Abstract
Background
Study on the relationship between glutathione-S-transferase Pi 1 (GSTP1) and P16 promoter region methylation and the risk of hepatitis B virus-related hepatocellular carcinoma (HBV-related HCC) has produced inconsistent results.
Objectives
To assess the correlation between GSTP1 and P16 promoter methylation frequency and HBV-related HCC susceptibility.
Methods
All relevant studies were identified by searching PubMed, Embase, Web of Science, and China National Knowledge Infrastructure literature databases before December, 2017. The OR and the corresponding 95% CI were calculated to investigate the risk of GSTP1 and P16 promoter methylation rate and HBV-related HCC. Sensitivity analysis was performed and publication bias was estimated using the Begg’s and Egger’s test.
Results
Our meta-analysis identified the relationships of GSTP1 (six studies including 213 HBV-related HCC tumor tissues) and P16 (nine studies with 287 HBV-related HCC tumor tissue) promoter methylation with HCC risk. Compared with normal liver tissue and cirrhosis, the pooled ORs of GSTP1 promoter region methylation in HBV-related HCC cancer tissues were 6.05 (95% CI =1.20–30.52) and 5.21 (95% CI =2.19–12.41), respectively. Compared with paracancerous tissue, normal liver tissue, cirrhosis, and chronic hepatitis B as controls, the pooled ORs of P16 promoter region methylation in HBV-related HCC cancer tissues were 7.18 (95% CI =2.31–22.33), 24.89 (95% CI =3.38–183.03), 5.92 (95% CI =1.78–19.68), and 12.12 (95% CI =0.75–196.50).
Conclusion
In summary, our meta-analysis found strong associations between GSTP1 and P16 gene promoter methylation and an increased HBV-related HCC susceptibility. Moreover, GSTP1 and P16 methylation in promoter region could obviously increase the risk of HBV-related HCC in patients with cirrhosis, indicating that these would be promising biomarkers for early clinical diagnosis of HBV-related HCC.
Introduction
Hepatocellular carcinoma (HCC), one of the leading malignancies, is a major cause of cancer-related death worldwide.Citation1 As is known to all, HCC is a consequential complication of cirrhosis.Citation2 In addition, epidemiological studies have provided overwhelming evidence that hepatitis B virus (HBV) infection is the main risk factor associated with the occurrence of chronic hepatitis B (CHB), cirrhosis, and HCC. Globally, 257 million people are chronic HBV carriers, and nearly one-quarter of them will develop HCC.Citation3–Citation5 Therefore, the progression of HCC in persons persistently infected with HBV is of great significance. However, the mechanisms contributing to the HBV-related HCC have not been fully elucidated.
DNA methylation, a common epigenetic alteration, plays an important role in the regulation of gene expressions and other life activities. Previous studies have shown that aberrant promoter hypermethylation of many tumor suppressor genes (TSGs), which will lead to transcriptional repression and loss of gene function, may play a major role in the progression of tumorigenesis.Citation6–Citation8 In recent years, several aberrant methylations of tumor-associated genes have been observed in HCC.Citation9–Citation11 In consideration of the significance of HBV in the occurrence of HCC, it is necessary to further study whether aberrant DNA methylation of specific genes exerts a potential role in early detection of HBV-related HCC.
The glutathione S-transferase pi 1 (GSTP1) gene is considered to be a major TSG located on chromosome 16p13, belonging to a family of phase II metabolic enzymes, and plays a critical role in the prevention of hepatocyte mutation.Citation12 In addition, the p16INK4A gene is a cell cycle-related gene located on chromosome 9p21 and plays a key role in cell cycle regulation.Citation13,Citation14 Preliminary reports have reported that GSTP1 and P16 promoter hypermethylation leads to the downregulation of gene expressions and contributes to an increase in the incidence of HBV-related HCC.Citation15 However, the relationship of GSTP1 and P16 promoter methylation with HBV-related HCC risk and pathogenesis remains controversial.Citation15–Citation26 Therefore, we carried out the current meta-analysis to comprehensively assess the associations of GSTP1 and P16 promoter methylation with the risk of HBV-related HCC.
Methods
Search strategy
Relevant literature was retrieved from PubMed, Embase, Web of Science, and China National Knowledge Infrastructure databases with a combination of the following words: “Hepatitis B Virus” or “HBV” and “hepatocellular carcinoma” or “HCC” and “GSTP1” or “glutathione-S-transferase P1” or “P16” and “methylation”. All relevant articles were published before December 2017, without language restrictions. Literature searches were conducted independently by two reviewers using a standardized approach (Qin Li and Cunliang Deng). The references from relevant primary articles were manually searched to identify potential studies. Any disagreement was adjudicated by a third investigator (Xiang Li) after referring to original articles.
Inclusion criteria and exclusion criteria
We used the following inclusion criteria for our research: 1) studies that have a case-control design; 2) studies in which patients diagnosed with HBV-related HCC, cirrhosis, and CHB were accurately diagnosed according to the diagnostic criteria; 3) studies in which patients with HBV infection were diagnosed using a HBV surface antigen (HBsAg) serological assay; 4) methods suitable for the methylation detection were confined to MethyLight array, pyrosequencing, and methylation-specific polymerase chain reaction (MSP); and 5) studies primarily evaluating the frequency of GSTP1 or P16 promoter methylation in HBV-related HCC tissues with control group. Studies were excluded if they met one of the following criteria: 1) overlapping data or review articles; 2) studies in which patients had other severe diseases, such as other malignancies, heart failure, or kidney failure; and 3) studies in which patients received a combination of other treatments.
Data extraction
We recorded the following information: first author, publication year, geographical location, ethnicity, detection method of methylation, and frequency of methylation. The case numbers of HBV-related HCC, cirrhosis, CHB, paracancerous tissue, and normal liver tissue were separately extracted from the overall population after fully understanding the paper.
Statistical analysis
Meta-analyses were performed using STATA 12.0 (Stata Corporation, College Station, TX, USA). The strength of the associations between GSTP1 and P16 promoter methylation and the risk of HBV-related HCC was expressed as the ORs and 95% CIs. The Z-test was applied to estimate the statistical significance of pooled ORs. Between-study heterogeneity was evaluated by the Cochrane Q-statistic and I2 test. The random-effects model was chosen when heterogeneity existed among studies (I2>50%). Otherwise, a fixed-effects model was applied. Sensitivity analysis was carried out to investigate the influence of single study on the final results. Egger’s test, Begg’s test, and funnel plots were performed to examine the publication bias. The results were displayed as forest plots and identified to be statistically significant when P<0.05.
Results
Characteristics of studies
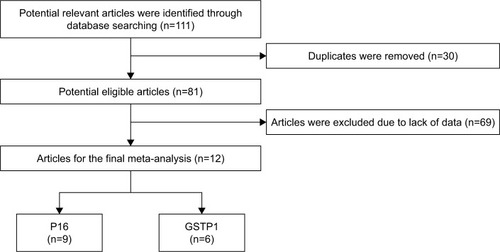
The process of literature search is summarized in . The original search yielded a total of 111 articles related to the searched keywords. According to the inclusion criteria, a total of 429 HCC samples with HBV infection in 12 studies were included; additionally, 173 adjacent samples, 167 normal samples, 241 cirrhosis samples, and 190 CHB samples were included. The publication year of the studies ranged from 2003 to 2017.
The geographic setting of the studies was heterogeneous: two studies were from Korea, eight were from China, one was from Japan, and one each from France and Thailand. The MSP method used for this meta-analysis was adopted in eight studies, one study was detected by pyrosequencing, and the other three studies were conducted using MethyLight array. The characteristics of the studies are summarized in .
Table 1 Clinicopathological features of eligible studies
Association of GSTP1 promoter region methylation with the risk of HBV-related HCC
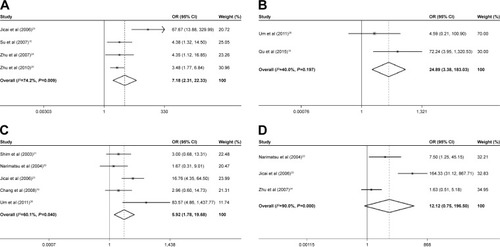
Six studies were included to assess the potential role of GSTP1 promoter methylation in the risk of HCC in patients with HBV infection. A random-effects model was used where significant heterogeneity was found among the studies. Our findings demonstrated that the frequency of the GSTP1 gene methylation in neoplasm tissues was significantly higher than in the normal and cirrhosis tissues (neoplasm tissue vs normal tissue: OR =6.05, 95% CI =1.20–30.52, P<0.05, and ; neoplasm tissue vs cirrhosis tissue: OR =5.21, 95% CI =2.19–12.41, P<0.01, and ).
Table 2 Meta-analysis of the associations of GSTP1 and P16 gene methylation with HBV-related HCC pathogenesis
Figure 2 Forest plots of GSTP1 promoter region methylation and the risk of HBV-related HCC.
Abbreviations: GSTP1, glutathione-S-transferase Pi 1; HBV, hepatitis B virus; HCC, hepatocellular carcinoma.

Association of P16 promoter region methylation with the risk of HBV-related HCC
The pooled OR of P16 methylation in HBV-related HCC tissues was 7.18, with corresponding 95% CI = 2.31–22.33, compared with adjacent tissues in the random-effects model (P<0.01; and ). Compared with normal tissues, the fixed-effects model was chosen to evaluate the association between the frequency of P16 methylation and the risk of HBV-related HCC, and a significant result was found (OR =24.89, 95% CI=3.38–183.03, P<0.01; and ). Moreover, the pooled OR was 5.92 in HBV-related HCC tumor tissues vs cirrhosis tissues (95% CI =1.78–19.68, P<0.01; and ), indicating that P16 methylation in promoter region could obviously increase the risk of HBV-related HCC in patients with cirrhosis. However, no significant association was found between HBV-related HCC tumor tissues and CHB tissues (OR =12.12, 95% CI =0.75–196.50, P>0.05; and ).
Figure 3 Forest plots of P16 promoter region methylation and the risk of HBV-related HCC.
Abbreviations: CHB, chronic hepatitis B; HBV, hepatitis B virus; HCC, hepatocellular carcinoma.
Sensitivity analysis
Sensitivity analysis was performed to evaluate the effect of each study on overall meta-analysis by excluding one study at a time, but the pooled ORs were always constant, indicating that no individual study significantly affected the overall pooled estimates, and our results were robust.
Publication bias
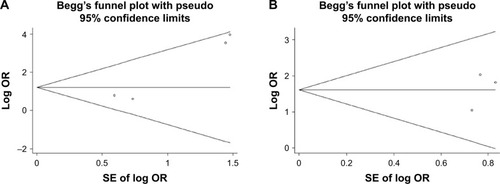
Begg’s and Egger’s tests were used to investigate the publication bias. Begg’s funnel plot showed no publication bias for the association between the methylation of GSTP1 and the risk of HBV-related HCC in cancerous and normal tissue, while the Egger’s test indicated an opposite result. Moreover, results from our meta-analysis showed that there was no publication bias when assessing the GSTP1 methylation in HBV-related HCC tumor tissues vs cirrhosis tissues ( and ).
Figure 4 Begg’s funnel plot analysis for publication bias between GSTP1 methylation and HBV-related HCC susceptibility.
Abbreviations: GSTP1, glutathione-S-transferase Pi 1; HBV, hepatitis B virus; HCC, hepatocellular carcinoma.
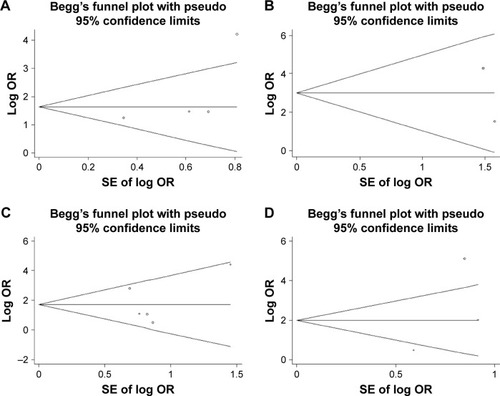
With regard to the association between the methylation of P16 and the risk of HBV-related HCC, the funnel plots presented no apparent asymmetry, and the Egger’s tests showed no publication bias in HBV-related HCC tumor tissues vs adjacent tissues, normal tissues, cirrhosis tissues, and CHB tissues ( and ).
Figure 5 Begg’s funnel plot analysis for publication bias between P16 methylation and HBV-related HCC susceptibility.
Abbreviations: CHB, chronic hepatitis B; HBV, hepatitis B virus; HCC, hepatocellular carcinoma.
Discussion
To the best of our knowledge, HBV infection is one of the most associated factors in hepatocarcinogenesis. HBV has been proved to integrate into the host genome and interfere with cell proliferation and invasion, DNA repair, as well as epigenetic modification.Citation27 In areas with high prevalence of HBV infection, a spectrum of liver disease has been formed, extending from CHB to cirrhosis, even to HCC.Citation28,Citation29 Therefore, it is of great importance to explore the molecular mechanisms underlying HBV-related HCC.
Previous studies demonstrated that HBV infection could lead to hypermethylation, and aberrant DNA methylation has been proved to be an early event in hepatocarcinogenesis.Citation30–Citation32 Consequently, the analysis of DNA methylation could be an effective method for assessing reliable and powerful biomarkers for HBV-related HCC, which will greatly improve the diagnosis and treatment of it.
The GSTP1 is a gene encoding an enzyme with detoxifiation and protein-binding functions. In addition to the overexpression of GSTP1 observed in many neoplasms, GSTP1 genetic polymorphisms and abnormal CpG island methylation have also been thought to function in susceptibility to several cancers, including breast, lung, and liver cancers.Citation33–Citation36 According to the previous studies, GSTP1 promoter methylation may be involved in the occurrence and development of HBV-related HCC.Citation15,Citation16,Citation18,Citation19 However, there are contradictory results concerning the role of GSTP1 promoter methylation in the progression of HBV-related HCC as well.Citation17,Citation20 In view of the conflicting evidence on this issue, we performed this meta-analysis to evaluate the exact relationship between GSTP1 promoter methylation and increased risk of HBV-related HCC. Consequently, our results confirmed that the methylation rates of GSTP1 gene in cancer tissues were considerably higher than those of normal controls (OR =6.05, 95% CI =1.20–30.52) as well as cirrhosis tissues (OR =5.21, 95% CI =2.19–12.41), suggesting the importance of GSTP1 promoter region methylation in the development of HBV-related HCC.
In addition, P16 is another important TSG for the suppression of tumor growth.Citation37,Citation38 P16 gene promoter region methylation represents the most common mechanism of P16 inactivation in HBV-induced human HCC.Citation39 As several studies demonstrated, the methylation of P16 may participate in the development of HBV-related HCC.Citation18,Citation19,Citation21,Citation22 However, the negative results indicated that the exact relationship of P16 gene promoter region methylation with HBV-related HCC susceptibility was still inconclusive.Citation17,Citation20 In the present study, an apparently increased risk of HBV-related HCC was found in tumor tissue in comparison with paracancerous tissue (OR =7.18, 95% CI =2.31–22.33), normal liver tissue (OR =24.89, 95% CI =3.38–183.03), and cirrhosis (OR =5.92, 95% CI =1.78–19.68), conforming that P16 promoter methylation is a risk factor for HBV-related HCC.
In this meta-analysis, the publication bias was examined in the eligible studies using Begg’s test and Egger’s test, and the funnel plots showed that the data did not have a considerable discrepancy among studies. Furthermore, we conducted sensitivity analysis and consistent results were found, which revealed that no single study could affect the overall conclusion.
However, several potential limitations must be empha-sized in this study. First, the limited studies with small sample size may limit the power of statistics. Second, moderate heterogeneity across included studies existed in separate comparisons, which may have an influence on the interpretation of our results. Third, as the pathogenesis of HBV-related HCC has a significant difference in diverse countries and regions, HBV genotypes B and C are common in Asia while genotypes A and D are prevalent in Europe, but the ethnicity of the population in this meta-analysis was mainly Asian. Therefore, the results of this meta-analysis were mainly adapted to Asian population, and large-scale subjects with multi-ethnic populations are needed.
Conclusion
This meta-analysis showed that GSTP1 and P16 promoter methylation might play an important role in HBV-related HCC initiation and progression, and GSTP1 and P16 methylation in promoter region could obviously increase the risk of HBV-related HCC in patients with cirrhosis, which might be promising biomarkers for the early clinical diagnosis of HBV-related HCC. However, in consideration of the limitations mentioned earlier, more large-scale and well-designed studies will provide more insights into the role of GSTP1 and P16 promoter methylation in the risk and pathogenesis of HBV-related HCC.
Acknowledgments
This project was supported by the Sichuan Science and Technology Department Project (14JC0086).
Disclosure
The authors report no conflicts of interest in this work.
References
- LiWLiLHanJWuHLiver transplantation vs liver resection in patients with HBV-related hepatocellular carcinoma beyond Milan criterion: A meta-analysisClin Transplant2018323e1319329315813
- El-SeragHBEpidemiology of viral hepatitis and hepatocellular carcinomaGastroenterology201214261264127322537432
- ManiSKKAndrisaniOHepatitis B virus-associated hepatocellular carcinoma and hepatic cancer stem cellsGenes201893E13729498629
- GanemDPrinceAMHepatitis B virus infection – natural history and clinical consequencesN Engl J Med2004350111118112915014185
- TianYYangWSongJWuYNiBHepatitis B virus X protein-induced aberrant epigenetic modifications contributing to human hepatocellular carcinoma pathogenesisMol Cell Biol201333152810281623716588
- DexheimerGMAlvesJReckziegelLLazzarettiGAbujamraALDNA methylation events as markers for diagnosis and management of acute myeloid leukemia and myelodysplastic syndromeDis Markers2017201754728931429038614
- LorinczMCSchübelerDEvidence for converging DNA methylation pathways in placenta and cancerDev Cell201743325725829112847
- WeisenbergerDJLiangGLenzHJDNA methylation aberrancies delineate clinically distinct subsets of colorectal cancer and provide novel targets for epigenetic therapiesOncogene201837556657728991233
- HardyTMannDAEpigenetics in liver disease: from biology to therapeuticsGut201665111895190527624887
- NishidaNKudoMEpigenetic regulation and development of hepatocellular carcinomaNihon Shokakibyo Gakkai Zasshi2016113577578427151473
- ZhangCLiJHuangTMeta-analysis of DNA methylation biomarkers in hepatocellular carcinomaOncotarget2016749812558126727835605
- JainSChenSChangKCImpact of the location of CpG methylation within the GSTP1 gene on its specificity as a DNA marker for hepatocellular carcinomaPLoS One201274e3578922536438
- PiepkornMMelanoma genetics: an update with focus on the CDKN2A(p16)/ARF tumor suppressorsJ Am Acad Dermatol2000425 Pt 170572610775844
- LukasJParryDAagaardLRetinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16Nature199537565315035067777060
- SuPFLeeTCLinPJDifferential DNA methylation associated with hepatitis B virus infection in hepatocellular carcinomaInt J Cancer200712161257126417534893
- ChangHYiBLiLMethylation of tumor associated genes in tissue and plasma samples from liver disease patientsExp Mol Pathol20088529610018691570
- FengQSternJEHawesSELuHJiangMKiviatNBDNA methylation changes in normal liver tissues and hepatocellular carcinoma with different viral infectionExp Mol Pathol201088228729220079733
- LambertMPPaliwalAVaissièreTAberrant DNA methylation distinguishes hepatocellular carcinoma associated with HBV and HCV infection and alcohol intakeJ Hepatol201154470571521146512
- QuZJiangYLiHYuDCDingYTDetecting abnormal methylation of tumor suppressor genes GSTP1, P16, RIZ1, and RASSF1A in hepatocellular carcinoma and its clinical significanceOncol Lett20151042553255826622888
- DongXHouQChenYWangXDiagnostic value of the methylation of multiple gene promoters in serum in hepatitis B virus-related hepatocellular carcinomaDis Markers201720172929381628951629
- ShimYHYoonGSChoiHJChungYHYuEp16 Hypermethylation in the early stage of hepatitis B virus-associated hepatocarcinogenesisCancer Lett2003190221321912565176
- NarimatsuTTamoriAKohNp16 promoter hypermethylation in human hepatocellular carcinoma with or without hepatitis virus infectionIntervirology2004471263115044833
- ZhangJCYuZTLuJPersistent infection of hepatitis B virus is involved in high rate of p16 methylation in hepatocellular carcinomaMol Carcinog200645753053616649250
- ZhuRLiBZLiHAssociation of p16INK4A hypermethylation with hepatitis B virus X protein expression in the early stage of HBV-associated hepatocarcinogenesisPathol Int200757632833617539963
- ZhuYZZhuRFanJHepatitis B virus X protein induces hypermethylation of p16(INK4A) promoter via DNA methyltransferases in the early stage of HBV-associated hepatocarcinogenesisJ Viral Hepat20101729810719732323
- UmTHKimHOhBKAberrant CpG island hypermethylation in dysplastic nodules and early HCC of hepatitis B virus-related human multistep hepatocarcinogenesisJ Hepatol201154593994721145824
- GengMXinXBiLQZhouLTLiuXHMolecular mechanism of hepatitis B virus X protein function in hepatocarcinogenesisWorld J Gastroenterol20152138107321073826478665
- FungSKLokASManagement of patients with hepatitis B virus-induced cirrhosisJ Hepatol200542Suppl 1S54S6415777573
- TanakaMKatayamaFKatoHHepatitis B and C virus infection and hepatocellular carcinoma in China: a review of epidemiology and control measuresJ Epidemiol201121640141622041528
- HermanJGBaylinSBGene silencing in cancer in association with promoter hypermethylationN Engl J Med2003349212042205414627790
- EggerGLiangGAparicioAJonesPAEpigenetics in human disease and prospects for epigenetic therapyNature2004429699045746315164071
- KewMCHepatitis B virus x protein in the pathogenesis of hepatitis B virus-induced hepatocellular carcinomaJ Gastroenterol Hepatol201126Suppl 114415221199526
- NakazatoHSuzukiKMatsuiHAssociation of genetic polymorphisms of glutathione-S-transferase genes (GSTM1, GSTT1 and GSTP1) with familial prostate cancer risk in a Japanese populationAnticancer Res2003233C2897290212926131
- ChenGZhangHSunLPrognostic significance of GSTP1 in patients with triple negative breast cancerOncotarget2017840686756868028978147
- WangYRenBUZhangLGuoZCorrelation between metabolic enzyme GSTP1 polymorphisms and susceptibility to lung cancerExp Ther Med20151041521152726622518
- HannHWJainSParkGSteffenJDSongWSuYHDetection of urine DNA markers for monitoring recurrent hepatocellular carcinomaHepatoma Res2017310511128795155
- Mohd RidahLJA TalibNMuhammadNHussainFAZainuddinNp16 Tumor suppressor gene methylation in diffuse large B cell lymphoma: a study of 88 cases at two hospitals in the east coast of MalaysiaAsian Pac J Cancer Prev201718102781278529072413
- SunGZhangCFengMMethylation analysis of p16, SLIT2, SCARA5, and Runx3 genes in hepatocellular carcinomaMedicine20179641e827929019900
- LvXYeGZhangXHuangTp16 Methylation was associated with the development, age, hepatic viruses infection of hepatocellular carcinoma, and p16 expression had a poor survival: a systematic meta-analysis (PRISMA)Medicine20179638e810628930859