Abstract
We first describe the application of natural orifice specimen extraction surgery in the treatment of a rectal implantation metastasis tumor from ovarian cancer. One patient diagnosed with recurrent rectal implantation metastasis 1 year after the removal of ovarian cancer successfully underwent transanal specimen extraction via laparoscopic rectectomy without an abdominal incision at the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College in March 2017. The operation time was 118 minutes, and the intraoperative blood loss was 5 mL. The specimen was extracted via the anus during the operation, and the resection margin was negative. The patient recovered well without complications. Anal function was normal, and the stoma and abdominal incision were well healed after 1 month of follow-up. This study supports the idea that the application of natural orifice specimen extraction surgery for rectal implantation metastasis from ovarian cancer is safe and feasible and can achieve satisfactory outcomes.
Introduction
Ovarian cancer has four different types of spread: peritoneal implantation, lymphatic, direct, and hematogenous. The most common forms of dissemination are peritoneal implantation and lymphatic drainage.Citation1 The incidence of colorectal implantation metastases is as high as 30%–39% in advanced stage ovarian cancer.Citation2 Therefore, the resection of colorectal implantation metastases is an important component of tumor reductive surgery in the treatment of ovarian cancer. Natural orifice specimen extraction surgery (NOSES), considered a prequel of natural orifice transluminal endoscopic surgery (NOTES), eliminates morbidity and postoperative pain related to the extraction surgical site and has been applied in the treatment of selected patients with colorectal carcinoma.Citation3,Citation4 Here, we present a case of recurrent rectal implantation metastasis 1 year after the removal of ovarian cancer where the patient successfully underwent transanal specimen extraction via laparoscopic rectectomy without an abdominal incision. The patient involved in this study gave her written informed consent authorizing the use and disclosure of the details and accompanying images published.
Case report
Medical history and examination
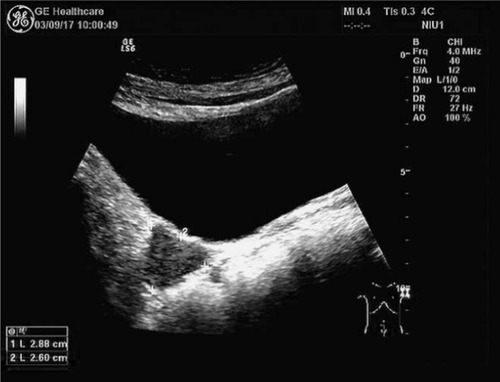
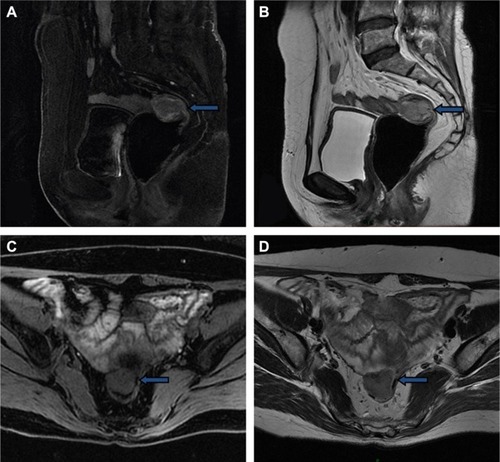
A 57-year-old woman was diagnosed with ovarian cancer and underwent laparoscopic extensive total hysterectomy, ovariectomy, pelvic lymphadenectomy, periaortic lymph node biopsy, omentectomy, and appendectomy in December 2015. Operative exploration showed mild ascites in the pelvic cavity. The right ovary had adhered to the right fallopian tube and presented as an 8×7×6 cm3 irregular mass. Some cystic neoplasms covered the mass and adhered to the rectum closely. A 3×2×2 cm3 cauliflower-like mass was found at the surface of the rectum after separation of the right ovary and fallopian tube. All the aforementioned masses were removed completely. Postoperative pathology showed right ovarian high-grade serous adenocarcinoma, and the tumor had invaded the serosal layer of the fallopian tube. No lymph node metastases were detected. The patient then received six cycles of chemotherapy with paclitaxel and cisplatin, with the last cycle completed on April 28, 2016. She had no follow-up issues until February 2017, when blood tests showed an increased CA12-5 tumor marker with a level of 44.81 U/mL. As a result, the patient underwent positron emission tomography/computed tomography (PET/CT). A 2.8×2.8 cm2 nodule with increased glucose uptake was found at the right front site of the upper rectum, and this nodule clung to the right wall of the rectum; the maximum standard uptake value was 16.8. No other signs of recurrence or metastasis were detected. A 2.9×2.6 cm2 hypoechoic solid tumor with abundant blood flow signal was observed behind the bladder via abdominal ultrasound (). Preoperative pelvic magnetic resonance imaging showed that a 3.1×2.6 cm2 nodule at the right front wall of the upper rectum with a blurred outer edge and a well-defined inner edge had invaded the rectum with a slightly high T2weighted image/fat suppression signal and a low diffusion-weighed imaging signal (). The lesion also showed significantly circular enhancement. The patient had no previous history of an abnormality in this region. No discrete mass was observed on vagino-recto-abdominal examination.
Surgery and pathology
The patient underwent transanal specimen extraction via laparoscopic rectectomy without an abdominal incision on March 14, 2017.
Preoperative preparation
From 2 pm to 6 pm on the day prior to surgery, 70 mg of polyethylene glycol Macrogol per 1 L of water was given to the patient four times (1 L per hour).
Trocar placement
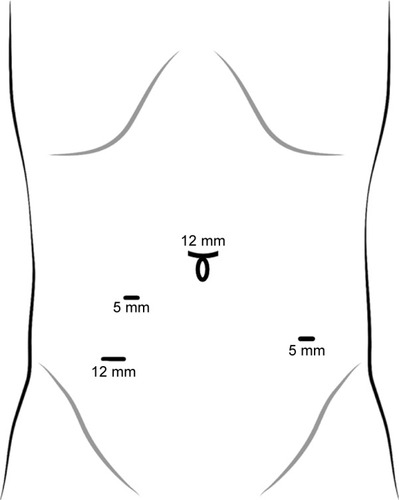
Under general anesthesia, the patient was placed in the Trendelenburg position. A 12-mm observation port was inserted under the umbilicus, and then three ports were placed after the establishment of pneumoperitoneum. One 12-mm diameter port was placed in the right lower abdomen, one 5-mm port was placed in the right paraumbilicus, and a 5-mm port was placed in the left lower abdomen ().
Excision of tumor
Operative exploration showed mild ascites and no deposits in the pelvic cavity. No abnormality was detected in the liver, gallbladder, pancreas, or spleen. The mass (with a diameter of 4 cm) was located at the right front wall of the middle rectum and had invaded the gut cavity (). A cytological examination of the pelvic lavage fluid and a biopsy examination of the remaining omentum and pelvic lateral peritoneum were performed prior to total mesorectal excision. The sigmoid mesocolon and mesorectum were dissected along the inner side of the ureters by harmonic scalpel. The inferior mesenteric artery was ligated distal to the origin of the ascending branch of the left colic artery with Hem-o-lock, and the inferior mesenteric vein was ligated at the corresponding site. Intestines were dissected at 3 cm from the distal margin of the tumor and 3 cm from the proximal margin of the tumor via harmonic scalpel ().
Figure 4 Transanal specimen extraction via laparoscopic rectectomy without an abdominal incision.
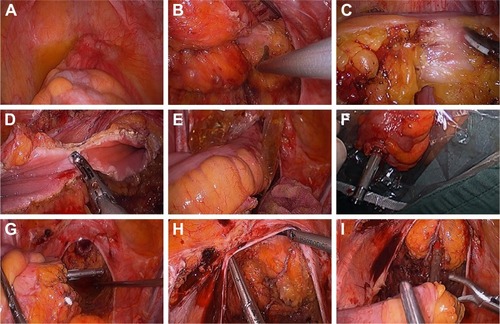
Specimen extraction and digestive tract reconstruction
After copious rectal stump irrigation by iodophor diluent, the distal rectum was dissected circularly 2 cm from the distal margin of the tumor by a harmonic scalpel and the broken ends were sterilized by iodophor gauze (). A sterile protector was introduced into the peritoneal cavity via trocar and then extracted through the rectal stump before the specimen was extracted and obtained for further pathological evaluation. The transected bowel was pulled out in continuity via the anus using sponge forceps (). The distal circular stapling device anvil was fixed extracorporeally with a purse-string suture (). The colon was then repositioned into the abdomen through the rectal stump (). Next, the rectal stump was closed (), the proximal anvil of the circular stapling device in the rectum was opened, and a straight end-to-end circular anastomosis was performed (). Finally, one drainage tube was placed in the pelvic cavity surrounding the anastomosis site, and another transanal drainage tube was placed at the proximal site of the anastomosis in the rectal stump.
Surgical and pathological outcome
The patient successfully underwent transanal specimen extraction via laparoscopic rectectomy without an abdominal incision or conversion to laparotomy. The operation time was 118 minutes, and intraoperative blood loss was 5 mL. The time to ambulation was 1 day after surgery, and the time to first flatus was 50 hours after surgery. The patient recovered smoothly, and postoperative pain was minimal. The transanal drainage tube was removed 7 days after surgery, and the peritoneal drainage tube was removed 10 days after surgery. Postoperative pathology revealed poorly differentiated adenocarcinoma measuring 5×3.5×2.2 cm3 invading from the rectal serosa into the submucosa (). The tumor cells were arranged in nests, and vessel invasion was present (). The tumor was classified as an implantation metastasis from ovarian serous carcinoma on the basis of medical history and immunochemistry. There was no invasion of the remaining omentum or pelvic lateral peritoneum. No tumor cells were detected in the pelvic lavage fluid, and the resection margins were free of tumor. Among the 14 lymph nodes removed, none contained metastatic cancer. The immunochemical staining () was AE1/AE3(3+), CA125(3+), CK7(3+), P16(3+), WT1(3+), PAX2(3+), PAX8(1+), CK5&6(−), CR(−), MC(−), P63(−), CDX2(−), and CK20(−).
Figure 5 Macroscopic observation of rectal neoplasm.
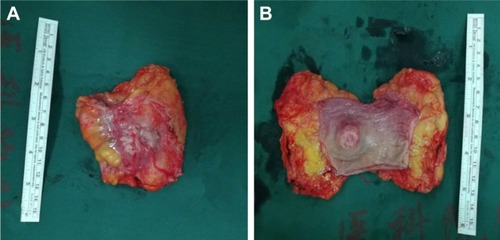
Figure 6 Microscopic observation and immunochemistry of rectal neoplasm.
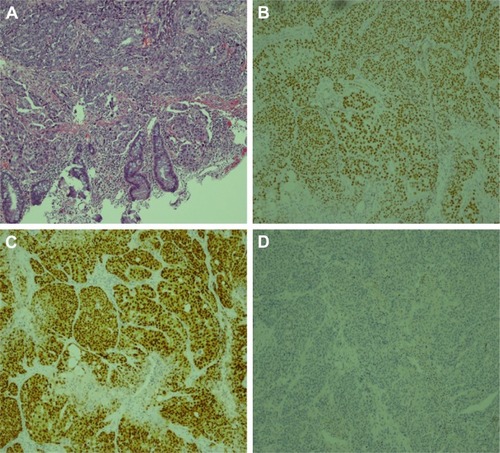
Followup
During hospitalization, the surgeon assessed the patient’s recovery state during daily rounds. Follow-up after discharge was performed by telephone and outpatient visits. The last date of follow-up was April 20, 2017. The level of CA12-5 decreased to 13.69 U/mL half a month after surgery. No postoperative complications such as intraperitoneal bleeding, digestive tract bleeding, abdominal infection, pulmonary infection, organ dysfunction, bowel obstruction, anastomotic leakage, and anastomotic stenosis occurred, and digestive function was well recovered 1 month after surgery. In addition, defecation was under satisfactory control, with well-healed anastomotic stoma and trocar ports postoperatively.
Discussion
One of the main means of spread of ovarian cancer is peritoneal implantation metastasis. The standard treatment of this disease is maximal removal of resectable primary and metastatic lesions even when complete gross resection is not feasible,Citation5 because satisfactory surgical cytoreduction is considered to be a significant predictor for prognosis in patients with the disease.Citation6 In this case, a solitary nodule invading the rectum with increased glucose uptake was detected by PET/CT, suspicious for implantation metastasis from ovarian cancer. As a result, surgical resection was the most optimal treatment option.
NOSES combines the scarless idea of NOTES with the operating advantage of laparoscopic surgery, creating a bridge between routine laparoscopic colorectal surgery and NOTES.Citation7 In addition to better cosmetic results,Citation3 the absence of an abdominal incision leads to less postoperative pain, early return of gastrointestinal function, and early ambulation. Moreover, NOSES precludes surgical site infection and incisional hernia.Citation8–Citation10
Although many studies have reported primary colorectal carcinoma treated by NOSES,Citation3,Citation4,Citation7–Citation12 the application of NOSES in the treatment of rectal implantation metastasis of ovarian cancer has not been described. We successfully performed transanal specimen extraction via laparoscopic rectectomy without an abdominal incision in this patient with recurrent rectal implantation from ovarian cancer. In addition, the specimen was extracted from the rectal stump completely with negative resection margins. No intraoperative and postoperative complications occurred in this patient, and the postoperative pain was minimal. Therefore, NOSES is a safe and feasible option in the treatment of rectal implantation metastasis from ovarian cancer. However, the use of NOSES is highly dependent on the patient’s clinical condition and is suitable only for small, solitary resectable metastatic tumors. In addition, experienced laparoscopic operational skills, tacit surgical cooperation, adequate preoperative bowel preparation, and aseptic and nontumor intraoperative techniques are strictly required. We have previously reported on our experience with NOSES in the treatment of primary colorectal cancer.Citation13 We also minimized the risk of contamination by using adequate bowel preparation with polyethylene glycol Macrogol and by performing a distal rectal washout before removing the surgical specimen via the rectum.
Conclusion
Our study supports the idea that NOSES for rectal implantation metastasis from ovarian cancer is safe and feasible and obtains a satisfactory cosmetic result with less invasiveness in well-selected patients. With the growing popularity of laparoscopic surgery, we believe that NOSES will be increasingly applied in colorectal disease, benefiting more patients.
Author contributions
HS collected the data and drafted the manuscript; HZ designed the study and helped revise the manuscript; BL collected the surgical specimens; WR participated in the discussions of the postoperative pathology; MB, ZZ, XW, and QL conceived the study and participated in its coordination; PW participated in the data interpretation. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This research was supported by The Terry Fox Run Foundation from Cancer Foundation of China (LC2016B10) and CAMS Initiative for Innovative Medicine (CAMS-2017-I2M-4–002). The funders had no role in study design, data collection and analysis, decision to publish, or in the preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- TrastourCRahiliASchumackerCEffiABBernardJLHematogenous rectal metastasis 20 years after removal of epithelial ovarian cancerGynecol Oncol200494258458815297210
- ZhangSZhiXLiuNLiuXIntestinal metastasis of ovarian cancer: clinical analysis of 64 cases (in Chinese)Chin J Curr Adv Gen Surg200692110112
- WolthuisAMFieuwsSvan den BoschAde Buck van OverstraetenAD’HooreARandomized clinical trial of laparoscopic colectomy with or without natural-orifice specimen extractionBr J Surg2015102663063725764376
- WangXAdvances of minimally invasive technique in colorectal cancer surgeryZhonghua Wei Chang Wai Ke Za Zhi201619662162327353095
- BlandAEEverettENPastoreLMAndersenWATaylorPTPredictors of suboptimal surgical cytoreduction in women with advanced epithelial ovarian cancer treated with initial chemotherapyInt J Gynecol Cancer200818462963617986246
- WallaceSKumarAMc GreeMEfforts at maximal cytoreduction improve survival in ovarian cancer patients, even when complete gross resection is not feasibleGynecol Oncol20171451212628159407
- LeungALCheungHYLiMKAdvances in laparoscopic colorectal surgery: a review on NOTES and transanal extraction of specimenAsian J Endosc Surg201471111624165166
- FranklinMELiangSRussekKIntegration of transanal specimen extraction into laparoscopic anterior resection with total mesorectal excision for rectal cancer: a consecutive series of 179 patientsSurg Endosc201327112713222833263
- SaurabhBChangSCKeTWNatural Orifice Specimen Extraction With Single Stapling Colorectal Anastomosis for Laparoscopic Anterior Resection: Feasibility, Outcomes, and Technical ConsiderationsDis Colon Rectum2017601435027926556
- WolthuisAMPenninckxFD’HooreALaparoscopic sigmoid resection with transrectal specimen extraction has a good short-term outcomeSurg Endosc20112562034203821136110
- TelemDAHanKSKimMCTransanal rectosigmoid resection via natural orifice translumenal endoscopic surgery (NOTES) with total mesorectal excision in a large human cadaver seriesSurg Endosc2013271748022752277
- LiSYChenGBaiXAnus-preserving rectectomy via telescopic colorectal mucosal anastomosis for low rectal cancer: experience from a Chinese cohortWorld J Gastroenterol201319243841384623840123
- ZhouHTZhouZXLiangJWWangZZhangXMHuJJAnalysis of 21 cases treated by total laparoscopic rectosigmoid cancer surgery with transanal natural orifice specimen extractionZhonghua Yi Xue Za Zhi201393262082208424169293