Abstract
Purpose
Strategies to increase radiosensitivity are urgently needed. Combining radiosensitizing reagents with radiotherapy could improve the outcome of cancer treatment. Some preclinical studies showed that sepantronium bromide (YM155) could sensitize cancer cells to radiation by inhibiting the survivin protein. In this study, we try to investigate the function of YM155 on radiosensitivity of esophageal squamous cell carcinoma (ESCC) cells.
Materials and methods
ESCC cell lines were treated with radiation and YM155, and the radiation efficacy was evaluated by cell counting kit-8 assay and clonogenic survival assay. Cell senescence was measured by senescence-associated β-galactosidase staining. Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling assay, fluorescein isothiocyanate-labeled Annexin V/propidium iodide assay, and poly ADP-ribose polymerase cleavage were used to detect apoptosis. KYSE150 xenografts model was used to test the efficacy of radiation combined with YM155.
Results
YM155 could inhibit the upregulation of survivin induced by radiation in all ESCC cell lines, but the efficacy of radiosensitization varied in different cell lines. Radiation-induced senescence in KYSE150 and KYSE410 cells, and the combination with YM155 inhibited senescence and promoted apoptosis of ESCC cells, thereby enhancing radiosensitivity. Combination with YM155 and radiation delayed the growth of KYSE150 xenografts in nude mice by switching radiation-induced senescence to apoptosis. When p21 was inhibited in KYSE150 cells, radiation did not induce senescence, and the radiosensitization of YM155 was also attenuated. In KYSE510 and KYSE180 cells, radiation did not induce senescence, and YM155 could not enhance the radiosensitivity.
Conclusion
Our results suggest a new mechanism that YM155 might sensitize ESCC cells to radiation by switching radiation-induced senescence to apoptosis. The major determinant of radiosensitization by YM155 might be the induction of senescence by radiation.
Introduction
Esophageal cancer is a common cancer in China that is reported to have an incidence rate of 477.9 per 100,000 in 2015.Citation1 Surgery is the primary treatment for esophageal cancer; however, radiotherapy and chemotherapy are also widely used in combination based on the clinical stage.Citation2–Citation4 Radiotherapy is usually combined with chemotherapy and targeted therapy in the clinic. However, the radiotherapeutic effect remains unsatisfactory, and radioresistance is highly common in clinical treatment, leading to possible treatment failure. Therefore, strategies to increase radiosensitivity are urgently needed.
Radiation mainly induces DNA damage, following which cells enter apoptosis, necrosis, mitotic catastrophe, autophagy, cell cycle arrest, and/or senescence.Citation5 Several aspects of these pathways contribute to the effects of radiotherapy, such as p53,Citation6 EGFR,Citation7 miRNAs,Citation8 and certain immune factors.Citation9 Several approaches have been evaluated to enhance the efficiency of radiotherapy. MiR-338-5p enhanced the radiosensitivity of esophageal squamous cell carcinoma (ESCC) by inducing apoptosis through targeting survivin.Citation10 Downregulation of ROGDI can mediate radiosensitivity by blocking cells at G2/M, the most radiosensitive phase of the cell cycle.Citation11 Sunitinib sensitized ESCC cells to radiation by inducing DNA double-strand breaks.Citation12 Cordycepin produced radiosensitization by inducing p53-mediated apoptosis and modulating the expression of cell cycle checkpoint molecules.Citation13 However, to date, notably few approaches have advanced to clinical trials.
Survivin is a multifunctional protein involved in apoptosis, cell division, and senescence.Citation14,Citation15 Survivin is overexpressed in multiple types of cancers, and survivin overexpression predicts resistance to chemotherapy and radiotherapy.Citation16,Citation17 Survivin appears to be an attractive target in cancer treatment, and various strategies have been used to target survivin.Citation18,Citation19 YM155 is the first small-molecule inhibitor to be developed, which could inhibit survivin expression by inhibiting the survivin upstream transcription factor Sp1.Citation20 YM155 has been tested in clinical studies as a single agent or combined with chemotherapy.Citation21–Citation23
Recently, some preclinical studies showed that YM155 could sensitize cancer cells to radiation by inhibiting the survivin protein. The molecular mechanism included inhibition of DNA repair, enhancement of apoptosis, and abrogation of G2 checkpoint.Citation24–Citation26 We previously reported that a high expression of survivin predicts poor prognosis in ESCC following radiotherapy.Citation27 In this study, we investigated the function of YM155 in sensitizing ESCC cells to radiation in vitro and in vivo. We observed that YM155 treatment led to different consequences in different ESCC cell lines and concluded that the molecular mechanism contributed to the difference in efficacies. Our results provided an evidence for the potential use of YM155 in certain cancers to enhance radiosensitivity.
Materials and methods
Cell culture and transfection
The human ESCC cell lines KYSE150, KYSE410, KYSE180, and KYSE510, generously provided by Dr Yutaka Shimada,Citation28 were cultured in RPMI1640 medium containing 10% fetal bovine serum and supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C with 5% CO2. Transfection was performed in 70%–80% confluent cells using Attractene Transfection Reagent (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions.
Reagents and plasmids
Sepantronium bromide (YM155) was purchased from Selleck Chemicals (Houston, TX, USA). For in vitro experiments, YM155 was dissolved in saline and diluted with culture medium. For in vivo experiments, YM155 was dissolved and diluted in saline immediately before administration. pSilencer3.0H1-shRNA-p21 was constructed as described previously.Citation29
Cell viability analysis
A total of 4 × 103 cells/well were seeded in 96-well plates, incubated overnight, and treated with YM155 at various concentrations (0, 5, 10, 25, 50, and 100 nmol/L). Forty-eight hours later, cell viability was assessed using the cell counting kit-8 (CCK-8) assay (Dojindo, Kumamoto, Japan). The absorbance was determined at 450 nm using an iMark microplate reader (Bio-Rad Laboratories Inc., Hercules, CA, USA) according to the manufacturer’s instructions.
Clonogenic survival
The ESCC cells were seeded at a density of ~5 × 102 cells per well into 6-well plates. After cell adhesion, the cells were treated with YM155 for 24 h and subjected to irradiation with 4 Gy X-rays. The cells were later washed with PBS for three times, cultured in drug-free medium for ~10 days, fixed with methanol, and stained with Giemsa (Solarbio Life Sciences, Beijing, China). Only colonies containing more than 50 cells were scored. All experiments were conducted at least three times.
Western blot analysis
Cells were harvested and washed in PBS, homogenized in radio immunoprecipitation assay buffer (Cell Signaling Technology, Danvers, MA, USA), and centrifuged at 14,000 × g for 15 min at 4°C. After the concentrations were determined using a BCA Protein Quantification Kit (Thermo Fisher Scientific, Waltham, MA, USA), the extracted proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked with Tris-buffered saline with Tween 20 (TBS-T) buffer containing 5% nonfat milk for 1 h at room temperature and incubated overnight at 4°C with the following primary antibodies: survivin (1:1,000; Cell Signaling Technology), p53 (1:1,000; Proteintech), p21 (1:1,000; Proteintech), poly ADP-ribose polymerase (PARP) (1:1,000; Cell Signaling Technology), β-actin (Sigma-Aldrich Co., St Louis, MO, USA), and caspase-8 (1:1,000; Cell Signaling Technology). After being washed with TBS-T buffer, the membranes were incubated with secondary antibodies (ZSGB Biotechnology, Beijing, China) for 1 h at room temperature. The signals were detected using detection reagents (Engreen Biosystem Co. Ltd., Beijing, China) according to the manufacturer’s instructions.
Senescence-associated β-galactosidase (SA-β-Gal) detection
Cells treated under different conditions were subjected to SA-β-Gal staining using the Senescence Cells Staining Kit (Sigma-Aldrich Co.). Briefly, after being washed with PBS for three times, the cells were fixed with a fixative solution for 5–10 min followed by incubation with the staining solution at 37°C for 2–24 h. Green-stained senescent cells were identified under a light microscope (Olympus CX41).
Annexin V-FITC/PI assay
For the fluorescein isothiocyanate (FITC)-labeled annexin V (Annexin V-FITC)/PI (propidium iodide) assay, we used the Apoptosis Detection Kit (BD Biosciences, San Jose, CA, USA). In brief, the cells were collected by trypsinization and washed with PBS for three times. After centrifugation at 120 × g, the cells were suspended and incubated with 1 mL of Annexin V-FITC solution for 30 min at room temperature in dark, and PI was added to a final concentration of 1 mg/mL. The samples were then analyzed by flow cytometer LSR II (BD Biosciences) using the FlowJo software (Tree Star Inc., Ashland, OR, USA).
Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay
Apoptosis was detected using the In Situ Cell Death Detection Kit, AP (Hoffman-La Roche Ltd., Basel, Switzerland). In brief, fixed cells or frozen slides were permeabilized with TritonX-100 in 0.1% sodium citrate for 2 min on ice. The slides were later incubated with TdT enzyme at 37°C for 1 h and exposed to Converter-AP at 37°C for 30 min, after which the samples were incubated with the substrate solution for 10 min at 15°C–25°C in the dark. The labeling on the cells was examined by light microscopy (Olympus CX41).
Immunohistochemistry staining
Sections (5 μm thick) of paraffin-embedded tissue were placed on glass slides, rehydrated, incubated with 3% hydrogen peroxide to quench endogenous peroxidase activity, and blocked by incubation with 5% bovine serum albumin in PBS. The sections were subsequently incubated with the survivin (1:200; Cell Signaling Technology) and p21 (1:200; Proteintech) antibodies. After washing, the slides were incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody for 1 h at room temperature. The slides were incubated with 3,3′-diaminobenzidine (ZSGB Biotechnology) and counterstained with hematoxylin (37%). The sections were analyzed under a light microscope (Olympus CX41), and the quantitation data were analyzed by Image Pro Plus software.
Xenograft tumor studies
All experimental procedures using animals were reviewed and approved by the Institutional Animal Care Committee at the Cancer Hospital of the Chinese Academy of Medical Science and our protocols followed the guidelines in China and the AAALAC protocol for the Care and Use of Animals in Research. Five- to six-week-old female BALB/C nude mice were provided by Beijing HFK Bioscience Co. Ltd (Beijing, China). A suspension of 1 × 106 KYSE150 cells in 0.1 mL saline was injected subcutaneously. The tumor volume was determined by caliper measurement of tumor length (L) and width (W) according to the formula LW2/2. Twenty nude mice with established tumors (all 150–200 mm3) were divided into four groups and treated with 1) vehicle (saline) alone; 2) a single dose of 10 Gy irradiation (IR); 3) YM155 alone (5 mg/kg as 7-day continuous intraperitoneal injections); or 4) YM155 plus IR (a single fraction of 10 Gy IR delivered on day 3 of drug treatment). X-ray radiation was delivered by a 6 MV linear accelerator (Varian Medical Systems, Palo Alto, CA, USA) at a dose rate of 300 cGy/min with a source-to-target distance of 100 cm. For tumor irradiation, animals were positioned to place the tumor in the center of a 1.0 × 1.0 cm radiation field with the remaining mice being shielded from radiation. Body weight and tumor diameter were measured three times per week. The efficacy of each treatment was evaluated by the volume change during the treatment period. The first day of treatment was designed as day 0, and observation continued until day 18. At the end of the observation, the mice were sacrificed, and the tumors were fixed in 10% formalin.
Statistical analysis
All data with error bars are presented as the mean ± SD. The significance of differences between treatment and control mean values in all experiments was determined by the two-tailed Student’s t-test, and p < 0.05 was considered to be statistically significant. All calculations were performed using GraphPad Prism software.
Results
YM155 inhibited the upregulation of survivin induced by radiation
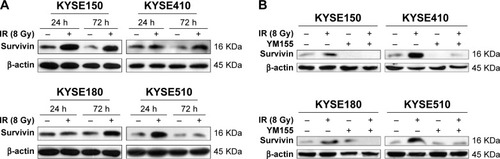
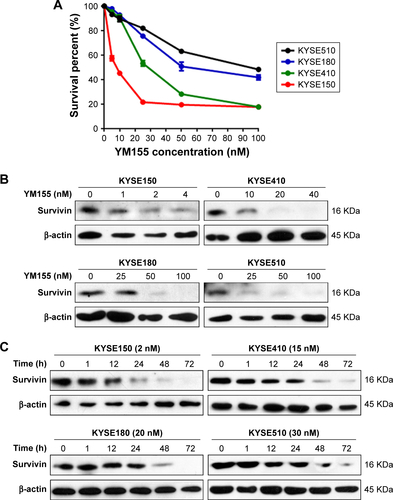
First, ESCC cells were treated with radiation, and Western blot analysis showed that survivin could be upregulated by 8 Gy radiation in all four ESCC cell lines (). The effect of YM155 on cell survival was evaluated. The CCK-8 assay showed that after 48 h of YM155 treatment, YM155 inhibited cell survival in a dose-dependent manner (). Then, the effect of YM155 on the survivin expression was evaluated; the effective inhibition concentration of YM155 varied in different cell lines (). Thus, the subtoxic concentrations of YM155 were chosen for treatment of the cells (2, 15, 20, and 30 nM for KYSE150, KYSE410, KYSE180, and KYSE510, respectively) in the following experiments. Western blot analysis showed that the survivin protein decreased 48–72 h after YM155 treatment (). Moreover, YM155 inhibited survivin upregulation induced by radiation (). These results indicated that survivin was upregulated by radiation and that YM155 inhibited the upregulation.
Figure 1 YM155 inhibited the upregulation of survivin induced by radiation in ESCC cells. (A) ESCC cells were harvested and the cell lysates were subjected to immunoblot analysis with antibodies against survivin. (B) Survivin was detected 72 h after 8 Gy radiation with or without YM155 treatment. β-actin was used as an internal control.
Radiosensitization of YM155 varied in different ESCC cell lines
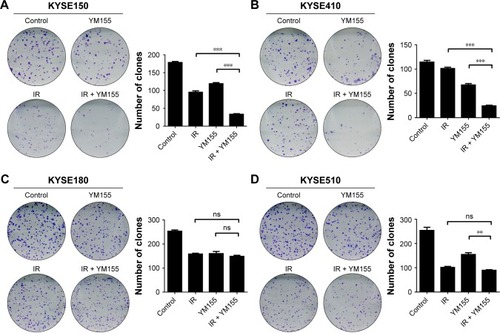
Next, the efficacy of YM155 as a radiosensitizer in ESCC cells was evaluated. The effect of YM155 on cell survival was assessed by the colony formation assay in a panel of four ESCC cell lines. Compared with the control cells, the survival fraction was significantly decreased after radiation combined with YM155 in the KYSE150 and KYSE410 cells () but not in the KYSE180 and KYSE510 cells (). Therefore, YM155 could sensitize KYSE150 and KYSE410 cells to radiation, but combination with YM155 could not enhance the effect of radiation in KYSE180 and KYSE510 cells. These results for the first time indicated that the radiosensitization effect of YM155 varied in different ESCC cell lines.
Figure 2 Radiosensitization of YM155 varied in different ESCC cell lines. Long-time viability of cells was determined by the colony-formation assay. The cells were treated with YM155, irradiated as illustrated and fixed after incubation for 10–14 days. The number of clones is shown in the histogram (right) (**p < 0.01; ***p < 0.001). The data are represented as the mean from three independent experiments. (A) KYSE150, (B) KYSE410, (C) KYSE180, and (D) KYSE510. Values are represented as the mean ± SD (n = 3) for each treatment.
YM155 could enhance radiosensitivity of ESCC cells when radiation induced senescence
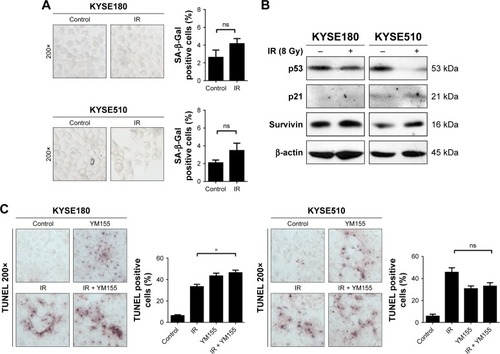
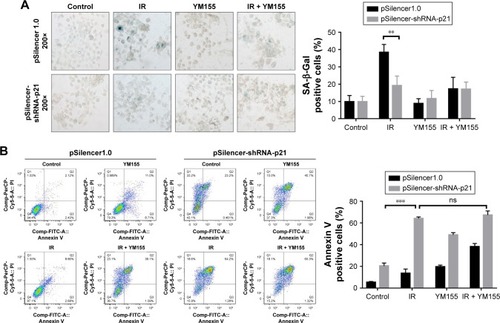
Cell senescence was checked by cytochemical detection of the activity of SA-β-Gal. The result showed that 53% of KYSE150 and 65% of KYSE410 cells were SA-β-Gal positive, 72 h after the 8 Gy irradiation. With the YM155 treatment, the SA-β-Gal positive cells decreased to 9% and 16% in the KYSE150 and KYSE410 cells, respectively (). Western blot analysis showed that p53 and p21 were upregulated after 8 Gy irradiation and that YM155 inhibited the upregulation ().
Figure 3 Radiation induced senescence in KYSE150 and KYSE410 cells and YM155 reduced senescence but enhanced apoptosis. (A) Representative images of SA-β-Gal (green) staining for the KYSE150 and KYSE410 cells. The cells were treated with YM155 1 day before exposure to 8 Gy radiation. The cells were subjected to SA-β-Gal staining 3 days later; the quantifications are shown on the right. Values are represented as the mean ± SD (n = 3) for each treatment (**p < 0.01, ***p < 0.001). (B) The protein levels of p53, p21, and survivin in cells with or without treatment were evaluated by Western blotting, and β-actin was used as an internal control. (C) Representative images of TUNEL assay (purple) staining for the KYSE150 and KYSE410 cells; the quantifications are shown on the right. Values are represented as the mean ± SD (n = 3) for each treatment (**p < 0.01, ***p < 0.001). (D) The protein levels of PARP and caspase-8 in cells with or without treatment were evaluated by Western blotting, and β-actin was used as an internal control. (E) The cells were stained by Annexin V/PI and analyzed by flow cytometry. Values are represented as the mean ± SD (n = 3) for each treatment (***p < 0.001).
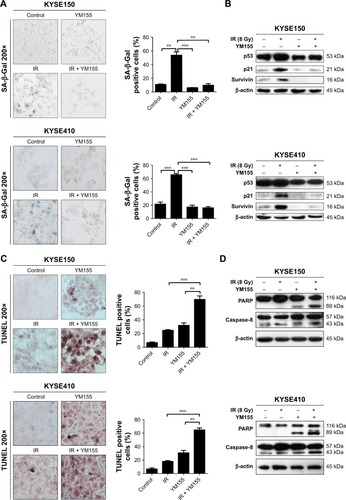
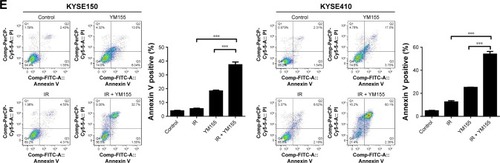
Additionally, cell apoptosis induced by radiation was detected. The TUNEL assay showed that radiation and YM155 could both induce apoptosis, but the combination of YM155 with radiation significantly increased the percentage of apoptotic cells from 24% to 69% for the KYSE150 cells and 18% to 65% for the KYSE410 cells (). PARP cleavage was significantly induced after YM155 treatment combined with radiation in the KYSE150 and the KYSE410 cells (). In agreement with the TUNEL assay result, Annexin V/PI staining showed that the combination of YM155 with radiation significantly increased the percentage of double-staining cells in the KYSE150 and KYSE410 cells (). These data indicated that YM155 could inhibit cell senescence and promote apoptosis of ESCC cells when radiation induced cell senescence.
YM155 could not enhance radiosensitivity of ESCC cells when radiation did not induce senescence
Cell senescence was evaluated in the KYSE180 and KYSE510 cells, and few SA-β-Gal positive cells were observed after radiation (). Additionally, Western blot analysis showed no p53 or p21 upregulation (). The TUNEL assay showed that radiation and YM155 could both induce apoptosis, but the combination of YM155 with radiation did not increase the percentage of apoptosis cells in the KYSE180 and KYSE510 cells (). The data suggest that when radiation did not induce senescence, as in the KYSE180 and KYSE510 cells, YM155 could not enhance radiosensitivity.
Figure 4 YM155 could not enhance apoptosis in KYSE510 and KYSE180 cells. (A) Representative images of SA-β-Gal (green) staining for the KYSE510 and KYSE180 cells. The cells were treated with YM155 1 day before exposure to 8 Gy radiation. The cells were subjected to SA-β-Gal staining 3 days later; the quantifications are shown on the right. Values are represented as the mean ± SD (n = 3) for each treatment (ns, p > 0.05). (B) The protein levels of p53, p21, and survivin were evaluated by Western blotting. (C) Representative images of TUNEL assay (purple) staining for the KYSE510 and KYSE180 cells; the quantifications are shown on the right. Values are represented as the mean ± SD (n = 3) for each treatment (*p < 0.05).
YM155 enhanced radiation-induced tumor inhibition of KYSE150 cells in xenograft model
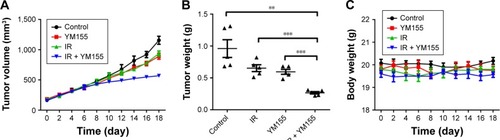
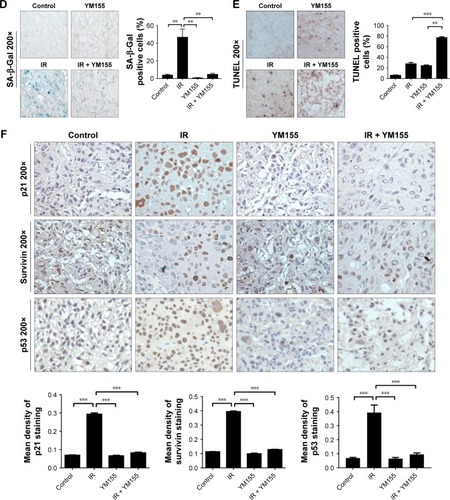
To test the effects of the radiosensitivity of YM155 in vivo, KYSE150 cells were subcutaneously injected into nude mice. After tumor formation, the mice were treated as described in the “Materials and methods” section. As shown in , tumor volumes and tumor weights were significantly inhibited in the group treated with YM155 in combination with radiation. No significant body weight loss was observed in the mice treated with this combination compared with those treated with YM155 alone (). In agreement with the in vitro results, SA-β-Gal staining was observed after radiation, and the SA-β-Gal positive cells decreased in tumors treated with YM155 and radiation (). In contrast, the TUNEL assay showed that the combination of YM155 with radiation significantly increased the percentage of apoptosis (). Immunohistochemical analysis showed that p53, p21, and survivin were upregulated in irradiated tumor cells but inhibited when radiation was combined with the YM155 treatment (). These data show that radiation could induce cell senescence in the KYSE150 xenograft, and YM155 could enhance tumor inhibition by reducing senescence and promoting apoptosis.
Figure 5 YM155 could enhance radiosensitivity of KYSE150 xenografts. (A) Tumor volume was measured at the indicated times after the onset of treatment. Values are represented as the mean ± SD from five mice per group. (B) The mice were sacrificed, and tumor weight was measured. Values are represented as the mean ± SD from five mice per group. (C) Mice body weight was measured at the indicated times after the onset of treatment. (D) Representative images of SA-β-Gal (green) staining for tumor sections of different treatments; quantifications are shown on the right. (E) Representative images of TUNEL assay (purple) staining for tumor sections of different treatments; quantifications are shown on the right. (F) Immunohistochemistry analysis of p53, p21, and survivin expression in tumor sections after different treatments; quantifications are shown below the images. **p < 0.01; ***p < 0.001.
Radiosensitization of YM155 was dependent on radiation-induced senescence
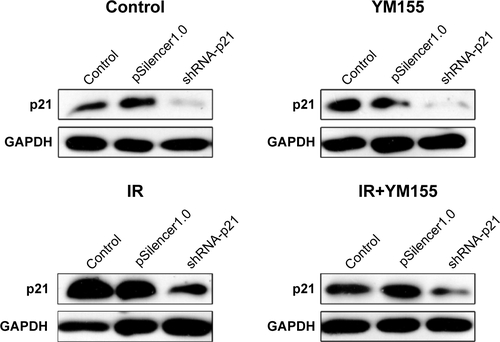
To determine whether the radiosensitization of YM155 was dependent on radiation-induced senescence, pSilencer3.0H1-shRNA-p21 was transfected into KYSE150 cells. As shown in , p21 was inhibited in the KYSE150 cells before and after irradiation. SA-β-Gal staining showed that the percentage of radiation-induced senescent cells decreased when p21 was inhibited (). Next, cell apoptosis was evaluated by using Annexin V-PI staining, and the result showed increased apoptosis in the p21-knockdown cells; however, the apoptotic ratio after radiation in the p21-knockdown KYSE150 cells showed no significant difference with or without YM155 treatment (). Therefore, p21 inhibition could enhance radiation-induced apoptosis, but YM155 could not enhance the radiation efficacy in the KYSE150 cells without radiation-induced senescence.
Figure 6 Knocking down the expression of p21 diminished the radiosensitization of YM155 in KYSE150 cells. (A) Representative images of SA-β-Gal (green) staining for KYSE150 cells of different treatments; quantifications are shown on the right. (B) Representative images of Annexin V/PI staining for cells under different conditions; quantifications are shown on the right. **p < 0.01; ***p < 0.001.
Discussion
Because of survivin’s specific expression in cancer cells and correlation with poor prognosis of patients, this protein has been a very attractive therapeutic target in several types of cancers. Inhibition of survivin as a therapeutic strategy has been evaluated in in vitro and in vivo studies.Citation19 In addition to targeting survivin alone in clinical trials, combination treatment with chemotherapy and radiotherapy has also been considered an attractive option. It has been reported that downregulation and inhibition of survivin could enhance the radiosensitivity of cancer cells.Citation30,Citation31
YM155, a small-molecule inhibitor of survivin, could sensitize cancer cells to radiation both in vitro and in vivo.Citation24–Citation26 Radiation could upregulate survivin in the ESCC cell lines TE4 and TE8.Citation32 In our study, survivin was upregulated by radiation in all ESCC cell lines; therefore, we used YM155 as a survivin inhibitor and confirmed that YM155 could inhibit the upregulation of survivin induced by radiation. But the radiosensitization effect of YM155 varied in different ESCC cell lines. This study is the first that demonstrates the difference in efficacy of YM155 in promoting the radiosensitivity of ESCC cells. Recently published data reported that YM155 could promote radiation-induced clonogenic cell death of Eca109 and TE3 cells, but the sensitization enhancement ratio of the two cell lines exhibited no large difference.Citation24 Targeted therapy was only effective in specific conditions. For example, herceptin was used in breast cancer patients with ErbB2 overexpression;Citation33 EGFR with specific mutations conferred sensitivity to gefitinib and erlotinib,Citation34 and EGFR monoclonal antibody could be combined with radiotherapy when EGFR is overexpressed.Citation35 A previous study showed that YM155 could suppress survivin expression and therefore inhibit the growth of cancer cells.Citation36,Citation37 YM155 could reverse cisplatin resistance in head and neck cancer cellsCitation38 and enhance the sensitivity of docetaxel in human malignant melanoma models.Citation39 Additionally, YM155 could enhance radiosensitization in ESCC and NSCLC cells.Citation24–Citation26 However, no study discussed differences in inhibition by YM155. Because we observed a difference in radiosensitization efficacy, we explored the mechanism and determined the cause of this difference. We have detected the expression of survivin in ESCC cell lines;Citation40 however, the radiosensitization efficacy was not consistent with survivin expression. Therefore, we supposed that the radiosensitive efficiency of YM155 was not determined by survivin expression alone.
Depending on the level of DNA damage and cell type, radiation induces cell cycle arrest, DNA repair, senescence, or apoptosis.Citation41 In our study, the apoptosis and senescence of ESCC cells was tested. We observed that YM155 could promote radiosensitivity only when radiation induced senescence of ESCC cells. When the senescence pathway was inhibited by inhibiting p21 in the KYSE150 cells, the radiosensitization efficiency of YM155 was diminished. These results suggested that survivin was important in radiation-induced senescence and that inhibition of survivin by YM155 could inhibit senescence and promote apoptosis of ESCC cells. In senescent cells treated with a low-dose doxorubicin, survivin was upregulated to maintain their survival by inhibiting apoptosis.Citation42 An anti-survivin siRNA nanodrug (MN-siBIRC5) could enhance the therapeutic efficacy of low-dose doxorubicin when doxorubicin induced senescence-like morphological changes.Citation43 Therefore, the major determinant of radiosensitization by YM155 could be the ability of radiation to induce senescence.
Cell senescence could be induced by several stimuli and is usually controlled by p53 or p16-Rb pathway.Citation44 Ionizing radiation induced senescence primarily through the p53–p21 pathways.Citation41,Citation44,Citation45 We observed that p53 and p21 were upregulated in radiation-induced senescent cells, but there was no significant p16 upregulation. The result indicated that senescence induced by 8 Gy radiation in ESCC cells mainly depended on the p53–p21 pathways. When p21 protein upregulation was inhibited by RNAi, radiation did not induce senescence in the KYSE150 cells, and the radiosensitization efficiency of YM155 was diminished.
p53 status in cancer cells could determine the response of cancer therapy.Citation46,Citation47 It is generally accepted that tumors with wild-type p53 show a p53-mediated senescence response to DNA damage.Citation48–Citation50 Disruptive TP53 mutations increased radioresistance via the inhibition of senescence in headneck squamous cell carcinoma tumors.Citation51 Cells with mutant p53 underwent mitotic catastrophe and apoptosis after chemotherapy.Citation52 After sequenced p53 exons of ESCC cells used in our study, we observed deletion mutations in exon7 of p53 in KYSE510 and KYSE180 cells, leading to amino acid deletion in the DNA binding site of the p53 protein (data not shown). According to our results, KYSE150 and KYSE410 attained senescence after radiation, but the KYSE510 and KYSE180 cells did not. Therefore, we could suppose that the cells of patients with wide-type p53 were more likely to be induced to senescence and might show better response to YM155 treatment combined with radiation. Although additional studies are required to test the correlation of p53 status and the radiosensitive efficiency of YM155, our study has discussed a potential strategy of combining YM155 treatment with radiotherapy in ESCC patients.
Conclusion
We found the efficacy of YM155 radiosensitizing activity varied in different ESCC cell lines and the difference was not determined by survivin expression only. Our results suggest a new pathway through which YM155 could partially sensitize ESCC cells to radiation by inhibiting radiation-induced senescence and enhancing apoptosis. The major determinant of radiosensitization by YM155 might be the induction of senescence by radiation.
Author contributions
HZ conceived the idea of the project and designed most of the experiments. XL conducted most of the experiments, analyzed the results, and wrote the paper. YZ is the main designer of animal experiment. MH contributed to the preparation of figures. WZ and YG provided a lot of experience and intellectual support in the process of the experiment. ML, ZX, NX, and SL provided expertise and feedback. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work. All authors approved the final submitted version.
Acknowledgments
This work was supported by the National Natural Science Foundation (31171322, 81272512, 81321091) and CAMS Innovation Fund for Medical Sciences (2016-I2M-1-001).
Supplementary materials
Figure S1 YM155 suppressed protein levels of survivin in a dose-dependent and time-dependent manner in human ESCC cells. (A) Dose–response curve for four ESCC cell lines after YM155 treatment for 48 h. The survival curves of these cells were constructed by CCK-8 assay. (B) ESCC cells were treated with the indicated concentrations of YM155 for 48 h and then total cell lysates were assayed for survivin by Western blotting. (C) ESCC cells were treated with YM155 for the indicated times, and the expression of survivin was analyzed by immunoblotting. β-actin was used as an internal control.
Disclosure
The authors report no conflicts of interest in this work.
References
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- MarietteCPiessenGTribouletJPTherapeutic strategies in oesophageal carcinoma: role of surgery and other modalitiesLancet Oncol20078654555317540306
- OhashiSMiyamotoSKikuchiOGotoTAmanumaYMutoMRecent advances from basic and clinical studies of esophageal squamous cell carcinomaGastroenterology201514971700171526376349
- GoenseLvan RossumPSKandiolerDStage-directed individualized therapy in esophageal cancerAnn N Y Acad Sci201613811506527384385
- DelochLDererAHartmannJFreyBFietkauRGaiplUSModern radiotherapy concepts and the impact of radiation on immune activationFront Oncol2016614127379203
- ZhangJShenLSunLQThe regulation of radiosensitivity by p53 and its acetylationCancer Lett2015363210811825911240
- SakiMMakinoHJavvadiPEGFR mutations compromise hypoxia-associated radiation resistance through impaired replication fork-associated DNA damage repairMol Cancer Res201715111503151628801308
- MuellerAKLindnerKHummelRHaierJWatsonDIHusseyDJMicroRNAs and their impact on radiotherapy for cancerRadiat Res2016185666867727223830
- DererAFreyBFietkauRGaiplUSImmune-modulating properties of ionizing radiation: rationale for the treatment of cancer by combination radiotherapy and immune checkpoint inhibitorsCancer Immunol Immunother201665777978626590829
- ParkMYoonHJKangMCKwonJLeeHWMiR-338-5p enhances the radiosensitivity of esophageal squamous cell carcinoma by inducing apoptosis through targeting survivinSci Rep2017711093228883406
- ChenYFChoJJHuangTHTsengCNHuangEYChoCLDown-regulation of a novel human gene, ROGDI, increases radiosensitivity in cervical cancer cellsCancer Biol Ther201619
- DingYQZhuHCChenXCSunitinib modulates the radio-sensitivity of esophageal squamous cell carcinoma cells in vitroDis Esophagus20162981144115126542732
- Seong daBHongSMuthusamiSKimWDYuJRParkWYCordycepin increases radiosensitivity in cervical cancer cells by overriding or prolonging radiation-induced G2/M arrestEur J Pharmacol2016771778326688569
- AltieriDCSurvivin, versatile modulation of cell division and apoptosis in cancerOncogene200322538581858914634620
- WangQWuPCRobersonRSSurvivin and escaping in therapy-induced cellular senescenceInt J Cancer201112871546155820503268
- AltieriDCValidating survivin as a cancer therapeutic targetNat Rev Cancer200331465412509766
- RodelFHoffmannJDistelLSurvivin as a radioresistance factor, and prognostic and therapeutic target for radiotherapy in rectal cancerCancer Res200565114881488715930309
- AltieriDCSurvivin, cancer networks and pathway-directed drug discoveryNat Rev Cancer200881617018075512
- PeeryRCLiuJYZhangJTTargeting survivin for therapeutic discovery: past, present, and future promisesDrug Discov Today201722101466147728577912
- ChengQLingXHallerASuppression of survivin promoter activity by YM155 involves disruption of Sp1-DNA interaction in the survivin core promoterInt J Biochem Mol Biol20123217919722773958
- GiacconeGZatloukalPRoubecJMulticenter phase II trial of YM155, a small-molecule suppressor of survivin, in patients with advanced, refractory, non-small-cell lung cancerJ Clin Oncol200927274481448619687333
- ClemensMRGladkovOAGartnerEPhase II, multicenter, open-label, randomized study of YM155 plus docetaxel as first-line treatment in patients with HER2-negative metastatic breast cancerBreast Cancer Res Treat2015149117117925547219
- KudchadkarRErnstSChmielowskiBA phase 2, multicenter, open-label study of sepantronium bromide (YM155) plus docetaxel in patients with stage III (unresectable) or stage IV melanomaCancer Med20154564365025533314
- QinQChengHLuJSmall-molecule survivin inhibitor YM155 enhances radiosensitization in esophageal squamous cell carcinoma by the abrogation of G2 checkpoint and suppression of homologous recombination repairJ Hematol Oncol201476225139395
- IwasaTOkamotoISuzukiMRadiosensitizing effect of YM155, a novel small-molecule survivin suppressant, in non-small cell lung cancer cell linesClin Cancer Res200814206496650418927289
- HuSFuSXuXThe mechanism of radiosensitization by YM155, a novel small molecule inhibitor of survivin expression, is associated with DNA damage repairCell Physiol Biochem20153731219123026418254
- ZhuHWangQHuCHigh expression of survivin predicts poor prognosis in esophageal squamous cell carcinoma following radiotherapyTumour Biol20113261147115321826474
- ShimadaYImamuraMWagataTYamaguchiNTobeTCharacterization of 21 newly established esophageal cancer cell linesCancer19926922772841728357
- ZhangJLouXYangSBAG2 is a target of the c-Myc gene and is involved in cellular senescence via the p21(CIP1) pathwayCancer Lett20123181344122146591
- HuJPanJLuoZTaoZDownregulation of survivin by shRNA inhibits invasion and enhances the radiosensitivity of laryngeal squamous cell carcinomaCell Biochem Biophys201572125125725701406
- Ferreiro-NeiraITorresNELiesenfeldLFXPO1 inhibition enhances radiation response in preclinical models of rectal cancerClin Cancer Res20162271663167326603256
- SugaseTTakahashiTSeradaSSOCS1 gene therapy improves radiosensitivity and enhances irradiation-induced DNA damage in esophageal squamous cell carcinomaCancer Res201777246975698629042418
- ShakSOverview of the trastuzumab (Herceptin) anti-HER2 monoclonal antibody clinical program in HER2-overexpressing metastatic breast cancer. Herceptin Multinational Investigator Study GroupSemin Oncol1999264 Suppl 12717710482196
- RielyGJPaoWPhamDClinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinibClin Cancer Res2006123 Pt 183984416467097
- BasavarajCSierraPShivuJMelarkodeRMonteroENairPNimotuzumab with chemoradiation confers a survival advantage in treatment-naive head and neck tumors over expressing EGFRCancer Biol Ther201010767368120647773
- NakaharaTKitaAYamanakaKYM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenograftsCancer Res200767178014802117804712
- KitaANakaharaTYamanakaKAntitumor effects of YM155, a novel survivin suppressant, against human aggressive non-Hodgkin lymphomaLeuk Res201135678779221237508
- KumarBYadavALangJCYM155 reverses cisplatin resistance in head and neck cancer by decreasing cytoplasmic survivin levelsMol Cancer Ther20121191988199822723337
- YamanakaKNakaharaTYamauchiTAntitumor activity of YM155, a selective small-molecule survivin suppressant, alone and in combination with docetaxel in human malignant melanoma modelsClin Cancer Res201117165423543121737502
- WangYZhuHQuanLDownregulation of survivin by RNAi inhibits the growth of esophageal carcinoma cellsCancer Biol Ther20054997497816082195
- ErikssonDStigbrandTRadiation-induced cell death mechanismsTumour Biol201031436337220490962
- MaKXuQWangSNuclear accumulation of Yes-Associated Protein (YAP) maintains the survival of doxorubicin-induced senescent cells by promoting survivin expressionCancer Lett20163751849126944315
- GhoshSKYigitMVUchidaMSequence-dependent combination therapy with doxorubicin and a survivin-specific small interfering RNA nanodrug demonstrates efficacy in models of adenocarcinomaInt J Cancer201413471758176624114765
- CampisiJd’Adda di FagagnaFCellular senescence: when bad things happen to good cellsNat Rev Mol Cell Biol20078972974017667954
- MirzayansRAndraisBScottAMurrayDNew insights into p53 signaling and cancer cell response to DNA damage: implications for cancer therapyJ Biomed Biotechnol2012201217032522911014
- MirzayansRAndraisBScottAWangYWMurrayDIonizing radiation-induced responses in human cells with differing TP53 statusInt J Mol Sci20131411224092243524232458
- BiegingKTMelloSSAttardiLDUnravelling mechanisms of p53-mediated tumour suppressionNat Rev Cancer201414535937024739573
- Tonnessen-MurrayCALozanoGJacksonJGThe regulation of cellular functions by the p53 protein: cellular senescenceCold Spring Harb Perspect Med201772 pii:a026112
- WidelMLalikAKrzywonAPoleszczukJFujarewiczKRzeszowska-WolnyJThe different radiation response and radiation-induced bystander effects in colorectal carcinoma cells differing in p53 statusMutat Res2015778617026099456
- MirzayansRScottACameronMMurrayDInduction of accelerated senescence by gamma radiation in human solid tumor-derived cell lines expressing wild-type TP53Radiat Res20051631536215606307
- SkinnerHDSandulacheVCOwTJTP53 disruptive mutations lead to head and neck cancer treatment failure through inhibition of radiation-induced senescenceClin Cancer Res201218129030022090360
- VarnaMLehmann-CheJTurpinE p53 dependent cell-cycle arrest triggered by chemotherapy in xenografted breast tumorsInt J Cancer2009124499199719048622