Abstract
Background and aim
Cyclins have been reported to be overexpressed with poor prognosis in several human cancers. However, limited numbers of studies evaluated the expressions and prognostic roles of cyclins in gastric cancer (GC). We aim to evaluate the expressions and prognostic roles of cyclins. Also, further efforts were made to explore biological function of the differentially expressed cyclins.
Methods
Cyclins expressions were analyzed by Oncomine and The Cancer Genome Atlas datasets, and the prognostic roles of cyclins in GC patients were investigated by the Kaplan–Meier Plotter database. Then, a comprehensive PubMed literature search was performed to identify expression and prognosis of cyclins in GC. Biological functions of the differentially expressed cyclins were explored through Enrich R platform, and KEGG and transcription factor were analyzed.
Results
The expression levels of CCNA2 (cyclin A2), CCNB1 (cyclin B1), CCNB2 (cyclin B2), and CCNE1 (cyclin E1) mRNAs were identified to be significantly higher in GC tissues than in normal tissues in both Oncomine and The Cancer Genome Atlas datasets. High expressions of CCNA2, CCNB1, and CCNB2 mRNAs were identified to be related with poor overall survival in Kaplan–Meier Plotter dataset. Evidence from clinical studies showed that CCNB1 was related with overall survival in GC patients. Cyclins were associated with several biological pathways, including cell cycle, p53 signaling pathway, FoxO signaling pathway, viral carcinogenesis, and AMPK signaling pathway. Enrichment analysis also showed that cyclins interacted with some certain transcription factors, such as FOXM1, SIN3A, NFYA, and E2F4.
Conclusion
Based on our results, high expressions of cyclins were related with poor prognosis in GC patients. The above information might be useful for better understanding the clinical and biological roles of cyclins mRNA and guiding individualized treatments for GC patients.
Introduction
Gastric cancer (GC) is estimated to be one of the most leading causes of all deaths worldwide.Citation1 Every year, nearly one million new cases occur and around 7,00,000 people die of GC.Citation2 Although there is a decrease in incidence in the recent years, patients with GC still suffer from a poor prognosis, which brought great burden to global health and economy.Citation3,Citation4 Tumor resection or lymphadenectomy is one of the main treatments for advanced GC.Citation5 However, surgical treatment alone is associated with high recurrence rates.Citation6,Citation7 Therefore, identification of the molecular mechanism during GC pathogenesis, as well as identification of potential prognostic biomarkers and drug targets is still needed.
Cyclins, including cyclin A, B, D, and E, act as key controlling elements of the eukaryotic cell cycle.Citation8 Considerable efforts have been expended in order to understand the potential biological mechanism and clinical significance of cyclins in human cancers.Citation8–Citation11 Many studies demonstrated that the expressions of cyclins were overexpressed in human cancers and correlated with poor clinical outcomes in lung cancer,Citation12–Citation17 breast cancer,Citation18–Citation28 and ovarian cancer.Citation29–Citation34 However, limited numbers of studies evaluated the expressions and prognostic roles of cyclins in GC patients,Citation35–Citation37 and additional efforts are still needed to explore their biological function.
Oncomine (http://www.oncomine.org),Citation38 a cancer microarray database and web-based data mining platform, could automatically compute differential expression profiles between cancer and normal tissues for a gene of interest. Kaplan–Meier Plotter (KM Plotter; http://kmplot.com/analysis/) is capable of assessing the effect of 54,675 genes on survival using 1,065 GC samples.Citation39 In the present study, we aim to evaluate the expressions and prognostic roles of different cyclins in GC patients through the above two databases. For further biological function analysis of cyclins, enrichment analysis was performed by Enrich platform.Citation40 In this study, we aim to study the clinical significance and biological mechanism of cyclins during GC pathogenesis through bioinformatics methods.
Materials and methods
Oncomine database analysis
Oncomine (http://www.oncomine.org), which contains 715 datasets and 86,733 samples, was utilized to examine the cyclins mRNA expressions between GC and normal tissues.Citation38 The thresholds were restricted to the following: P-value: 0.05; fold change: 2.0; gene rank: all; and data type: mRNA. We performed comparisons by cancer vs normal analysis. Fold change, P-value, gene rank, and sample sizes were obtained from studies that showed statistically significant differences.
Then, the GSE13861, GSE27342, GSE13911, and GSE19826 datasets were employed from Oncomine database, and the raw data of cyclins expressions were downloaded. After normalization, a meta-analysis was conducted by Review Manager. The standardized mean difference was calculated. P-value <0.05 was considered to be statistically significant. Heterogeneity among studies was evaluated by the Cochran’s Q test and I2 statistic. The P-value for Cochran’s Q test <0.1 or I2>50% was considered to be heterogeneous. Pooled analysis was applied with a fixed-effects model if no heterogeneity was detected. Random-effects model was applied if any heterogeneity existed.
For validation, the expressions of cyclins for GC patients in The Cancer Genome Atlas (TCGA; https://cancergenome.nih.gov/) dataset were analyzed. The TCGA GC data included 32 normal and 375 GC samples.
KM Plotter database analysis
KM Plotter (http://kmplot.com/analysis/) is capable of assessing the effect of 54,675 genes on survival using 10,461 cancer samples.Citation39 Currently, it contains 5,143 breast, 1,618 ovarian, 2,437 lung, and 1,065 GC patients with a mean follow-up of 69/40/49/33 months, respectively. The prognostic roles of cyclins in GC were assessed by KM Plotter. GC patients were divided into high- and low-expression groups by the median values of mRNA expression. The overall survival (OS) of GC patients was measured by calculating logrank P-value and hazard ratio (HR) with 95% CI. Number of cases, HRs with 95% CIs, and P-values were extracted from the KM Plotter webpage. In this study, “array quality control” was selected, and “exclude biased arrays” without follow-up. The GSE14210, GSE15459, GSE22377, GSE29272, and GSE51105 data-sets were employed for pooled survival analysis from KM Plotter database.
Literature search of expression and prognosis of cyclins in GC
A comprehensive PubMed literature search was performed to identify expression and prognosis of cyclins in GC. The following keywords were used: “gastric cancer”, “stomach adenocarcinoma”, “gastric adenocarcinoma”, “survival”, “prognosis”, “gene expression”, “CCNA2”, “CCNB1”, “CCNB2”, and “CCNE1”. The prognosis roles of cyclins were reviewed by comprehensive literature search.
Enrichment analysis of cyclins
In order to obtain additional biological information of the differentially expressed cyclins, a comprehensive functional gene set analysis was performed using the EnrichR platform.Citation40 EnrichR platform includes 69 gene-set libraries, such as KEGG, WikiPathways, Gene Ontology, and transcription factor (TF). P-value and Z-score were calculated for enrichment analysis, and protein–protein interaction (PPI) network was constructed. The P-value is computed using a standard statistical method used by most enrichment analysis tools – Fisher’s exact test or the hypergeometric test. The Z-score is computed using a modification to Fisher’s exact test in which an Z-score was computed for deviation from an expected rank. For multiple hypotheses, Benjamini–Hochberg (BH) procedure was used.
Results
Cyclins mRNA expression in GC patients
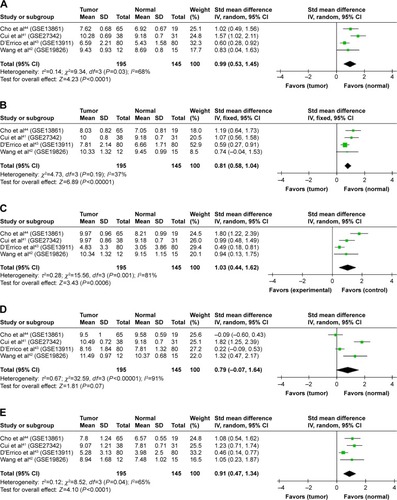
Using Oncomine analysis, we investigated mRNA levels of cyclins in GC patients. The differentially expressed cyclins in four datasetsCitation41–Citation44 are shown in . The mRNA expression levels of CCNA2, CCNB1, CCNB2, CCND2, and CCNE1 were significantly higher in GC than in normal tissues in each single dataset. CCNB1 and CCNE1 mRNA expressions were also significantly higher in gastric diffuse adenocarcinoma and gastric intestinal adenocarcinoma, respectively. The pooled analysis, by comparing different studies in Oncomine datasets, showed that CCNA2, CCNB1, CCNB2, and CCNE1 were significantly higher in GC than in normal tissues ().
Table 1 The differentially expressed cyclins mRNA in GC
Figure 1 Cyclins expression in gastric cancer in Oncomine datasets.
Abbreviations: GC, gastric cancer; std, standard.
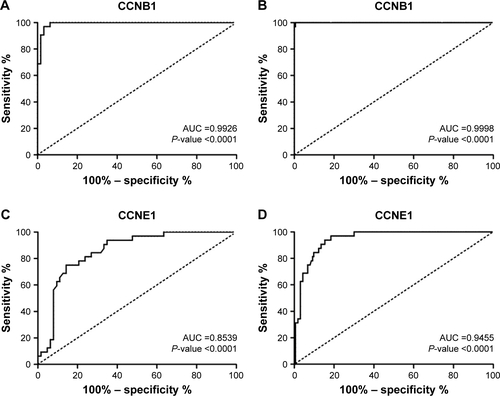
The expressions of cyclins were analyzed in TCGA GC dataset for validation. The mRNA expression levels of CCNA2, CCNB1, CCNB2, and CCNE1 were significantly higher in GC than in normal tissues, while CCND2 did not show the significance (). The receiver operating characteristic (ROC) curves of CCNA2, CCNB1, CCNB2, and CCNE1 were constructed for GC diagnosis (). Besides, CCNB1 and CCNE1 mRNA expressions were also significantly higher in diffuse gastric adenocarcinoma and gastric intestinal type adenocarcinoma, respectively (). Also, the ROC curves of CCNB1 and CCNE1 were constructed for GC Lauren subtype classification ().
Figure 2 Cyclins expression in GC in TCGA dataset.
Abbreviations: GC, gastric cancer; TCGA, The Cancer Genome Atlas.
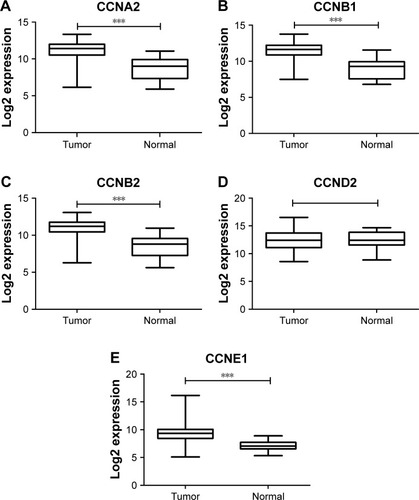
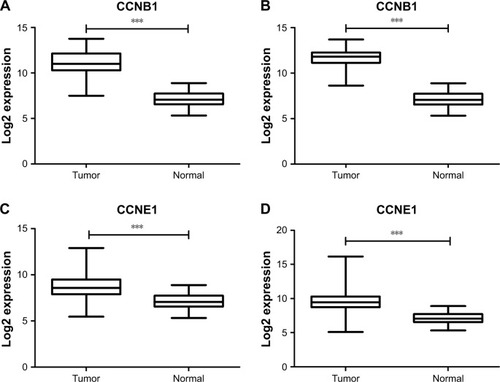
Figure 3 CCNB1 and CCNE1 expressions in GC Lauren subtype.
Abbreviations: GC, gastric cancer; TCGA, The Cancer Genome Atlas.
In summary, CCNA2, CCNB1, CCNB2, and CCNE1 were identified to be differentially expressed in GC tissues in both Oncomine and TCGA datasets. Furthermore, CCNB1 and CCNE1 expressions were also differentially expressed in GC Lauren subtype, which indicated that CCNB1 and CCNE1 might be helpful to classify GC Lauren subtypes.
Prognostic role of cyclins mRNA in GC patients
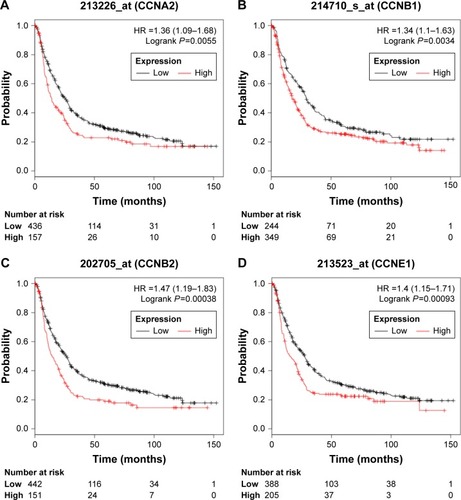
The prognostic roles of differentially expressed cyclins in GC were assessed by KM Plotter. Survival curves were plotted for GC patients. High expressions of CCNA2 (HR =1.36, 95% CI: 1.09–1.68, P=0.0055), CCNB1 (HR =1.34, 95% CI: 1.10–1.63, P=0.0034), CCNB2 (HR =1.47, 95% CI: 1.19–1.83, P=0.00038), and CCNE1 (HR =1.40, 95% CI: 1.15–1.71, P=0.00093) were related with poor OS ().
Figure 4 Prognosis roles of differentially expressed cyclins in GC patients in KM Plotter datasets.
Abbreviations: GC, gastric cancer; KM Plotter, Kaplan–Meier Plotter.
The prognostic role of differentially expressed cyclins was reviewed in the previously published studies for validation (). Up to now, there are no data about the prognosis roles of CCNA2 and CCNB2. Three studies evaluated the relationship between CCNB1 expression and clinicopathological features in GC. Three studies showed that CCNB1 high expression was related with lymph node metastasis. Two studies evaluated the prognosis role of CCNB1. The study from KimCitation53 did not indicate poor prognosis for CCNB1 overexpression GC patients. But Kim enrolled only 23 patients, and the results might not be reliable. The study involving 482 GC patients from Begnami et alCitation35 showed poor 5-year OS for CCNB1 high-expression patients. Only one study evaluated the prognosis role of CCNE1. CCNE1 was associated with Lauren classification, tumor size, lymphatic and vascular invasion, but not with OS.
Table 2 The relationship between differentially expressed cyclins and clinicopathological features in previously published studies
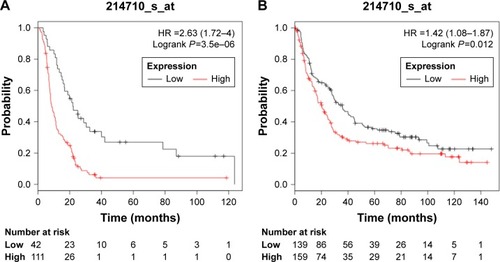
Then, further efforts were made to investigate the prognosis role of CCNB1 in GC. High expression of CCNB1 (HR =2.63, 95% CI: 1.72–4.00, P=3.5E–06) predicted poor OS in GC patients who received 5-FU-based chemotherapy treatment (). Furthermore, high expression of CCNE1 predicted poor OS in HER2-negative GC patients (HR =1.42, 95% CI: 1.08–1.87, P=0.0117; ). The above results indicated that CCNE1 might be useful for guiding individualized treatments for GC patients.
Figure 5 Prognosis roles of CCNB1 in different GC patients.
Abbreviations: GC, gastric cancer; KM Plotter, Kaplan–Meier Plotter.
Enrichment analysis of cyclins
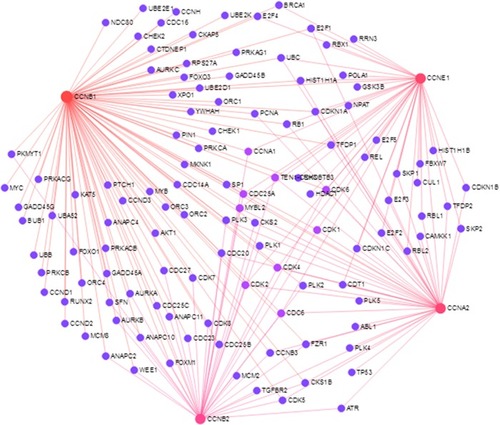
The PPI network constructed is shown in . In the PPI network, CDK1 and CDK2 were identified as PPI hub proteins. Several biological pathways were involved, including cell cycle, p53 signaling pathway, FoxO signaling pathway, viral carcinogenesis, and AMPK signaling pathway. Enrichment analysis also showed that cyclins were related with some TFs, such as FOXM1, SIN3A, NFYA, and E2F4 ().
Table 3 Enrichment analysis of cyclins
Discussion
Publicly available microarray data have vast potential to serve as a source of biomarker discovery because there is enormous quantity of existing gene expression data.Citation45,Citation46 Potential roles for biomarkers include early diagnosis of disease, disease classification, prediction of prognosis model, and response to drug target therapy.Citation47,Citation48 Identification of genes associated with clinical survival rate and target therapy might provide a novel insight into GC pathogenesis and guide individualized treatments. In our study, the expression and prognosis roles of cyclins expressions were analyzed, and biological function was evaluated by enrichment analysis. Our study showed that high expression of cyclins mRNA was related with GC prognosis.
CCNA2 is overexpressed in several human cancers and correlated with tumor progression and shorter survival in lung, breast, and colorectal cancer.Citation12,Citation18,Citation49–Citation51 Our study showed that CCNA2 overexpression was an indicator of poor prognosis. Up to now, there is no study evaluating the expression and prognostic role of CCNA2 in GC patients. More efforts are needed to confirm expression pattern and prognostic role of CCNA2 in GC patients.
CCNB1 and CCNB2, which are key regulators of progression through the G2/M transition during the cell cycle, played significant roles in the progression and development of human cancers.Citation13–Citation15,Citation19–Citation24,Citation35 In this report, CCNB1 and CCNB2 were upregulated in GC, which was consistent with previous studies.Citation35,Citation52–Citation54 The CCNB1 mRNA overexpression was also confirmed in different GC Lauren subtypes, which indicated that CCNB1 might be helpful to guide GC classification. Relevant research showed that overexpression of CCNB1 was associated with clinical stage,Citation54 lymph lode metastasis,Citation35,Citation53,Citation54 and poor survival in GC patients.Citation35 However, there are no data evaluating the prognostic role of CCNB2 in GC patients. In our study, high expression of CCNB1 and CCNB2 mRNAs predicted poor OS in GC patients through KM Plotter database analysis. ISL1, which could promote cell proliferation, is a novel regulator of the CCNB1 and CCNB2 genes.Citation55 Downregulation of CCNB1 expression contributes to oridonin-induced cell cycle arrest at G2/M phase and growth inhibition in SGC-7901 GC cells.Citation56 Inhibition of GC cell growth by CCNB1 and CCNB2 may explain poor prognosis in GC patients.
The expression pattern of CCND2 presented conflicting results in GC. Some studies demonstrated that absence of CCND2 expression is associated with promoter hypermethylation in GC,Citation57 as well as in breast, lung, prostate, and liver cancers.Citation58–Citation62 But some studies showed that CCND2 mRNA is overexpressed in GC.Citation36,Citation63–Citation65 Therefore, more studies are needed to evaluate the expression of CCND2 in GC to resolve the conflicting picture. In this report, there was no difference of CCND2 expression between GC and normal tissues. Relevant research showed overexpression of CCND2 was related with better differentiation, lymph node metastasis, and poor survival, which were confirmed in GC by immunohistochemistry analysis.Citation64,Citation65 Another research showed that high expression of CCND2 mRNA was related with depth of invasion, lymph node metastasis, distant metastasis, and TNM stage and predicted poor prognosis in GC patients.Citation36 But the samples involved in the above studies that evaluated the prognostic role of CCND2 were small. Therefore, more studies are needed to evaluate the expression and prognostic role of CCND2 in GC.
CCNE1 is considered to be involved in the oncogenic process.Citation9,Citation66 CCNE1 overexpressions are significantly correlated with unfavorable prognosis in lung and breast cancers.Citation25–Citation27,Citation31–Citation34 Relevant research demonstrated that CCNE1 in both mRNA and protein levels was associated with intestinal Lauren classification.Citation37 In our study, CCNE1 was upregulated and predicted poor prognosis for GC patients. Recent evidence showed that CCNE1 was the target gene of microRNA-25, and cell apoptosis was observed when microRNA-25 and CCNE1 expressions downregulated,Citation67 which might explain poor prognosis in GC patients with high CCNE1 mRNA expression.
HER2 is a 185-kDa protein, encoded by a gene located on chromosome 17q21. HER2 overexpression was detected in 6%–23% of GC patients.Citation68,Citation69 HER2-targeted therapies, using trastuzumab in addition to chemotherapy, showed significant benefit for patients with advanced GC.Citation70 In this study, high expression of CCNB1 predicted poor OS in HER2-negative GC patients, which indicated that CCNB1 might be useful for guiding individualized treatments for GC patients. 5-FU adjuvant therapy of GC significantly improves OS.Citation71 In this study, we found that high expression of CCNB1 predicted poor OS in GC patients who receive 5-FU-based chemotherapy treatment. Therefore, CCNB1 might be a potential therapy target for GC patients who receive 5-FU-based chemotherapy treatment.
In order to gain further insight into the biological mechanism of cyclins, enrichment analysis was performed. Cell cycle and p53 signaling pathway were identified as the most significant pathways that were related with cyclins. Dysregulated cell cycle progression is an important biological event during cancer pathogenesis. Present evidences have indicated that cyclins, CDKs, E2Fs, and p53 play an important role in cell cycle regulation.Citation8 The above cell cycle regulators were the target molecules of most oncogenes. Recent studies showed that several molecules could inhibit cell proliferation and induce cell cycle arrest at G2/M phase in GC cell lines, with downregulation of CDK1 and CCNB1 expressions but upregulation of p21 Waf1/Cip1, p27 Kip1, and p53 expressions.Citation72–Citation74 Another study showed that SPPH-induced S-phase arrest was mediated by upregulating the expression of E2F1 and downregulating those of CCNE1, CDK2, and CCNA2.Citation75 FOXM1 was identified as the most significant TF through Enrich R platform. FOXM1 has been shown to regulate transcription of cell cycle genes, including Cdc25B, CDK1, cyclin B, and p27 Kip.Citation76,Citation77 Knockdown of FOXM1 resulted in enhanced cell growth inhibition in AGS cells.Citation77 The above results provided a novel insight of cyclins into GC pathogenesis, which might be helpful for better understanding the biological mechanism of cyclins.
While the present study provides important insights into expression and prognostic roles of cyclins in GC patients at the mRNA level, it would be sensible to highlight the strengths and limitations of our study. Firstly, the publicly available databases were employed to analyze the expressions and prognostic roles of cyclins in GC patients. We pooled four datasets to evaluate the expressions of cyclins through Oncomine database. To evaluate the prognosis roles of cyclins, five GEO datasets were pooled to calculate the pooled HR. All the results of expression pattern and prognostic roles of cyclins were not based on a single dataset. Secondly, we analyzed the expressions of cyclins in TCGA dataset for validation. Also, the prognosis roles of cyclins were reviewed by comprehensive literature search. However, we did not perform polymerase chain reaction (PCR) or immunohistochemical to validate our findings. Though we performed an enrichment analysis, there are no experimental data about the mechanism of how cyclins affect the prognosis of GC patients, and additional efforts are needed to explore the biological roles of cyclins during GC pathogenesis.
Conclusion
In summary, CCNA2, CCNB1, CCNB2, and CCNE1 mRNA expressions are significantly higher in GC than in normal tissues. Furthermore, CCNB1 and CCNE1 could be helpful to guide GC Lauren classification. High expression of CCNB1 mRNA predicted poor OS in GC patients who received 5-FU-based chemotherapy treatment. Besides, high expression of CCNB1 predicted poor OS in HER2-negative GC patients. Enrichment analysis showed that cyclins are related with several biological pathways and interacts with some certain TFs. The above information might be useful for better understanding the clinical and biological roles of cyclins mRNA and guiding individualized treatments for GC patients.
Supplementary materials
Figure S1 The ROC curves of CCNA2, CCNB1, CCNB2, and CCNE1 were constructed for GC diagnosis.
Abbreviations: AUC, area under curve; GC, gastric cancer; ROC, receiver operating characteristic.
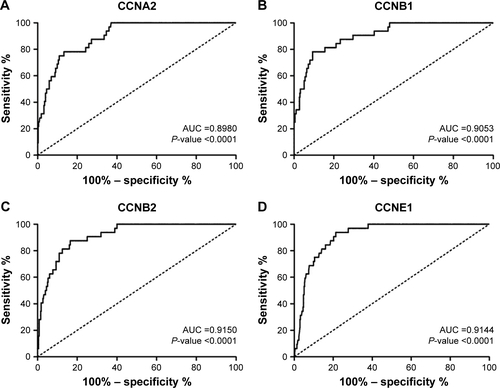
Figure S2 The ROC curves of CCNB1 and CCNE1 for GC Lauren subtype classification.
Abbreviations: AUC, area under curve; GC, gastric cancer; ROC, receiver operating characteristic.
Disclosure
The authors report no conflicts of interest in this work.
References
- MurrayCJVosTLozanoRDisability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010Lancet201238098592197222323245608
- JemalABrayFCenterMMFerlayJWardEFormanDGlobal cancer statisticsCA Cancer J Clin2011612699021296855
- FerlayJShinHRBrayFEstimates of worldwide burden of cancer in 2008: GLOBOCAN 2008Int J Cancer2010127122893291721351269
- FitzmauriceCAllenCBarberRMGlobal, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015JAMA Oncol20173452454827918777
- Japanese Gastric Cancer AssociationJapanese gastric cancer treatment guidelines 2014 (ver. 4)Gastric Cancer2017201119
- D’AngelicaMGonenMBrennanMFTurnbullADBainsMKarpehMSPatterns of initial recurrence in completely resected gastric adenocarcinomaAnn Surg2004240580881615492562
- YooCHNohSHShinDWChoiSHMinJSRecurrence following curative resection for gastric carcinomaBr J Surg200087223624210671934
- WimanKGZhivotovskyBUnderstanding cell cycle and cell death regulation provides novel weapons against human diseasesJ Intern Med2017281548349528374555
- StamatakosMPallaVKaraiskosICell cyclins: triggering elements of cancer or not?World J Surg Oncol2010811121176227
- von Bergwelt-BaildonMSKondoEKlein-GonzálezNWendtnerCMThe cyclins: a family of widely expressed tumor antigens?Expert Rev Vaccines201110338939521434806
- MuşatMMorrisDGKorbonitsMGrossmanABCyclins and their related proteins in pituitary tumourigenesisMol Cell Endocrinol20103261–2252920347931
- KoEKimYChoEYSynergistic effect of Bcl-2 and cyclin A2 on adverse recurrence-free survival in stage I non-small cell lung cancerAnn Surg Oncol20132031005101223115005
- CooperWAKohonen-CorishMRMccaughanBKennedyCSutherlandRLLeeCSExpression and prognostic significance of cyclin B1 and cyclin A in non-small cell lung cancerHistopathology2009551283619614764
- TakashimaSSaitoHTakahashiNStrong expression of cyclin B2 mRNA correlates with a poor prognosis in patients with non-small cell lung cancerTumour Biol20143554257426524375198
- QianXSongXHeYCCNB2 overexpression is a poor prognostic biomarker in Chinese NSCLC patientsBiomed Pharmacother20157422222726349989
- LeeEJinDLeeBBNegative effect of cyclin D1 overexpression on recurrence-free survival in stage II-IIIA lung adenocarcinoma and its expression modulation by vorinostat in vitroBMC Cancer20151598226681199
- XuPZhaoMLiuZElevated nuclear CCND1 expression confers an unfavorable prognosis for early stage lung adenocarcinoma patientsInt J Clin Exp Pathol2015812158871589426884860
- GaoTHanYYuLAoSLiZJiJCCNA2 is a prognostic biomarker for ER+ breast cancer and tamoxifen resistancePLoS One201493e9177124622579
- AgarwalRGonzalez-AnguloAMMyhreSIntegrative analysis of cyclin protein levels identifies cyclin b1 as a classifier and predictor of outcomes in breast cancerClin Cancer Res200915113654366219470724
- KoliadiANilssonCHolmqvistMCyclin B is an immunohistochemical proliferation marker which can predict for breast cancer death in low-risk node negative breast cancerActa Oncol201049681682020307242
- AaltonenKAminiRMHeikkiläPHigh cyclin B1 expression is associated with poor survival in breast cancerBr J Cancer200910071055106019293801
- DingKLiWZouZZouXWangCCCNB1 is a prognostic biomarker for ER+ breast cancerMed Hypotheses201483335936425044212
- Niméus-MalmströmEKoliadiAAhlinCCyclin B1 is a prognostic proliferation marker with a high reproducibility in a population-based lymph node negative breast cancer cohortInt J Cancer2010127496196719957331
- ShubbarEKovácsAHajizadehSElevated cyclin B2 expression in invasive breast carcinoma is associated with unfavorable clinical outcomeBMC Cancer201313123282137
- AlexanderAKarakasCChenXCyclin E overexpression as a biomarker for combination treatment strategies in inflammatory breast cancerOncotarget201789148971491128107181
- LundgrenCAhlinCHolmbergLAminiRMFjällskogMLBlomqvistCCyclin E1 is a strong prognostic marker for death from lymph node negative breast cancer. A population-based case-control studyActa Oncol201554453854425327158
- LuhtalaSStaffSTannerMIsolaJCyclin E amplification, over-expression, and relapse-free survival in HER-2-positive primary breast cancerTumour Biol20163779813982326810187
- CaldonCESergioCMKangJCyclin E2 overexpression is associated with endocrine resistance but not insensitivity to CDK2 inhibition in human breast cancer cellsMol Cancer Ther20121171488149922564725
- HashimotoTYanaiharaNOkamotoACyclin D1 predicts the prognosis of advanced serous ovarian cancerExp Ther Med20112221321922977490
- WangHWangHMakkiMSOverexpression of β-catenin and cyclin D1 predicts a poor prognosis in ovarian serous carcinomasInt J Clin Exp Pathol20147126427124427347
- AyhanAKuhnEWuRCCCNE1 copy-number gain and over-expression identify ovarian clear cell carcinoma with a poor prognosisMod Pathol201730229730327767100
- PilsDBachmayr-HeydaAAuerKCyclin E1 (CCNE1) as independent positive prognostic factor in advanced stage serous ovarian cancer patients – a study of the OVCAD consortiumEur J Cancer20145019911024176298
- NakayamaNNakayamaKShamimaYGene amplification CCNE1 is related to poor survival and potential therapeutic target in ovarian cancerCancer2010116112621263420336784
- KanskaJZakhourMTaylor-HardingBKarlanBYWiedemeyerWRCyclin E as a potential therapeutic target in high grade serous ovarian cancerGynecol Oncol2016143115215827461360
- BegnamiMDFregnaniJHNonogakiSSoaresFAEvaluation of cell cycle protein expression in gastric cancer: cyclin B1 expression and its prognostic implicationHum Pathol20104181120112720334896
- ShiHHanJYueSZhangTZhuWZhangDPrognostic significance of combined microRNA-206 and Cyclin D2 in gastric cancer patients after curative surgery: a retrospective cohort studyBiomed Pharmacother20157121021525960238
- KouraklisGKatsoulisIETheocharisSDoes the expression of cyclin E, pRb, and p21 correlate with prognosis in gastric adenocarcinoma?Dig Dis Sci20095451015102019058005
- RhodesDRKalyana-SundaramSMahavisnoVOncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profilesNeoplasia20079216618017356713
- SzászAMLánczkyANagyÁCross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patientsOncotarget2016731493224933327384994
- KuleshovMVJonesMRRouillardADEnrichr: a comprehensive gene set enrichment analysis web server 2016 updateNucleic Acids Res201644W1W90W9727141961
- CuiJChenYChouWCAn integrated transcriptomic and computational analysis for biomarker identification in gastric cancerNucleic Acids Res20113941197120720965966
- WangQWenYGLiDPUpregulated INHBA expression is associated with poor survival in gastric cancerMed Oncol2012291778321132402
- D’ErricoMde RinaldisEBlasiMFGenome-wide expression profile of sporadic gastric cancers with microsatellite instabilityEur J Cancer2009453461469
- ChoJYLimJYCheongJHGene expression signature-based prognostic risk score in gastric cancerClin Cancer Res20111771850185721447720
- RudyJValafarFEmpirical comparison of cross-platform normalization methods for gene expression dataBMC Bioinformatics20111246722151536
- RungJBrazmaAReuse of public genome-wide gene expression dataNat Rev Genet2013142899923269463
- SlonimDKYanaiIGetting started in gene expression microarray analysisPLoS Comput Biol2009510e100054319876380
- PepeMSFengZImproving biomarker identification with better designs and reportingClin Chem20115781093109521666069
- OhashiRGaoCMiyazakiMEnhanced expression of cyclin E and cyclin A in human hepatocellular carcinomasAnticancer Res2001211B65766211299822
- RenshawAALoughlinKRDuttaACyclin A and MIB1 (Ki67) as markers of proliferative activity in primary renal neoplasmsMod Pathol199811109639669796723
- HandaKYamakawaMTakedaHKimuraSTakahashiTExpression of cell cycle markers in colorectal carcinoma: superiority of cyclin A as an indicator of poor prognosisInt J Cancer199984322523310371338
- WangDGChenGWenXYWangDChengZHSunSQIdentification of biomarkers for diagnosis of gastric cancer by bioinformaticsAsian Pac J Cancer Prev20151641361136525743799
- KimDHPrognostic implications of cyclin B1, p34cdc2, p27(Kip1) and p53 expression in gastric cancerYonsei Med J200748469470017722244
- YasudaMTakesueFInutsukaSHondaMNozoeTKorenagaDOverexpression of cyclin B1 in gastric cancer and its clinicopathological significance: an immunohistological studyJ Cancer Res Clin Oncol2002128841241612200597
- ShiQWangWJiaZChenPMaKZhouCISL1, a novel regulator of CCNB1, CCNB2 and c-MYC genes, promotes gastric cancer cell proliferation and tumor growthOncotarget2016724364893650027183908
- GaoSYLiJQuXYZhuNJiYBDownregulation of Cdk1 and cyclinB1 expression contributes to oridonin-induced cell cycle arrest at G2/M phase and growth inhibition in SGC-7901 gastric cancer cellsAsian Pac J Cancer Prev201415156437644125124639
- YuJLeungWKEbertMPAbsence of cyclin D2 expression is associated with promoter hypermethylation in gastric cancerBr J Cancer200388101560156512771922
- TruongPKLaoTDDoanTPLeTALoss of expression of cyclin d2 by aberrant DNA methylation: a potential biomarker in vietnamese breast cancer patientsAsian Pac J Cancer Prev20151662209221325824739
- EvronEUmbrichtCBKorzDLoss of cyclin D2 expression in the majority of breast cancers is associated with promoter hypermethylationCancer Res20016162782278711289162
- SalskovAHawesSESternJEHypermethylation of CCND2 may reflect a smoking-induced precancerous change in the lungJ Oncol2011201195014095014921577262
- HenriqueRCostaVLCerveiraNHypermethylation of cyclin D2 is associated with loss of mRNA expression and tumor development in prostate cancerJ Mol Med2006841191191817016690
- TsutsuiMIizukaNMoribeTMethylated cyclin D2 gene circulating in the blood as a prognosis predictor of hepatocellular carcinomaClin Chim Acta20104117–851652020064498
- YuJMiehlkeSEbertMPExpression of cyclin genes in human gastric cancer and in first degree relativesChin Med J2002115571071512133540
- TakanoYKatoYvan DiestPJMasudaMMitomiHOkayasuICyclin D2 overexpression and lack of p27 correlate positively and cyclin E inversely with a poor prognosis in gastric cancer casesAm J Pathol2000156258559410666388
- TakanoYKatoYMasudaMOhshimaYOkayasuICyclin D2, but not cyclin D1, overexpression closely correlates with gastric cancer progression and prognosisJ Pathol1999189219420010547574
- KeyomarsiKHerliczekTWThe role of cyclin E in cell proliferation, development and cancerProg Cell Cycle Res199731711919552414
- ZhangYPengZZhaoYChenLmicroRNA-25 inhibits cell apoptosis of human gastric adenocarcinoma cell line AGS via regulating CCNE1 and MYCMed Sci Monit2016221415142027120728
- GravalosCJimenoAHER2 in gastric cancer: a new prognostic factor and a novel therapeutic targetAnn Oncol20081991523152918441328
- SchoppmannSFJeschBZacherlJHER-2 status in primary oesophageal cancer, lymph nodes and distant metastasesBr J Surg201198101408141321618213
- BangYJvan CutsemEFeyereislovaATrastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trialLancet2010376974268769720728210
- JácomeAASankarankuttyAKdos SantosJSAdjuvant therapy for gastric cancer: what have we learned since INT0116?World J Gastroenterol201521133850385925852269
- LeeMHChoYKimDHMenadione induces G2/M arrest in gastric cancer cells by down-regulation of CDC25C and proteasome mediated degradation of CDK1 and cyclin B1Am J Transl Res201581252465255
- YuanCXZhouZWYangYXInhibition of mitotic Aurora kinase A by alisertib induces apoptosis and autophagy of human gastric cancer AGS and NCI-N78 cellsDrug Des Devel Ther20159487508
- YuanCXZhouZWYangYXDanusertib, a potent pan-Aurora kinase and ABL kinase inhibitor, induces cell cycle arrest and programmed cell death and inhibits epithelial to mesenchymal transition involving the PI3K/Akt/mTOR-mediated signaling pathway in human gastric cancer AGS and NCI-N78 cellsDrug Des Devel Ther2015912931318
- LiXXieHChenYLangMChenYShiLSilkworm pupa protein hydrolysate induces mitochondria-dependent apoptosis and S phase cell cycle arrest in human gastric cancer SGC-7901 cellsInt J Mol Sci20181941013
- ZengJWangLLiQFoxM1 is up-regulated in gastric cancer and its inhibition leads to cellular senescence, partially dependent on p27 kip1J Pathol2009218441942719235838
- XiangHLLiuFQuanMFCaoJGLvY7-Difluoromethoxyl-5,4′-di-n-octylgenistein inhibits growth of gastric cancer cells through downregulating forkhead box M1World J Gastroenterol201218334618462622969238