its threshold value, extracellular molecules are incorporated into the cells via endocytosis.Citation26,Citation46 The transmembrane potential is theoretically given by ΔVm =1.5×E×a×cosθ; where E is the intensity of the applied electric field, a is the cell radius, and θ is the angle between the radial vector for a given location on cell membrane and the vector of electric field.Citation48 Thus, exposure of PANC-1 cells with a diameter of ≈34 µm to electric field strength of 60 V/cm used in our experiment leads to an induced transmembrane potential ΔV=153 mV, which is below the breakdown threshold of mammalian cell (200–1,500 mV).Citation49 It suggests that the observed increase of curcumin uptake into cells did not involve electroporation, but rather endocytosis under low intensity PEF treatment. Therefore, this non-invasive electro-endocytosis combined with curcumin could be effective and safe for treating cancers. Further studies are needed to evaluate the effects of pulse duration as well as frequency on cytotoxicity enhancement of curcumin in the future. We believe that this approach can be extended to other PEF therapy in fighting cancer, thereby widening the therapeutic window.
Conclusion
We show for the first time that the antiproliferative and pro-apoptotic effects of low-dose curcumin were strengthened when PANC-1 cells were exposed to non-invasive PEF in a non-contact manner. Our results demonstrated that PEF synergized with curcumin in inducing cell death via activation of extrinsic and intrinsic caspase pathways. Also, pro-apoptotic Bax and antiapoptotic Bcl-2 proteins were shown to be involved. Moreover, increased uptake of curcumin into cells under PEF exposure in combination treatment clearly gives evidence for enhanced cytotoxic effects. These findings may provide support to develop an application of non-invasive electric field for cancer therapy.
Acknowledgments
We would like to thank Technology Commons in College of Life Science, National Taiwan University for use of the flow cytometry system, and the staff of the imaging core at the First Core Labs, National Taiwan University Hospital for technical assistance. This work was supported by grants from the Ministry of Science and Technology (MOST105-2112 -M-002–006-MY3; CY Chao) and Ministry of Education (NTU-ICRP-103R7560-2; CY Chao) of Taiwan, Republic of China.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2016CA Cancer J Clin201666173026742998
- FryerRAGalustianCDalgleishAGRecent advances and developments in treatment strategies against pancreatic cancerCurr Clin Pharmacol20094210211219442075
- LiDXieKWolffRAbbruzzeseJLPancreatic cancerLancet200436394141049105715051286
- MansonMMCancer prevention – the potential for diet to modulate molecular signallingTrends Mol Med200391111812524205
- MaheshwariRKSinghAKGaddipatiJSrimalRCMultiple biological activities of curcumin: a short reviewLife Sci200678182081208716413584
- MilacicVBanerjeeSLandis-PiwowarKRSarkarFHMajumdarAPDouQPCurcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivoCancer Res200868187283729218794115
- BachmeierBEMohrenzIVMirisolaVCurcumin downregulates the inflammatory cytokines CXCL1 and -2 in breast cancer cells via NFkappaBCarcinogenesis200829477978917999991
- SahuRPBatraSSrivastavaSKActivation of ATM/Chk1 by curcumin causes cell cycle arrest and apoptosis in human pancreatic cancer cellsBr J Cancer200910091425143319401701
- ShehzadALeeJLeeYSCurcumin in various cancersBiofactors2013391566823303705
- MasuelliLBenvenutoMFantiniMCurcumin induces apoptosis in breast cancer cell lines and delays the growth of mammary tumors in neu transgenic miceJ Biol Regul Homeost Agents201327110511923489691
- BushJACheungKJLiGCurcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53Exp Cell Res2001271230531411716543
- MoragodaLJaszewskiRMajumdarAPCurcumin induced modulation of cell cycle and apoptosis in gastric and colon cancer cellsAnticancer Res2001212A87387811396178
- UddinSHussainARManogaranPSCurcumin suppresses growth and induces apoptosis in primary effusion lymphomaOncogene200524477022703016044161
- ShimJSLeeHJParkSSChaBGChangHRCurcumin-induced apoptosis of A-431 cells involves caspase-3 activationJ Biochem Mol Biol2001343189193
- MagalskaASliwinskaMSzczepanowskaJSalvioliSFranceschiCSikoraEResistance to apoptosis of HCW-2 cells can be overcome by curcumin- or vincristine-induced mitotic catastropheInt J Cancer200611981811181816721786
- WaltersDKMuffRLangsamBBornWFuchsBCytotoxic effects of curcumin on osteosarcoma cell linesInvest New Drugs200826428929718071634
- ZhaoZLiCXiHCurcumin induces apoptosis in pancreatic cancer cells through the induction of forkhead box O1 and inhibition of the PI3K/Akt pathwayMol Med Rep20151245415542226166196
- BalasubramanianSEckertRLCurcumin suppresses AP1 transcription factor-dependent differentiation and activates apoptosis in human epidermal keratinocytesJ Biol Chem200728296707671517148446
- PremanandCRemaMSameerMZSujathaMBalasubramanyamMEffect of curcumin on proliferation of human retinal endothelial cells under in vitro conditionsInvest Ophthalmol Vis Sci20064752179218416639030
- MagalskaABrzezinskaABielak-ZmijewskaAPiwockaKMosieniakGSikoraECurcumin induces cell death without oligonucleosomal DNA fragmentation in quiescent and proliferating human CD8+ cellsActa Biochim Pol200653353153816951739
- LiuALouHZhaoLFanPValidated LC/MS/MS assay for curcumin and tetrahydrocurcumin in rat plasma and application to pharmacokinetic study of phospholipid complex of curcuminJ Pharm Biomed Anal200640372072716316738
- MiklavcicDCorovicSPuciharGPavseljNImportance of tumour coverage by sufficiently high local electric field for effective electro-chemotherapyEur J Cancer Suppl20064114551
- SnojMCemazarMSlekovec KolarBSersaGEffective treatment of multiple unresectable skin melanoma metastases by electrochemotherapyCroat Med J200748339139517589984
- FulimotoTMaedaHKuboKEnhanced anti-tumour effect of cisplatin with low-voltage electrochemotherapy in hamster oral fibro-sarcomaJ Int Med Res200533550751216222883
- ShankayiZFiroozabadiSMHassanZSOptimization of electric pulse amplitude and frequency in vitro for low voltage and high frequency electrochemotherapyJ Membr Biol2014247214715424271721
- AntovYBarbulAMantsurHKorensteinRElectroendocytosis: exposure of cells to pulsed low electric fields enhances adsorption and uptake of macromoleculesBiophys J20058832206222315556977
- NovacBMBanakhrFASmithIRDemonstration of a novel pulsed electric field technique generating neither conduction currents nor joule effectsIEEE Trans Plasma Sci2014421216228
- FrelingerALGerritsAJGarnerALModification of pulsed electric field conditions results in distinct activation profiles of platelet-rich plasmaPLoS One2016118e016093327556645
- RobinsonVSGarnerALLovelessAMNeculaesVBCalculated plasma membrane voltage induced by applying electric pulses using capacitive couplingBiomed Phys Eng Express201732025016
- KunwarABarikAPandeyRPriyadarsiniKITransport of liposomal and albumin loaded curcumin to living cells: an absorption and fluorescence spectroscopic studyBiochim Biophys Acta20061760101513152016904830
- RamachandranRPMadhivananSSundararajanRLinCWYSankaranarayananKAn in vitro study of electroporation of leukemia and cervical cancer cellsSundararajanRElectroporation-Based Therapies for Cancer: From Basics to Clinical ApplicationsCambridgeWoodhead Publishing Ltd2014161183
- CamarilloIGXiaoFMadhivananSLow and high voltage electro-chemotherapy for breast cancer: an in vitro model studySundararajanRElectroporation-Based Therapies for Cancer: From Basics to Clinical ApplicationsCambridgeWoodhead Publishing Ltd201455102
- HottingerAFPachecoPStuppRTumor treating fields: a novel treatment modality and its use in brain tumorsNeuro Oncol201618101338134927664860
- HarakawaSInoueNHoriTEffects of a 50 Hz electric field on plasma lipid peroxide level and antioxidant activity in ratsBioelectromagnetics200526758959416037959
- HaraAOgawaYElectric field therapy apparatusUS Patent19894802470
- ParasramkaMAGuptaSVSynergistic effect of garcinol and curcumin on antiproliferative and apoptotic activity in pancreatic cancer cellsJ Oncol2012201270973922685460
- JutooruIChadalapakaGLeiPSafeSInhibition of NFkappaB and pancreatic cancer cell and tumor growth by curcumin is dependent on specificity protein down-regulationJ Biol Chem201028533253322534420538607
- OstermanCJLynchJCLeafPCurcumin modulates pancreatic adenocarcinoma cell-derived exosomal functionPLoS One2015107e013284526177391
- SaczkoJKamińskaIKotulskaMCombination of therapy with 5-fluorouracil and cisplatin with electroporation in human ovarian carcinoma model in vitroBiomed Pharmacother201468557358024975085
- VásquezJLGehlJHermannGGElectroporation enhances mitomycin C cytotoxicity on T24 bladder cancer cell line: a potential improvement of intravesical chemotherapy in bladder cancerBioelectrochemistry20128812713322940093
- NikoletopoulouVMarkakiMPalikarasKTavernarakisNCrosstalk between apoptosis, necrosis and autophagyBiochim Biophys Acta20131833123448345923770045
- FerreiraCGSpanSWPetersGJKruytFAGiacconeGChemotherapy triggers apoptosis in a caspase-8-dependent and mitochondria-controlled manner in the non-small cell lung cancer cell line NCI-H460Cancer Res200060247133714111156422
- ElmoreSApoptosis: a review of programmed cell deathToxicol Pathol200735449551617562483
- RaisovaMHossiniAMEberleJThe Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosisJ Invest Dermatol2001117233334011511312
- YounsMFathyGMUpregulation of extrinsic apoptotic pathway in curcumin-mediated antiproliferative effect on human pancreatic carcinogenesisJ Cell Biochem2013114122654266523794119
- RosembergYKorensteinRIncorporation of macromolecules into cells and vesicles by low electric fields: induction of endocytotic-like processesBioelectrochem Bioenerg1997422275281
- KotnikTFreyWSackMHaberl MegličSPeterkaMMiklavčičDElectroporation-based applications in biotechnologyTrends Biotechnol201533848048826116227
- DebruinKAKrassowskaWModeling electroporation in a single cell. I. Effects of field strength and rest potentialBiophys J19997731213122410465736
- GowrishankarTRPliquettULeeRCDynamics of membrane sealing in transient electropermeabilization of skeletal muscle membranesAnn N Y Acad Sci199988819521010842634
