Abstract
Background
The present meta-analysis was aimed to evaluate the effects of postoperative adjuvant chemotherapy/transarterial chemoembolization (TACE) on the survival/disease-free survival (DFS) rate in hepatocellular carcinoma (HCC) patients with portal vein tumor thrombosis (PVTT).
Methods
The relevant trials were collected using a database search of MEDLINE, Embase, Cochrane Library, Web of Science, ScienceDirect, the China Journal Full-text Database, and the National Institute of Health Clinical Trials Database. The 1-, 3-, and 5-year survival/DFS rates were considered to be the primary end points. A sensitivity analysis was conducted by reanalyzing the data using different statistical approaches.
Results
Eight studies met the inclusion criteria. When compared with surgery alone, the pooled OR showed that the postoperative adjuvant therapy significantly increased the 1-, 3-, and 5-year survival rates for HCC patients with PVTT (the pooled OR and 95% CI of the 1-, 3-, and 5-year survival rates, respectively, were as follows: 2.72, 1.98–3.74; 1.62, 1.13–2.33; 1.99, 1.20–3.29). In addition, when compared with surgery alone, subgroup analysis showed that the postoperative chemotherapy improved the 1-, 3-, and 5-year survival rates of HCC patients with PVTT.
Conclusion
Compared with surgery alone, postoperative adjuvant chemotherapy can improve the 1-, 3- and 5-year survival rates of HCC patients with PVTT. However, postoperative TACE can only increase the 1-year survival rate. However, due to the limitations of this meta-analysis, additional relevant trials are required to confirm these findings.
Background
Hepatocellular carcinoma (HCC) is the fifth most common neoplasm and the second most frequent cause of cancer mortality worldwide.Citation1,Citation2 Globally, over 700,000 new cases are diagnosed every year, with an age-related incidence of 16 cases per 100,000 persons.Citation3 Regarding advanced HCC, portal vein tumor thrombosis (PVTT) has been found in a substantial percentage of HCC patients who are alive and in up to 44% in HCC patients who died.Citation4 HCC patients with PVTT have generally very poor prognosis when the treatment is delayed (median survival time: 2.7–4.0 months).Citation5 According to Barcelona Clinic Liver Cancer (BCLC) staging system, HCC patients with PVTT are classified as BCLC stage C with various treatment options, such as chemotherapy, transarterial chemoembolization (TACE), molecular-targeted therapy, surgical resection, and liver transplantation.Citation6 Although sorafenib target therapy is recommended to be the first-line treatment for BCLC criteria, operative resection and liver transplantation are still considered to be curative options.Citation7,Citation8 The efficacy of postoperative sorafenib vs surgery/sorafenib therapy has not been evaluated until now. The limited number of trials using sorafenib suggests that only descriptive analyses can be performed. Thus, additional trials are needed to assess the efficacy of postoperative sorafenib therapy and to compare the efficacy between adjuvant TACE/chemotherapy and sorafenib.
In recent years, the benefit of postoperative TACE and transarterial embolization has been confirmed in non-advanced HCC cases.Citation9–Citation14 In addition, a meta-analysis confirmed that postoperative TACE is a potential option for the curative resection of HCC with a mean tumor size larger than 5 cm.Citation15 Furthermore, some literature sources have reported that multimodality treatment can prolong the survival rate of HCC patients with PVTT.Citation16–Citation26 The multimodality treatment includes postoperative hepatic artery infusion (HAI), portal vein chemotherapy/chemobiotherapy (PVC), and TACE. Nevertheless, the efficacy of the treatment methods mentioned above remains controversial. Due to potential increased risk of liver failure caused by TACE and chemotherapy, only a few literature sources have evaluated the efficacy and safety of TACE compared with other treatments in HCC patients with PVTT.Citation18–Citation26 This meta-analysis was aimed to evaluate the effects of postoperative adjuvant chemotherapy/TACE on survival time in HCC patients with PVTT.
Methods
Search strategy and selection criteria
We identified the studies by searching MEDLINE, Embase, Cochrane Library (CENTRAL) databases, Web of Science, ScienceDirect, the China Journal Full-text Database, and the National Institute of Health Clinical Trials Database (www.ClinicalTrials.gov) with the following keywords: “hepatocellular carcinoma” or “HCC” or “hepatic tumor” or “hepatic cancer” or “liver cancer” and “PVTT” or “portal vein tumor thrombi” or “portal vein tumor thrombosis” and “chemotherapy” and “TACE” or “chemoembolization” or “embolization” and “clinical trials” in the English language and in non-English languages (limits of the search period: from January 1990 to October 2014). The references of the retrieved literature were also screened. The search strategies are shown in .
The inclusion criteria were as follows:
Trials: both randomized controlled studies (RCTs) and retrospective studies
Patients: diagnosed with HCC with PVTT and without sex and age limits
Interventions: postoperative adjuvant chemotherapy/TACE plus surgery vs surgery alone
Chemotherapy: HAI and PVC
Surgery: both hepatectomy and PVTT removal were conducted
Follow-up: data available on the 1-, 3-, or 5-year survival rates and/or DFS rates
The exclusion criteria were as follows:
Studies that included cholangiocellular carcinoma patients
Studies that did not conduct PVTT removal
Studies that involved duplicative publication
Studies that did not report the 1-, 3-, or 5-year overall survival (OS) rates and/or DFS rates.
Data extraction and quality assessment
Two authors (Shang H and Zhang YF) independently extracted information and data from the studies including the author’s first name, year of publication, study methods, patient’s characteristics, interventions, and 1-, 3-, and 5-year OS rates (). Disagreements were resolved by consensus.
Table 1 Characteristics of the clinical trials included in the meta-analysis Child–Pugh class (A) and treatment (B)
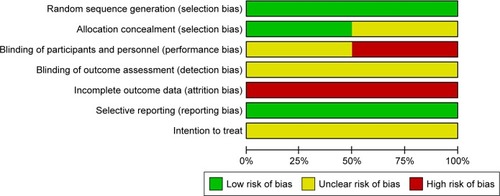
Furthermore, we evaluated the methodological quality of each RCT according to the method described in the Cochrane Handbook for Systematic Reviews of Interventions.Citation27 Random sequence generation, allocation concealment, blinding (patients, personnel, and outcomes assessment), incomplete outcome data, selective reporting, and whether the analysis referred to intention-to-treat were assessed to examine all of the included RCTs and were assigned values of “low risk”, “high risk”, or “unclear” (). This five-point quality scale includes points for randomization (0–2 points), double blinding (0–2 points), and withdrawals and dropouts (0–1 point). Total scores of 0–2 indicated low quality, whereas studies with total scores ≥3 were defined as high quality. However, the quality of each retrospective study was assessed by the Newcastle–Ottawa Quality Assessment Scale, and studies were classified as high quality if they received more than seven stars, medium quality if they received between four and six stars, and poor quality if they received fewer than four stars ().
Table 2 Newcastle–Ottawa Scale for assessing the quality of no-cohort studies
Statistical analysis
The effects of the treatment options were evaluated by the 1-, 3-, and 5-year survival rates using the ORs with 95% CIs. The 1-, 3-, and 5-year survival rates were considered the primary end points. The homogeneity of the included trials was analyzed by the chi-squared test with Cochran’s Q statistic and I2 (statistical significance set at P>0.1 or I2 <50%). The Mantel–Haenszel method with a fixed-effects model was used to analyze the pooled data when the homogeneity was significant. However, when heterogeneity between trials was observed, then instead of determining the causes of the heterogeneity, a random-effects model was used to analyze the data. Sensitivity analyses were performed to determine the stability of the overall treatment effects. The point estimate of the OR was used to assess the statistical significance of the outcomes (statistical significance was set at P<0.05 if the 95% CI did not include the value 1). The analyses of the data were performed using Review Manager, version 5.2 (The Cochrane Collaboration, Software Update, Oxford, UK).
Results
Eligible studies
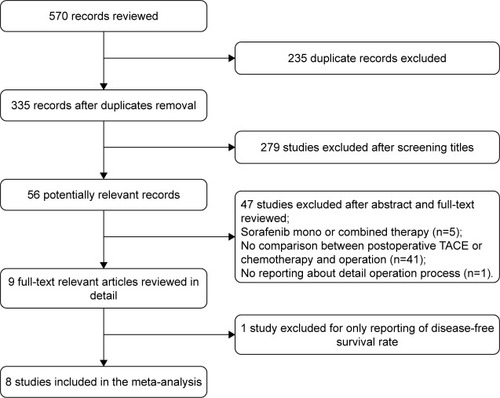
The search strategies and processes are shown in . Fifty-six potentially relevant records were identified, of which 47 studies were excluded after the abstract and full-text review for the following reasons: mono or combined sorafenib treatment was included (n=5), no comparison between postoperative TACE/chemotherapy and surgery (n=41), and no reporting about the details of the surgical procedure (n=1). Finally, only one study with a reported DFS rate was excluded that could not be subjected to a meta-analysis.Citation18 A total of eight studies involving 697 patients were included to compare surgery plus postoperative adjuvant chemotherapy/TACE with surgery alone. Only these eight studies reported the OS rates.Citation19–Citation26 In addition, three articles that were reported in the Chinese language were retrieved using Web of Science and were included in the meta-analysis after evaluating the quality. The data of an article included both postoperative TACE and chemotherapy (we marked Li et al1 for TACE and Li et al2 for chemotherapy).Citation23 Postoperative adjuvant chemotherapy included the following: HAI and PVC. The operative methods used in the included trials were hepatectomy and PVTT removal. The drugs, courses, and dosages of chemotherapy and TACE are listed in . Of the eight studies included, three articles reported 1-, 3-, and 5-year survival rates;Citation20,Citation23,Citation24 three articles reported 1- and 3-year survival rates;Citation19,Citation21,Citation25 and two articles reported only the 1-year survival rate.Citation22,Citation26 The characteristics of the included studies are shown in .
Table 3 Interventions of the included studies
Methodological quality of the included studies
The quality of each included study is shown in . The methodological quality of each included study was evaluated by the previously introduced principles. Three trials were considered to be of high quality, and five trials were considered to be of medium quality (). No signs of selective reporting were observed, and all of the trials did not state the intention-to-treat principle clearly.
Meta-analysis of the 1-, 3-, and 5-year survival rates
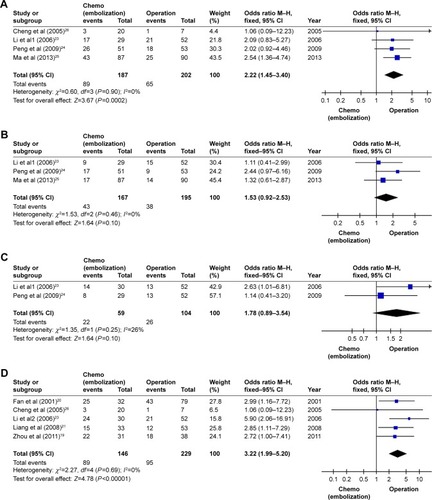
All of the eight included trials provided the 1-year survival rate. There was significant homogeneity among the eight studies (I2=0%, P=0.81), and a fixed-effects model was used to analyze the data. The pooled OR (2.72, 95% CI=1.98–3.74) showed a significantly increased 1-year survival rate for HCC patients with PVTT following postoperative adjuvant chemotherapy/TACE compared with surgery alone (P<0.00001). Similarly, six trials reported the 3-year survival rate and three trials reported the 5-year survival rate. Their pooled ORs calculated using the fixed-effect model showed a significantly increased 3- and 5-year survival rates for HCC patients with PVTT following postoperative adjuvant chemotherapy/TACE compared with surgery alone (P=0.009 and P=0.007, respectively), and the homogeneity was significant (3-year survival rate: pooled OR=1.62, 95% CI=1.13–2.33, I2=0%, P=0.69; 5-year survival rate: pooled OR=1.99, 95% CI=1.20–3.29, I2=0%, P=0.64) ().
Figure 3 Forest plot of the analysis of the 1-, 3-, and 5-year survival rates (chemo [embolization], chemotherapy, and/or transarterial chemoembolization).
Abbreviations: TACE, transarterial chemoembolization; M–H, Mantel–Haenszel test.
![Figure 3 Forest plot of the analysis of the 1-, 3-, and 5-year survival rates (chemo [embolization], chemotherapy, and/or transarterial chemoembolization).](/cms/asset/33ca5a75-b960-47db-9bd5-860a5c6701e4/dott_a_171612_f0003_b.jpg)
Subgroup analysis
A total of four trials reported the 1-year survival rate, which was compared between postoperative TACE and surgery alone for HCC patients with PVTT. Additionally, three trials reported the 3-year survival rate and two trials reported the 5-year survival rate. There was significant homogeneity among them, and fixed-effect models were used to analyze the data. The pooled OR calculated by the fixed-effect model indicated that postoperative adjuvant TACE significantly increased the 1-year survival rate compared with surgery alone for HCC patients with PVTT. However, the pooled OR of postoperative TACE regarding the 3- and 5-year survival rates showed that there were no statistical significances between postoperative TACE and surgery alone for HCC patients with PVTT because the 95% CI included the value 1 ().
Figure 4 Forest plot of the subgroup analysis of the 1-, 3-, and 5-year survival rates.
Abbreviations: TACE, transarterial chemoembolization; M–H, Mantel–Haenszel test.
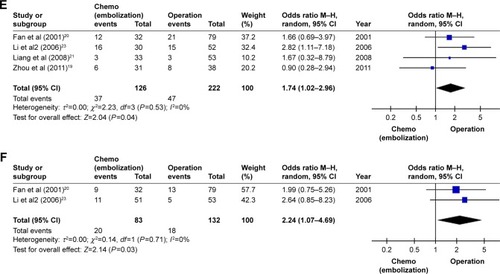
Regarding postoperative adjuvant chemotherapy, the pooled ORs of the 1-, 3-, and 5-year survival rates indicated that postoperative adjuvant chemotherapy could significantly increase 1-, 3-, and 5-year survival rates compared with surgery alone for HCC patients with PVTT (1-year survival rate: pooled OR=3.22, 95% CI=1.99–5.20, I2=0%, P=0.69; 3-year survival rate: pooled OR=1.74, 95% CI=1.02–2.96, I2=0%, P=0.53; 5-year survival rate: pooled OR=2.24, 95% CI=1.07–4.69, I2=0%, P=0.71) ().
Sensitivity analysis
Sensitivity analysis was performed using the fixed-effects model and random-effects model for the quality of the included studies and the effects of postoperative adjuvant chemotherapy/TACE and subgroup analysis. The change of the effects model did not result in the statistical significance of the pooled OR, indicating that the meta-analysis had good reliability.
Discussion
From 1990 to 2013, liver cancer ranked 21st among the leading causes of death worldwide. Furthermore, death due to liver cancer in all ages has increased from 510.1/1,000 to 818.0/1,000, and the age-standardized death rate has decreased from 13.7/100,000 to 13.0/100,000.Citation28 All of the above indicate that, although the morbidity of liver cancer has been younger, the efficacy of the treatment has improved. Based on the BCLC staging system, HCC patients with PVTT are classified as BCLC stage C.Citation6 The recommended treatment is sorafenib therapy. However, some studies have shown that hepatic arterial infusion chemotherapy (HAIC), radiotherapy (RT), TACE + RT, and sorafenib + TACE + RT can be an alternative treatment to sorafenib therapy in HCC patients with PVTT.Citation18,Citation29–Citation33 In addition, an article reports that TACE–sorafenib combination treatment may improve OS in HCC patients with PVTT compared with patients who underwent TACE alone.Citation34 Although the above-mentioned treatments have achieved satisfactory efficacy, operative resection and liver transplantation are still considered to be the curative options.Citation7 Moreover, one case report indicates that sorafenib therapy combined with intermittent cisplatin HAIC is considered to be an effective therapy for advanced HCC with PVTT in the major trunk.Citation35 Additionally, another case report involving two cases showed that preoperative sorafenib treatment provides an opportunity for HCC patients with PVTT to have curative resection.Citation36 However, the efficacy of postoperative sorafenib therapy vs surgery/sorafenib therapy has not been evaluated until now. The limited number of trials concerning sorafenib suggests that we can only perform a descriptive analysis. Thus, additional trials are needed to assess the efficacy of postoperative sorafenib therapy and to compare the efficacy between adjuvant TACE/chemotherapy and sorafenib therapy.
Although there are various treatment options, the prognosis of HCC patients with PVTT remains very poor. This meta-analysis aimed to evaluate the effects of postoperative adjuvant chemotherapy and/or TACE on the survival time in HCC patients with PVTT. We systematically searched the main databases (MEDLINE, Embase, Cochrane Library [CENTRAL], Web of Science, ScienceDirect, the China Journal Full-text Database, and the National Institute of Health Clinical Trials Database), and eight studies were included in our meta-analysis. All of the included studies fulfilled the inclusion criteria: RCT or retrospective study; patients were diagnosed with HCC with PVTT and without sex and age limits; postoperative adjuvant chemotherapy/TACE plus surgery vs surgery alone was performed; both hepatectomy and PVTT removal were conducted; and data available on the 1-, 3-, or 5-year survival rates and/or DFS rates. For the statistical homogeneity of the included studies, outcomes were analyzed using the fixed-effects model. The fixed-effects model was used to analyze sensitivity and the random-effects model was used to evaluate the reliability of the meta-analysis. This meta-analysis indicated that postoperative adjuvant therapy showed improvement in the 1-year survival rate when compared to surgery alone. Similarly, benefits of the 3- and 5-year survival rates were also found (1-year survival rate: pooled OR=2.72, 95% CI=1.98–3.74, I2=0%, P=0.81; 3-year survival rate: pooled OR=1.62, 95% CI=1.13–2.33, I2=0%, P=0.69; 5-year survival rate: pooled OR=1.99, 95% CI=1.20–3.29, I2=0%, P=0.64). In subgroup analysis, the meta-analysis indicated that postoperative chemotherapy could increase the 1-, 3-, and 5-year survival rates compared with surgery alone for HCC patients with PVTT. However, postoperative TACE could only improve the 1-year survival rate, and no statistical significance was found in the improvement of the 3- and 5-year survival rates compared with surgery alone for HCC patients with PVTT. The results are partly consistent with a previous study.Citation15 It was found that postoperative TACE could significantly improve the DFS and OS compared with surgery alone when the mean tumor size was bigger than 5 cm. However, the study failed to group OS by time.
Furthermore, of the included studies, Fan et al’s study evaluated the differences among different treatments in HCC patients with PVTT in the first branch and main trunk tumor thrombosis groups.Citation20 The outcomes reported that postoperative TACE and chemotherapy showed better 1-, 3-, and 5-year survival rates than the others in both the first branch tumor thrombosis group and main trunk tumor thrombosis group. Additionally, with the same treatment, the outcomes indicated that the first branch tumor thrombosis group had better 1-, 3-, and 5-year survival rates than the main trunk tumor thrombosis group. Furthermore, in the study by Li et al,Citation23 the 1-, 3-, and 5-year DFS rates in the surgery group were 50.7%, 17.8%, and 0% vs 62.3%, 23.7%, and 4.0% in the postoperative TACE group, respectively, indicating that postoperative TACE could increase the 1-, 3-, and 5-year DFS rates. These findings could guide the surgical method and subsequent treatment options for the different tumor thrombosis types in HCC patients with PVTT.
However, although TACE and chemotherapy can improve the survival of HCC patients with PVTT, the mild and severe adverse events should also be considered. The adverse events of TACE included the following: ascites, transient liver failure, deterioration of liver function, progression of tumor or metastases before surgery, bacteremia, and bleeding from the femoral puncture site.Citation23,Citation24 The adverse events of chemotherapy included nausea, loss of appetite, leukopenia, alopecia, drug fever, and deterioration of liver or renal function.Citation20–Citation22 All of the mild adverse events were common and easily resolved. However, although the severe adverse events were rare, they should be considered and treated well.
Finally, the limitations of this meta-analysis were analyzed. First, only two RCTs were analyzed, which possibly caused selection bias (). Second, due to the lack of relevant studies, the conclusions of the subgroup analysis of postoperative TACE and postoperative chemotherapy were limited. Third, only three high-quality articles were included. Fourth, all of the included studies were from Asia. Therefore, more clinical trials and more relevant studies from different countries are needed to provide additional evidence to support the meta-analysis. Due to the limitations above, the outcomes and conclusion of the present meta-analysis should be interpreted cautiously. However, in the present study the effects of postoperative adjuvant chemotherapy/TACE on survival time in HCC patients with PVTT are evaluated and a valuable conclusion is drawn, which provided a good guiding principle for clinical application.
Conclusion
Our meta-analysis showed that postoperative adjuvant therapy could increase the 1-, 3-, and 5-year survival rates of HCC patients with PVTT. In subgroup analysis, postoperative chemotherapy can improve the 1-, 3-, and 5-year survival rates of HCC patients with PVTT vs surgery alone. Postoperative TACE can only increase the 1-year survival rate, and no statistical significance was found in the improvement of the 3- and 5-year survival rates compared with surgery alone for HCC patients with PVTT. However, due to the limitations of this meta-analysis, more relevant RCTs from different countries are required to further confirm these findings. Meanwhile, a subgroup analysis of postoperative TACE and postoperative chemotherapy should be performed. Based on the BCLC staging system, the efficacy between adjuvant TACE/chemotherapy and sorafenib should be compared to provide evidence of the clinical options.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (grant no 81170454).
Disclosure
The authors report no conflicts of interest in this work.
References
- ParkinDMBrayFFerlayJPisaniPEstimating the world cancer burden: Globocan 2000Int J Cancer200194215315611668491
- JemalABrayFCenterMMFerlayJWardEFormanDGlobal cancer statisticsCA Cancer J Clin2011612699021296855
- FerlayJShinHRBrayFFormanDMathersCParkinDMEstimates of worldwide burden of cancer in 2008: GLOBOCAN 2008Int J Cancer2010127122893291721351269
- PirisiMAvelliniCFabrisCPortal vein thrombosis in hepatocellular carcinoma: age and sex distribution in an autopsy studyJ Cancer Res Clin Oncol199812473974009719503
- MinagawaMMakuuchiMTreatment of hepatocellular carcinoma accompanied by portal vein tumor thrombusWorld J Gastroenterol200612477561756717171782
- FornerAReigMEde LopeCRBruixJCurrent strategy for staging and treatment: the BCLC update and future prospectsSemin Liver Dis2010301061074
- LlovetJMRicciSMazzaferroVSorafenib in advanced hepatocellular carcinomaN Engl J Med2008359437839018650514
- FanSTLoCMLiuCLHepatectomy for hepatocellular carcinoma: toward zero hospital deathsAnn Surg1999229332233010077043
- IzumiRShimizuKIyobeTPostoperative adjuvant hepatic arterial infusion of Lipiodol containing anticancer drugs in patients with hepatocellular carcinomaHepatology19942022953018045490
- LiJQZhangYQZhangWZYuanYFLiGHRandomized study of chemoembolization as an adjuvant therapy for primary liver carcinoma after hepatectomyJ Cancer Res Clin Oncol199512163643667541051
- TacherVLinMBhagatNDual-phase cone-beam computed tomography to see, reach, and treat hepatocellular carcinoma during drug-eluting beads transarterial chemo-embolizationJ Vis Exp2013828250795
- ZhongCGuoRPLiJQA randomized controlled trial of hepatectomy with adjuvant transcatheter arterial chemoembolization versus hepatectomy alone for Stage III A hepatocellular carcinomaJ Cancer Res Clin Oncol2009135101437144519408012
- LiQWangJSunYPostoperative transhepatic arterial chemoembolization and portal vein chemotherapy for patients with hepatocellular carcinoma: a randomized study with 131 casesDig Surg200623423524016943671
- JiangJHGuoZLuHFAdjuvant transarterial chemoembolization after curative resection of hepatocellular carcinoma: propensity score analysisWorld J Gastroenterol201521154627463425914472
- ChengXSunPHuQGSongZFXiongJZhengQCTransarterial (chemo)embolization for curative resection of hepatocellular carcinoma: a systematic review and meta-analysesJ Cancer Res Clin Oncol201414071159117024752339
- LiYZhengY-BZhaoWSorafenib in combination with transarterial chemoembolization and radiofrequency ablation in the treatment for unresectable hepatocellular carcinomaMed Oncol201330473024048774
- YangMFangZYanZTransarterial chemoembolisation (TACE) combined with endovascular implantation of an iodine-125 seed strand for the treatment of hepatocellular carcinoma with portal vein tumour thrombosis versus TACE alone: a two-arm, randomised clinical trialJ Cancer Res Clin Oncol2014140221121924374800
- SongDSSongMJBaeSHA comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosisJ Gastroenterol201550444545425027973
- ZhouQWangYZhouXPrognostic analysis for treatment modalities in hepatocellular carcinomas with portal vein tumor thrombiAsian Pac J Cancer Prev201112112847285022393952
- FanJWuZQTangZYMultimodality treatment in hepatocellular carcinoma patients with tumor thrombi in portal veinWorld J Gastroenterol200171283211819728
- LiangLJHuWJYinXYAdjuvant intraportal venous chemotherapy for patients with hepatocellular carcinoma and portal vein tumor thrombi following hepatectomy plus portal thrombectomyWorld J Surg200832462763118228094
- NigumaTMimuraTTutuiNAdjuvant arterial infusion chemotherapy after resection of hepatocellular carcinoma with portal thrombosis: a pilot studyJ Hepatobiliary Pancreat Surg200512324925315995815
- LiQWangJSunYEfficacy of postoperative transarterial chemoembolization and portal vein chemotherapy for patients with hepatocellular carcinoma complicated by portal vein tumor thrombosis – a randomized studyWorld J Surg2006301120042011 discussion 2012–200317058027
- PengBGHeQLiJPZhouFAdjuvant transcatheter arterial chemoembolization improves efficacy of hepatectomy for patients with hepatocellular carcinoma and portal vein tumor thrombusAm J Surg2009198331331819285298
- MaLJzYXiangBDFxWZhaoYNLqLImpact of treatment strategies on patients with hepatocellular carcinoma of less than 10 cm but with portal vein tumor thrombusChin J Hepatobiliary Surg2013319165170
- ChengSQWuMCChenHHepatocellular carcinoma with tumor thrombi in the portal vein. A comparison of therapeutic effects by different treatmentsZhonghua Zhong Liu Za Zhi200527318318515946574
- HigginsJGreenSCochrane Handbook for Systematic Reviews of InterventionsHobokenWiley2010
- NaghaviMWangHDLozanoRGBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013Lancet2015385996311717125530442
- NakazawaTHidakaHShibuyaAOverall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysisBMC Gastroenterol2014148424886354
- ChoJYPaikYHParkHCThe feasibility of combined transcatheter arterial chemoembolization and radiotherapy for advanced hepatocellular carcinomaLiver Int201434579580124350564
- LiYZhengYBZhaoWSorafenib in combination with transarterial chemoembolization and radiofrequency ablation in the treatment for unresectable hepatocellular carcinomaMed Oncol201330473024048774
- YehSAChenYSPerngDSThe role of radiotherapy in the treatment of hepatocellular carcinoma with portal vein tumor thrombusJ Radiat Res201556232533125411553
- YamadaRSatoMKawabataMNakatsukaHNakamuraKTakashimaSHepatic artery embolization in 120 patients with unresectable hepatomaRadiology198314823974016306721
- ZhuKChenJLaiLHepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib – a retrospective controlled studyRadiology2014272128429324708192
- IshizakiMKaiboriMMatsuiKA case of curative resection for advanced hepatocellular carcinoma with portal vein tumor thrombus after hepatic arterial infusion chemotherapyGan To Kagaku Ryoho201239121991199323267953
- IrtanSChopin-LalyXRonotMFaivreSParadisVBelghitiJComplete regression of locally advanced hepatocellular carcinoma induced by sorafenib allowing curative resectionLiver Int201131574074321457447