Abstract
Fruit seeds high in antioxidants have been shown to have anticancer properties and enhance host protection against microbial infection. Recently we showed that a single oral dose of Salmonella enterica serovar Typhimurium expressing a truncated human interleukin-2 gene (SalpIL2) is avirulent, immunogenic, and reduces hepatic metastases through increased natural killer cell populations in mice. To determine whether antioxidant compounds enhance the antitumor effect seen in SalpIL2-treated animals, we assayed black cumin (BC), black raspberry (BR), and milk thistle (MT) seed oils for the ability to reduce experimental hepatic metastases in mice. In animals without tumor, BC and BR oil diets altered the kinetics of the splenic lymphocyte response to SalpIL2. Consistent with previous reports, BR and BC seed oils demonstrated independent antitumor properties and moderate adjuvant potential with SalpIL2. MT oil, however, inhibited the efficacy of SalpIL2 in our model. Based on these data, we conclude that a diet high in antioxidant oils promoted a more robust immune response to SalpIL2, thus enhancing its antitumor efficacy.
Introduction
Attenuated strains of Salmonella enterica (S. enterica) serovar Typhimurium were first explored as delivery vectors for anticancer therapeutics in the mid 1990s.Citation1,Citation2 Since then, S. enterica tumor vaccines have shown efficacy in experimental animal models against multiple tumor types including colorectal, melanoma, breast, bone, spine, liver, pancreatic, prostate, and lung.Citation3–Citation11 In our experimental models, we have focused on the efficacy of an avirulent and highly immunogenic strain, S. enterica Typhimurium χ4550, that was engineered to express a human interleukin-2 (IL-2) gene in primary and experimental metastasis models.Citation12–Citation14 In these studies, S. enterica χ4550 expressing a human IL-2 gene was effective at reducing the number and volume of hepatic metastases both when administered before and after tumor introduction. Decreased tumor burden in these models was inversely correlated with increased hepatic natural killer (NK) cell populations and when depleted, S. enterica χ4550’s antitumor properties were abolished.Citation15 Most recently, we have demonstrated that S. enterica Typhimurium expressing a C-terminal truncated human IL-2 gene (SalpIL2) reduced osteosarcoma lung metastases and significantly increased the number of detectable NK cells in the lung as compared to S. enterica-vector control or saline gavaged animals.Citation16,Citation17 In addition, in the serum of animals with pulmonary osteosarcoma metastases, a single oral dose of SalpIL2 altered the detection of several proinflammatory cytokines associated with tumor progression.Citation18 In Rag-1 knockout mice, which do not have functional T-cells, SalpIL2 continued to decrease tumor burden. Thus, SalpIL2’s antitumor properties appear to be independent of T cell contribution.
Adjuvants, such as cycloxygenase-2 (Cox2) inhibitors appeared to enhance the antitumor efficacy of our attenuated S. enterica strain. When administered with Cox2 inhibitors, S. enterica significantly reduced the number and volume of hepatic metastases as compared to all other treatment types.Citation19 However, due to insolubility of Cox2 inhibitors in water, controlled dose delivery of these drugs in the drinking water of mice required multiple suspension doses daily. Similar to pharmaceutical Cox2 inhibitors, fruit seed extracts from black cumin (BC; Nigella sativa), black raspberry (BR; Rubus occidentalis), and milk thistle (MT; Silybum marianum) have been shown to contain high levels of antioxidants and have inhibitory effects on Cox2.Citation20–Citation23 In addition, BC and BR seed extracts suppress activity of NFκB, a proinflammatory transcription factor shown to be hyperactive in many cancers.Citation24,Citation25 Anticancer properties of extracts from BC, BR, and MT oils have been demonstrated by observation of decreased tumor burden in oil treated animals.Citation26–Citation28 The application of antioxidant compounds also appears to be clinically beneficial. Freeze-dried BR gels, when applied directly to premalignant oral lesions, induced regression of cancerous tissues.Citation21,Citation29 However, to the best of our knowledge, BR oil has not been reported to affect the growth of syngeneic tumors in organs, nor have BC, BR, and MT oils been shown to be efficacious as adjuvants with immunomodulatory anticancer therapies.
In these studies, we sought to test the hypothesis that BC, BR, and MT antioxidant oils would enhance the efficacy of SalpIL2 in an experimental murine model of hepatic metastases. In this report, we show that several antioxidant compounds augment the cell-mediated immune response to SalpIL2 in nontumor bearing mice. In addition, antioxidant oils provide adjuvant antitumor properties to SalpIL2 and appear to directly decrease the growth of experimental hepatic metastases. In contrast to BC and BR oils, MT oil abrogated the ability of SalpIL2 to reduce the number of hepatic tumors in our model.
Materials and methods
Quantification of lipid-soluble antioxidants in selected oils
Fruit seed oils provided by Botanic Oil Innovation (Spooner, WI) were examined for lipid-soluble antioxidant capacities using an ACL kit to measure the capacity of lipophilic substances following the directions as described by the manufacturer (Analytik Jena, Spring, TX). Briefly, fruit seed oils were dissolved in hexane (20%) and methanol (79%) and run in triplicate to determine Trolox equivalents, a derivative of vitamin E, by chemiluminescence using the Photochem instrument (Analytik Jena).
Bacteria
The avirulent, but highly immunogenic S. enterica serovar Typhimurium strain χ4550, a well-characterized adenylatecyclase, cyclic adenosine monophosphate receptor protein, and aspartate semialdehyde dehydrogenase (asd) deletion mutant, was a kind gift from Dr Roy Curtiss III (Washington University, St Louis, MO). Plasmid pIL2, constructed by insertion of a C-terminal truncated human IL-2 cDNA immediately downstream from the trc promoter in pYA292Citation30 which contains an S. enterica asd cDNA for stable maintenance, was electroporated into S. enterica Typhimurium χ4550 and the resultant strain was renamed SalpIL2. SalpIL2 was cultured overnight at 37°C in Luria-Bertani broth (Difco Laboratories, Detroit, MI), washed and reconstituted to approximately 109 mL−1 colony forming units (CFU) in Hank’s Balanced Salt Solution (HBSS). Bacteria aliquots were stored as a 15% glycerol stock in liquid nitrogen until used in experiments. On the day of oral inoculation, bacterial stocks were allowed to thaw to room temperature and CFU concentration was confirmed by serial dilution on MacConkey agar plates (Becton, Dickinson, and Company, Bedford, MA). The University of Minnesota Institutional Biosafety Committee approved the use of SalpIL2 in all experiments as described.
Mice
Female 6 to 8 week old C57BL/6 mice were purchased from Harlan Sprague Dawley (Indianapolis, IN) and allowed to acclimate for a minimum of 7 days in specific pathogen free conditions. After inoculation with SalpIL2, all experimental animals were transferred to biosafety level two for the remainder of the study. Antioxidant oils were mixed with ground standard mouse chow (Harlan) to make a homogenous 10% w/w meal. Experimental mice were given ground meal or meal mixed with antioxidant oil daily and drinking water was supplied ad libitum. All animals were housed in microisolator cages and cared for by trained personnel from Research Animal Resources. The University of Minnesota Institutional Animal Care and Use Committee approved all protocols.
Murine adenocarcinoma tumor model
MCA-38 syngeneic tumors were propagated in naïve C57BL/6 mice as previously described.Citation12 Briefly, 4 × 106 tumor cells were injected subcutaneously into the hind flank and allowed to grow until a palpable mass was present. Anesthetized animals were euthanized and the tumor mass was aseptically removed, minced and digested in 0.1% DNase, 0.1% hyaluronidase, and 0.1% collagenase (Sigma Co, St Louis, MO) in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum (Sigma) for 2 hours with gentle agitation. The tumor solution was filtered through 150 μm Nitex mesh (Sefar America, Kansas City, MO), centrifuged at 500 × g for 10 minutes and resuspended in HBSS twice before enumeration by trypan blue exclusion. 5 × 104 cells were injected into the spleen of anesthetized animals via transabdominal incision and allowed to circulate for 3 minutes. The spleens were removed and the animals were allowed to recover on warming pads until ambulatory. Animals were sacrificed and hepatic metastases were visually enumerated 14 days posttumor injection.
Splenic and hepatic lymphocyte enrichment
Spleens from nontumor bearing animals were collected for splenic lymphocyte population analysis on day 3, 7, 14, and 21-post oral gavage and start of antioxidant oil. After 14 days of tumor growth, tumor-burdened livers were ground in DMEM with 10% fetal goat serum using sterile glass stoppers. The tissue slurry was filtered through 150 μm Nitex mesh, layered on top of lymphocyte separation medium (Mediatech, Inc, Herndon, VA) and centrifuged at 300 × g for 1 hour. Splenic, hepatic, and tumor lymphocytes were collected from the Ficoll-hypaque layer and serially washed with phosphate buffered saline with 1% bovine serum albumin and 0.1% sodium azide (Sigma).
Flow cytometry
Splenic and hepatic lymphocytes were examined for cells reactive with anti-NK1.1, anti-CD4, and anti-CD8 fluorochrome-conjugated anti-mouse monoclonal antibodies (BD Biosciences Pharmingen, San Diego, CA). Lymphocytes were incubated with the antibodies at 4°C for 30 minutes. After washing, analysis was performed with FACS cancytofluorometer (Becton-Dickinson, Grenoble, France) using Cell Quest Pro software (Becton-Dickinson). Lymphocytes were gated by side and forward scatter profiles. Data presented is based on 10,000 gated events.
Statistical analyses
Tumor number, volume, and splenic and hepatic lymphocyte populations were recorded for each mouse and used to calculate the mean values for each experimental cohort. All differences between groups were determined by Fisher’s exact test and ANOVA using StatView software (v 5.0.1; SAS Institute, Cary, NC). In the following sections of the manuscript we describe the results as significant (P < 0.05) or “tended/marginally” significant as (P < 0.10).
Results
The lipid-soluble antioxidant capacities of BC, BR, BC+BR, and MT oils were examined. The combination BC+BR seed oil displayed higher antioxidant capacities (25.9 ± 0.2) in equal combination as compared to individual oils (BC, 21.0 ± 0.1 and BR, 19.8 ± 0.6). In contrast, MT seed oil displayed the lowest levels of lipid-soluble antioxidants (1.3 ± 0.2) of the oils tested.
BC and BR enhance cellular immune response to SalpIL2
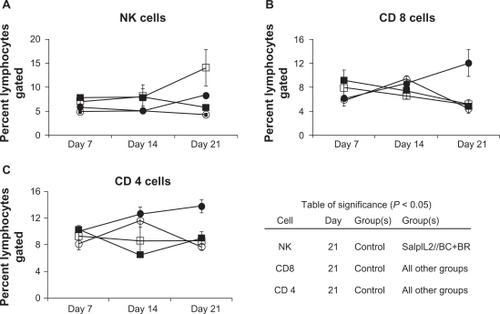
On day 14, NK cell populations were increased (P < 0.10) in animals administered SalpIL2 with or without BC+BR oil diet (10% w/w) compared to saline and oil-only groups (). On day 21, SalpIL2 with BC+BR significantly increased NK cell populations over control and combination oil-only groups. SalpIL2 with or without BC+BR increased CD8+ T cell populations significantly by day 7 over saline control and oil-only groups (). By day 21, however, all experimental groups had significantly lower splenic CD8+ and CD4+ T cell populations than control animals ().
Figure 1 Effect of combination antioxidant seed oil on the splenic lymphocyte response to SalpIL2 in mice. Splenic natural killer (NK) (A), CD 8+ T (B), and CD 4+ T cell populations (C) as determined by flow cytometry in response in animals fed a diet consisting of equal amounts of black raspberry (BR; Rubus occidentalis), black cumin (BC; Nigella sativa) seed oils (○), administered a single oral dose of SalpIL2 (□) or SalpIL2+BC+BR oil (▪) starting on day 0 as compared to control animals (•).
BC and BR enhance the NK cell response to SalpIL2 in tumor burdened animals
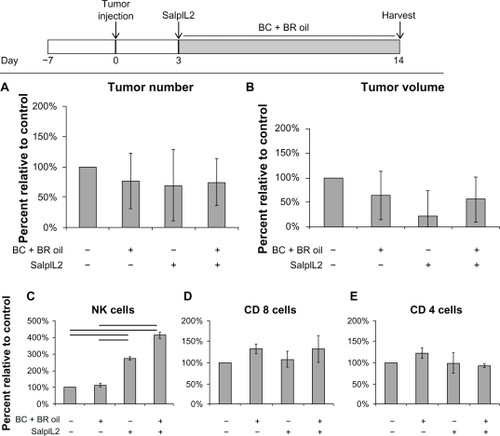
We next used a hepatic metastasis model to screen whether NK cell populations, increased from BC+BR and SalpIL2 administration () would reduce tumor burden in vivo. Consistent with our previous studies, SalpIL2 tended to reduce the volume of hepatic metastases by 78% in our model (P < 0.10). All experimental treatments, for example, BC+BR oil with and without SalpIL2, did not have significantly reduced tumor number and volume as compared to control animals (). Hepatic NK cell populations were significantly increased by SalpIL2 alone (272%) or SalpIL2 with BC+BR combination oil (412%) as compared to the controls (all P < 0.05). Mice given the combination of SalpIL2 with BC+BR oil tended to have increased NK cell populations compared to animals only given SalpIL2 (; P < 0.10). CD8+ and CD4+ T cells populations were not significantly different across the experimental groups ().
Figure 2 Effect of combination antioxidant seed oil on the SalpIL2 anti-tumor response in mice. On Day 0, 5 × 104 MCA-38 cells were injected intrasplenically to naïve animals. A single oral administration of SalpIL2 and initiation of a diet consisting of equal amounts of black raspberry (BR; Rubus occidentalis), black cumin (BC; Nigella sativa) seed oils 10% w/w was given to animals on Day 3. Animals were maintained for a total of 14 days prior to collection of tumor and hepatic lymphocyte data (see diagram above). Tumor number (A) and tumor volume (B) in animals fed a BC+BR with or without SalpIL2. Hepatic natural killer (NK) (C), CD8+ T (D), and CD4+ T cell response (E) to SalpIL2 and antioxidant oils in tumor burden mice at the experimental endpoint.
BC oil diet reduces hepatic metastases
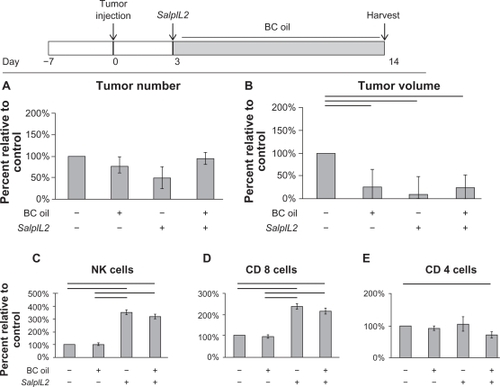
Although the combination BC+BR seed oils did not significantly decrease tumor number and volume in our hepatic metastasis model, we examined whether either BC or BR oil alone may enhance the antitumor efficacy of SalpIL2. Similar to the BC+BR combination oil diet, a BC seed oil diet did not significantly decrease the number of hepatic metastases. A diet with BC oil alone, however, reduced tumor volume by 74% and when administered with oral SalpIL2, significantly decreased tumor volume 75% as compared to control animals (; all P < 0.05). The reduction of tumor volume was similar to SalpIL2-only treated animals (91%). The antitumor effect observed by BC oil appeared to be independent of increased NK and CD8+ T cell populations. Only SalpIL2-treated animals, with or without BC oil, displayed significantly higher NK cell populations as compared to controls (; P < 0.05). Lastly, SalpIL2 with BC oil treated animals displayed a significant 29% reduction in CD4+ T cell population at the experimental endpoint () (P < 0.05).
Figure 3 Effect of black cumin (BC; Nigella sativa) seed oil on the SalpIL2 anti-tumor response in mice. On Day 0, 5 × 104 MCA-38 cells were injected intrasplenically to naïve animals. A single oral administration of SalpIL2 and initiation of BC (shaded area) seed oil diet 10% w/w was given to animals on Day 3. Animals were maintained for a total of 14 days prior to collection of tumor and hepatic lymphocyte data (see diagram above). Tumor number (A) and tumor volume (B) in animals fed a diet BC seed oil with and without SalpIL2. Hepatic natural killer (NK) (C), CD8+ T (D), and CD4+ T cell response (E) to SalpIL2 with and without BC oil on Day 14.
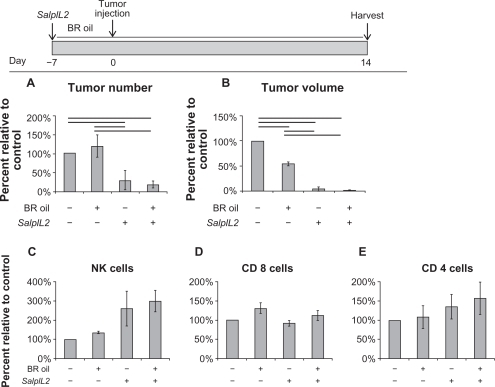
BR oil diet has treatment and preventative potential on hepatic metastases
In our model, a diet high in BR seed oil significantly decreased tumor volume, not tumor number, by 47% as compared to controls (; P < 0.05). No significant additional benefit in reduction of tumor volume was observed in animals administered SalpIL2 with (61%) or without BR seed oil (64%). In hepatic lymphocyte populations, however, only animals treated with SalpIL2 and BR oil had significantly increased NK cells (219%) in the tumor-burdened animals when compared to control (; P < 0.05). There were no significant changes in T cell populations between treatment groups. Previously, we showed that inoculation of mice with S. enterica 7 days prior to tumor injection provided protection against the establishment of hepatic metastases. Similar to administering a diet of BR oil 3 days after tumor injection, BR oil alone significantly reduced the volume not number of hepatic metastases by 45% as compared to controls (; P < 0.05). Animals given SalpIL2 with BR oil had similar reduction in tumor number and volume to SalpIL2 alone, 81%, 98% and 70%, 96% respectively as compared to controls (P < 0.10). Only animals administered SalpIL2 with BR oil showed a significant 298% increase in NK cell populations as compared to control animals (; P < 0.05). SalpIL2-only animals had a marginally significant increase of 260% in NK cell populations compared with controls (P < 0.10). Only CD8+ T cell populations were significantly altered between BR oil SalpIL2 treatment groups (; P < 0.05).
Figure 4 Effect of black raspberry (BR; Rubus occidentalis) seed oil on the SalpIL2 anti-tumor response in mice. On Day 0, 5 × 104 MCA-38 cells were injected intrasplenically to naïve animals. A single oral administration of SalpIL2 and initiation of BR (shaded area) seed oil diet 10% w/w was given to animals on Day 3. Animals were maintained for a total of 14 days prior to collection of tumor and hepatic lymphocyte data (see diagram above). Tumor number (A) and tumor volume (B) in animals fed a diet consisting of BR with and without SalpIL2. Hepatic natural killer (NK) (C), CD8+ T (D), and CD4+ T cell response (E) to SalpIL2 with and without BR oil on Day 14.
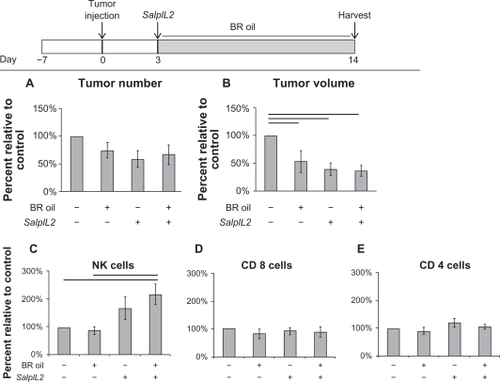
Figure 5 Effect of black raspberry (BR; Rubus occidentalis) seed oil and SalpIL2 on the prevention of experimental hepatic metastases in mice. A single oral administration of SalpIL2 and initiation of BR (shaded area) seed oil diet 10% w/w was given to animals on Day −7. On Day 0, 5x104 MCA-38 cells were injected intrasplenically and animals were maintained for 14 additional days. Tumor and hepatic lymphocyte data collected on Day 14 (see diagram above). Tumor number (A) and tumor volume (B) in animals fed a diet consisting of BR with and without SalpIL2. Hepatic natural killer (NK) (C), CD8+ T (D), and CD4+ T cell response (E) to SalpIL2 and BR oil in tumor burden mice on Day 21.
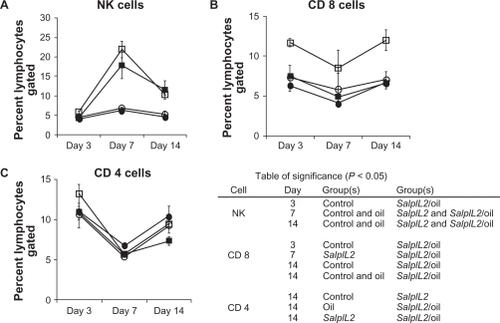
BR oil diet augmented cellular immunity to SalpIL2
To further elucidate the antitumor efficacy of BR seed oil, we examined the effect of BR oil diet on the splenic lymphocyte populations in SalpIL2-treated mice. BR oil with SalpIL2 increased NK cells by day 3 over control animals (5.7 to 4.7 respectively; ; P < 0.05), which has not previously been observed in SalpIL2-treated animals.Citation18 By days 7 and 14 however, increased NK-cell populations by SalpIL2 were independent of BR oil treatment. With respect to CD8+T cell populations, SalpIL2 with BR oil significantly increased CD8+T cell populations on days 3 and 14 compared to SalpIL2-treated and control animals (; P < 0.05). SalpIL2 and BR oil treatment also affected CD4+ T cell populations. By day 14, SalpIL2 significantly decreased CD4+ T cell populations (P < 0.05), while SalpIL2 with BR oil appeared to negate the SalpIL2-dependent drop in CD4+ T cells ().
Figure 6 Effect of black raspberry (BR; Rubus occidentalis) seed oil on the splenic lymphocyte response to SalpIL2 in mice. Splenic natural killer (NK) (A), CD8+ T (B), and CD4+ T cell populations (C) as determined by flow cytometry in response in animals fed a diet consisting of BR seed oil 10% w/w (○), administered as single oral dose of SalpIL2 (□) or SalpIL2 + BR oil (▪) starting on day 0 as compared to control animals (•).
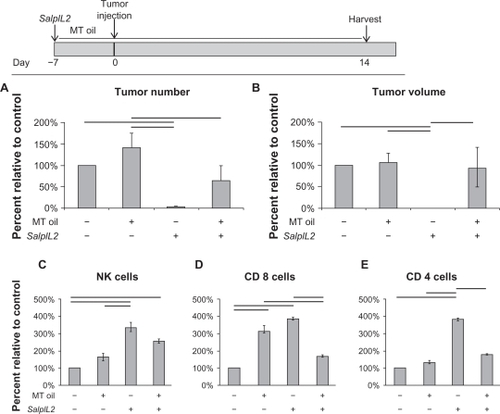
MT seed oil abrogates the antitumor efficacy of SalpIL2
As demonstrated in , the antitumor effect of SalpIL2 is most evident in animals when delivered prior to tumor administration. MT oil, however, appeared to inhibit SalpIL2’s ability to reduce tumor number (98% to 37%; P < 0.10; ) and volume (99% to 7%; P < 0.01; ). MT oil appeared to reduce the NK cell response to SalpIL2 as NK cell populations were significantly increased in animals given SalpIL2 with MT oil (255%) and SalpIL2 alone (335%; ). Interestingly both MT oil (315%) and SalpIL2 alone (387%) increased CD8+ T cell populations as compared to controls (), whereas, SalpIL2 with MT oil (176%) showed a significant reduction in CD8+ T cell populations as compared to SalpIL2 alone. SalpIL2 increased the number of hepatic CD4+T cells (376%) as compared to controls, which was significantly increased with respect to all other experimental groups.
Figure 7 Milk thistle (MT; Silybum marianum) seed oil inhibits SalpIL2-dependent prevention of experimental hepatic metastases in mice. A single oral administration of SalpIL2 and initiation of MT (shaded area) seed oil diet 10% w/w was given to animals on Day –7. On Day 0, 5 × 104 MCA-38 cells were injected intrasplenically and animals were maintained for 14 additional days. Tumor and hepatic lymphocyte data collected on Day 21 (see diagram above). Tumor number (A) and tumor volume (B) in animals fed a diet consisting of MT oil and or SalpIL2. Natural killer (NK) (C), CD8+ T (D), and CD4+ T cell response (E) to SalpIL2 and MT oil on Day 21.
Discussion
In this report, we investigated whether several known antioxidant seed oils could reduce hepatic metastasis or enhance the antitumor efficacy of attenuated S. enterica Typhimurium. Consistent with previous reports on synergistic antioxidant capacities upon mixing oils,Citation31 we show, compared to individual oils, the combination of BC+BR had increased antioxidant capacity. When given to mice without tumors, the combination of BC+BR oil with SalpIL2 resulted in an increase in splenic NK cells at day 21. The BC+BR combination oil was synergistic with SalpIL2 and increased hepatic NK cells populations, however, the number and volume of hepatic metastases were not significantly decreased. Perhaps due to the increased cell-mediated immune response to SalpIL2, the addition of BC+BR oil to the diet may have provided an increased clearance of SalpIL2. Thus, additional doses may be required to maintain the same level of SalpIL2 infection and demonstrate a significant difference between SalpIL2 with and without BC+BR oil diet.
Since, BC and BR oils individually have displayed significant antitumor properties in other models,Citation26,Citation27,Citation32 we next asked whether individual BC or BR oils have the ability to reduce tumor burden in our hepatic metastasis model. BC oil significantly reduced the volume, but not the number of hepatic tumors, suggesting that the consumption of BC oil can reduce tumor growth in the liver. The effect of BC oil in our model appears to be dose-dependent, as BC oil mixed equally with BR oil did not significantly reduce tumor burden without SalpIL2. The protective properties of BC oil against tumor initiation may be the result of the combination of antioxidant compounds within the extract,Citation33 and appears to not be directly correlated to its lipid-soluble antioxidant content. Thymoquinone (TQ), a component of BC seed oil, has demonstrated inhibitory effects on Cox-1 expression and prostaglandin E-2 production; necessary components of the Cox-dependent inflammatory response.Citation34 Similar to our data, TQ and BC oil reduced tumorigenesis and xenograft growth in animal models of colon and prostate cancer.Citation32,Citation35 The anti-inflammatory properties of BC oil may account for the decreased CD4+ T cell populations, however, increased NK or CD8+T cells in SalpIL2-treated animals may suggest a shift in the type of inflammatory response in tumor-bearing mice. Some CD4+ T cell populations, specifically “regulatory” T cells, appear to contribute to a tumor’s ability to resist killing by the immune system.Citation36 In contrast, regulatory T cells have also been correlated with less advanced colorectal disease.Citation37–Citation39 Therefore, depending on the severity of disease, regulatory T cells may be an attractive target for reprogramming local tumor immune cell populations. In order to more fully interpret our results in this regard, further characterization of tumor CD4+ T cells must be performed.
Similar to BC oil, BR oil alone significantly reduced tumor volume and appeared to enhance the NK cell response to SalpIL2 in mice with established metastases. Since tumor number was not significantly reduced, we interpreted these data to suggest that BR oil reduced the growth rather than induced regression of tumor. Although SalpIL2 alone marginally increased hepatic NK cells, the addition of BR oil significantly increased NK cells in tumor bearing mice, suggesting BR oil enhanced the NK cell response to SalpIL2. This observation was consistent in our experiments in nontumor burdened animals, as BR oil appeared to also increase the NK cell response to SalpIL2 (). Oral administration of SalpIL2, with and without BR oil, induced a significant increase in splenic NK cells after as short a time period as 3 days and peaked 7 days after oral inoculation (). Tumor delivery at the peak of the NK response to SalpIL2 and BR () resulted in greater than 90% reduction of tumor, though there was no statistical difference between the two Salmonella groups. BR oil alone, however, significantly reduced tumor volume suggesting BR oil can induce a preventive effect to the liver within 7 days to reduce tumor growth.
Not all oils tested in our models were shown to have measureable antitumor effects. MT oil, which had the least detectable amount of lipid soluble antioxidants, did not provide any protection against hepatic metastasis. Extracts from milk thistle seeds have shown antiproliferative effects with colon cancer cell lines and showed synergism with the chemotherapeutics doxorubicin and paclitaxel in vitro.Citation40 Most importantly milk thistle extracts have demonstrated promise as adjuvant treatment with minimal adverse effects in human clinical trials as reviewed by Tamayo and Diamond.Citation41 Silibinin, a flavinoid contained in MT seeds has been shown to inhibit tumorigenesis in ratsCitation42 and promotes cell cycle arrest of human colon cancer cells.Citation43 Our results with MT oil seem to contradict previous reports, however, mice were only fed the diet of MT oil 1 week prior to tumor injection, whereas others dosed silibinin for 3 weeks prior to prostate tumor xenograft.Citation44 Even though our tumor cells must survive metastasis from the spleen to the liver, the exposure to MT oil appeared to have little effect on tumor cell death or survival. In contrast, MT oil treatment alone slightly increased tumor burden and reversed the antitumor efficacy of SalpIL2 in our model. Silibinin has been described to be hepatoprotectant against infection and may have blocked the ability of SalpIL2 to mount an antitumor response, perhaps by enhancing host resistance to infection in the liver.Citation45,Citation46
In these studies, we show antioxidant oils with SalpIL2 induce a robust NK cell-mediated response and reduction of tumor burden. By administering SalpIL2 and the antioxidant oil diet 3 days after establishment of hepatic metastases, we model the clinical situation in which patients are diagnosed with distant metastases. In the clinical setting, soon after surgical resection, systemic chemotherapy is used to reduce undetectable micrometastases or unresectable metastatic lesions. Despite these efforts, patients diagnosed with metastatic colorectal cancer have an average 3-year survival rate of 11%.Citation47 Because there is a propensity for malignant colon cancers to form hepatic metastases, the use of S. enterica, whose primary site of infection is within the liver, is an ideal vector for colorectal hepatic metastases. Furthermore, based on the data in this report, the administration of antioxidant oils with SalpIL2 induces a more robust NK cell response as compared to SalpIL2 alone; even when tumors are not present. Hence, when administered prior to tumor development or under low metastatic tumor burden, SalpIL2 with antioxidant oils may create an environment in the liver that is abundant with NK cells and thus inhospitable for tumor metastasis. By reducing the occurrence of hepatic metastasis, SalpIL2 and antioxidant oils may provide a significant survival benefit, perhaps similar to the 90% 5-year survival of colon cancer patients without metastatic disease.Citation47 Therefore, additional studies on the mechanism by which antioxidant oils and SalpIL2 suppress metastatic tumors is warranted.
Acknowledgements
This study was supported by Botanic Oil Innovations and The Arnold S Leonard Cancer Research Fund. The Flow Cytometry Core Facility of the Masonic Cancer Center, a comprehensive cancer center designated by the National Cancer Institute, is supported in part by P30CA77598.
Disclosure
BS, AL, and DS are stockholders in Botanic Oil Innovation. All other authors certify that they have no commercial associations that may pose a conflict of interest in connection with this manuscript.
References
- EisensteinTKBushnellBMeisslerJJJrDalalNSchaferRHavasHFImmunotherapy of a plasmacytoma with attenuated salmonellaMed Oncol19951221031088535659
- SaltzmanDAHeiseCPHaszDEAttenuated Salmonella typhimurium containing interleukin-2 decreases MC-38 hepatic metastases: a novel anti-tumor agentCancer Biother Radiopharm199611214515310851531
- ZuoSGChenYWuZPOrally administered DNA vaccine delivery by attenuated Salmonella typhimurium targeting fetal liver kinase 1 inhibits murine Lewis lung carcinoma growth and metastasisBiol Pharm Bull201033217418220118536
- PawelekJMLowKBBermudesDTumor-targeted Salmonella as a novel anticancer vectorCancer Res19975720453745449377566
- LoefflerMLe’NegrateGKrajewskaMReedJCInhibition of tumor growth using salmonella expressing Fas ligandJ Natl Cancer Inst2008100151113111618664657
- HayashiKZhaoMYamauchiKSystemic targeting of primary bone tumor and lung metastasis of high-grade osteosarcoma in nude mice with a tumor-selective strain of Salmonella typhimuriumCell Cycle20098687087519221501
- KimuraHZhangLZhaoMTargeted therapy of spinal cord glioma with a genetically modified Salmonella typhimuriumCell Prolif2010431414819922490
- LeeCHWuCLShiauALSalmonella choleraesuis as an anticancer agent in a syngeneic model of orthotopic hepatocellular carcinomaInt J Cancer2008122493093517960612
- NagakuraCHayashiKZhaoMEfficacy of a genetically-modified Salmonella typhimurium in an orthotopic humanpancreatic cancer in nude miceAnticancer Res20092961873187819528442
- ZhaoMGellerJMaHYangMPenmanSHoffmanRMMonotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancerProc Natl Acad Sci U S A200710424101701017417548809
- ZuoSGChenYWuZPOrally administered DNA vaccine delivery by attenuated Salmonella typhimurium targeting fetal liver kinase 1 inhibits murine Lewis lung carcinoma growth and metastasisBiol Pharm Bull201033217418220118536
- SotoLJ3rdSorensonBSKimASFeltisBALeonardASSaltzmanDAAttenuated Salmonella typhimurium prevents the establishment of unresectable hepatic metastases and improves survival in a murine modelJ Pediatr Surg20033871075107912861543
- BarnettSJSotoLJ3rdSorensonBSNelsonBWLeonardASSaltzmanDAAttenuated Salmonella typhimurium invades and decreases tumor burden in neuroblastomaJ Pediatr Surg2005406993997 discussion 997–998.15991184
- FeltisBAMillerJSSaharDALiver and circulating NK1.1(+) CD3(–) cells are increased in infection with attenuated Salmonella typhimurium and are associated with reduced tumor in murine liver cancerJ Surg Res2002107110110712384070
- SaltzmanDAKatsanisEHeiseCPAntitumor mechanisms of attenuated Salmonella typhimurium containing the gene for human interleukin-2: a novel antitumor agent?J Pediatr Surg19973223013069044141
- SorensonBSBantonKLFrykmanNLLeonardASSaltzmanDAAttenuated Salmonella typhimurium with interleukin 2 gene prevents the establishment of pulmonary metastases in a model of osteosarcomaJ Pediatr Surg20084361153115818558199
- SorensonBSBantonKLFrykmanNLLeonardASSaltzmanDAAttenuated Salmonella typhimurium with IL-2 gene reduces pulmonary metastases in murine osteosarcomaClin Orthop Relat Res200846661285129118421536
- SorensonBBantonKAugustinLSafety and immunogenicity of Salmonella typhimurium expressing C-terminal truncated human IL-2 in a murine modelBiologics20104617320376175
- FeltisBASaharDAKimASSaltzmanDALeonardASSielaffTDCyclooxygenase-2 inhibition augments the hepatic antitumor effect of oral Salmonella typhimurium in a model of mouse metastatic colon cancerDis Colon Rectum20024581023102812195185
- ChenTRoseMEHwangHNinesRGStonerGDBlack raspberries inhibit N-nitrosomethylbenzylamine (NMBA)-induced angiogenesis in rat esophagus parallel to the suppression of COX-2 and iNOSCarcinogenesis200627112301230716777990
- MallerySRZwickJCPeiPTopical application of a bioadhesive black raspberry gel modulates gene expression and reduces cyclooxygenase 2 protein in human premalignant oral lesionsCancer Res200868124945495718559542
- KimSKimSHHurSMSilibinin prevents TPA-induced MMP-9 expression by down-regulation of COX-2 in human breast cancer cellsJ Ethnopharmacol2009126225225719715751
- ChehlNChipitsynaGGongQYeoCJArafatHAAnti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cellsHPB (Oxford)200911537338119768141
- SethiGAhnKSAggarwalBBTargeting nuclear factor-kappa B activation pathway by thymoquinone: role in suppression of antiapoptotic gene products and enhancement of apoptosisMol Cancer Res2008661059107018567808
- MadhusoodhananRNatarajanMSinghJVEffect of black raspberry extract in inhibiting NFkappa B dependent radioprotection in human breast cancer cellsNutr Cancer20106219310420043264
- DuncanFJMartinJRWulffBCTopical treatment with black raspberry extract reduces cutaneous UVB-induced carcinogenesis and inflammationCancer Prev Res (Phila)20092766567219584078
- KhanNSultanaSInhibition of two stage renal carcinogenesis, oxidative damage and hyperproliferative response by Nigella sativaEur J Cancer Prev200514215916815785320
- RainaKRajamanickamSSinghRPDeepGChittezhathMAgarwalRStage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate modelCancer Res200868166822683018701508
- ShumwayBSKrestyLALarsenPEEffects of a topically applied bioadhesive berry gel on loss of heterozygosity indices in premalignant oral lesionsClin Cancer Res20081482421243018413833
- GalanJENakayamaKCurtissR3rdCloning and characterization of the asd gene of Salmonella typhimurium: use in stable maintenance of recombinant plasmids in Salmonella vaccine strainsGene199094129352227450
- WangSMecklingKAMarconeMFKakudaYTsaoRSynergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacitiesJ Agric Food Chem201159396096821222468
- SalimEIFukushimaSChemopreventive potential of volatile oil from black cumin (Nigella sativa L.) seeds against rat colon carcinogenesisNutr Cancer200345219520212881014
- Al-JoharDShinwariNArifJRole of Nigella sativa and a number of its antioxidant constituents towards azoxymethane-induced genotoxic effects and colon cancer in ratsPhytother Res200822101311132318570215
- El MezayenREl GazzarMNicollsMRMareckiJCDreskinSCNomiyamaHEffect of thymoquinone on cyclooxygenase expression and prostaglandin production in a mouse model of allergic airway inflammationImmunol Lett20061061728116762422
- YiTChoSGYiZThymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathwaysMol Cancer Ther2008771789179618644991
- NishikawaHSakaguchiSRegulatory T cells in tumor immunityInt J Cancer2010127475976720518016
- SinicropeFARegoRLAnsellSMKnutsonKLFosterNRSargentDJIntraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinomaGastroenterology200913741270127919577568
- CorrealePRotundoMSDel VecchioMTRegulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapyJ Immunother201033443544120386463
- LoddenkemperCSchernusMNoutsiasMSteinHThielENagorsenDIn situ analysis of FOXP3+ regulatory T cells in human colorectal cancerJ Transl Med200645217166272
- ColomboVLupiMFalcettaFForestieriDD’IncalciMUbezioPChemotherapeutic activity of silymarin combined with doxorubicin or paclitaxel in sensitive and multidrug-resistant colon cancer cellsCancer Chemother Pharmacol4302010 [Epub ahead of print]
- TamayoCDiamondSReview of clinical trials evaluating safety and efficacy of milk thistle (Silybummarianum [L.] Gaertn.)Integr Cancer Ther20076214615717548793
- KohnoHTanakaTKawabataKSilymarin, a naturally occurring polyphenolic antioxidant flavonoid, inhibits azoxymethane-induced colon carcinogenesis in male F344 ratsInt J Cancer2002101546146812216075
- HoganFSKrishnegowdaNKMikhailovaMKahlenbergMSFlavonoid, silibinin, inhibits proliferation and promotes cell-cycle arrest of human colon cancerJ Surg Res20071431586517950073
- SinghRPDhanalakshmiSTyagiAKChanDCAgarwalCAgarwalRDietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice and increases plasma insulin-like growth factor-binding protein-3 levelsCancer Res200262113063306912036915
- PolyakSJMorishimaCLohmannVIdentification of hepato-protectiveflavonolignans from silymarinProc Natl Acad Sci U S A2010107135995599920231449
- FerenciPScherzerTMKerschnerHSilibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapyGastroenterology200813551561156718771667
- HornerMJRiesLAGKrapchoMSEER Cancer Statistics Review Updated 2009. Available at: http://seer.cancer.gov/csr/1975_2006/. Accessed April 7, 2011.