Abstract
Purpose
The purpose of this study was to estimate the relation between acute esophagitis (AE) and clinical, dosimetric, and position factors in patients with locally advanced non-small-cell lung cancer (NSCLC) receiving intensity-modulated (chemo)radiotherapy.
Materials and methods
A retrospective cohort analysis was performed to identify factors associated with Common Toxicity Criteria for Adverse Events grade 2 or worse AE (AE2+). A multivariable model was established including patient- and treatment-related variables and esophageal dose–volume histogram parameters. The esophagus was divided according to physiological anatomy, and logistic regression was used to analyze the position parameter for its correlation with AE2+.
Results
The incidence of AE2+ was 27.5%. All models included gender, concurrent chemo-radiotherapy (CCRT), position parameter, and one of the dosimetric variables. The model with mean dose showed the best goodness of fit. Gender (OR=2.47, P=0.014), CCRT (OR=3.67, P=0.015), mean dose (OR=1.33, P<0.001), and maximum radiation position (OR=1.65, P=0.016) were significantly related to AE2+.
Conclusion
Gender, concurrent chemotherapy, maximum radiation position, and mean dose were independent risk factors for AE2+. The upper part of the esophagus showed a higher sensitivity to radiation toxicity.
Introduction
Concurrent chemoradiotherapy (CCRT) is the preferred treatment for patients with advanced-stage non-small-cell lung cancer (NSCLC). Although CCRT brings survival benefit compared with sequential chemoradiation or radiotherapy (RT) alone, the risk of post-therapy toxicity as acute esophagitis (AE) may increase.Citation1 AE often occurs 2 weeks after the start of RT, including retrosternal pain, dysphagia, and odynophagia. Severe AE may require analgesics and parenteral nutrition and can even lead to treatment interruptions, which may reduce the quality of life of the patients and lower the chance of local tumor control.Citation2,Citation3
Previous studies have attempted to define clinical and dosimetric predictors of radiation-induced esophagitis. Factors found to correlate with AE include CCRT,Citation4,Citation5 lymphatic status,Citation6,Citation7 hyperfractionated RT (1.6 Gy/fraction twice daily),Citation8 esophageal length,Citation9 molecular markers,Citation10 and a number of dosimetric parameters.Citation11–Citation16 However, the clinical applicability of these studies’ findings remains restricted because of their various study populations, different RT techniques, and different end points. In addition, there are differences between the classification systems of the Radiation Therapy Oncology Group (RTOG) acute radiation morbidity scoring criteriaCitation17 and the Common Toxicity Criteria for Adverse Events (CTCAE) version. Most studies focus on three-dimensional conformal radiotherapy (3D-CRT), and the impact of intensity-modulated radiotherapy (IMRT) on AE is less reported. Research has shown that 3D-CRT toxicity prediction models are not suitable for predicting toxicity after IMRT.Citation18,Citation19
New research has shown that different parts of the esophagus have different sensitivities to AE.Citation20 However, the dosimetric predictors for AE in current studies are for the whole esophagus. In fact, most radiation areas for NSCLC cover only part of the esophagus. IMRT has advantages in reducing the exposure of organs at risk (OARs).Citation21–Citation23 However, it is difficult to constraint the dose of the whole esophagus and OARs together in some cases because of the anatomical positional relationship between OARs and tumors. Therefore, position factors should be regarded with care in the prediction of AE.
The objective of this study was to estimate the relation between AE and clinical, dosimetric, and position factors in patients with local regional advanced NSCLC receiving intensity-modulated (chemo)radiotherapy.
Materials and methods
Patients
In this retrospective study, we assessed a cohort of 193 consecutive patients who had undergone intensity-modulated (chemo)radiotherapy for stages IIa–IIIc NSCLC from March 2015 to December 2017 at a single center. This investigation was approved by the local institutional review board. The inclusion criteria histologically or cytologically confirmed NSCLC, with full dose–volume histogram (DVH) and clinical data available for evaluation. The exclusion criteria were postoperative radiation therapy for lung cancer, previous irradiation of chest, history of esophageal surgery, and esophageal cancer.
Radiation simulation, planning, and delivery
Patients were immobilized in the supine position and underwent a contrast-enhanced computed tomography (CT) scan with 5 mm slices from cricoid cartilage to mid-abdomen. The image data sets were transferred to the RayStation planning system (Raysearch Radiation Oncology Systems). Gross tumor volume (GTV) included the primary tumor and suspicious lymph nodes (confirmed by histopathology after endobronchial/endoesophageal ultrasonography, with malignant features on CT scan, and/or fluorodeoxyglucose– positron emission tomography positive). Clinical target volumes (CTVs) enclosed the GTV of the primary tumor and lymph nodes with 8-mm and 5-mm margins, respectively. The internal target volume (ITV) was delineated from four-dimensional CT images, which involved assessing the CTV on expiratory-phase images and then registering the outline to the images to create a union of target contours enclosing all possible positions of the target. The planning target volume (PTV) was created by an isotropic 5 mm expansion of the CTV. Patients were treated on a Synergy linac (Elekta Ltd, Crawley, UK). The prescribed dose to the PTV was 60 Gy in 30 (once-daily) fractions using IMRT with seven to nine coplanar fields or volumetric modulated arc therapy (VMAT). Weekly online image-guided RT for setup verifi-cation was required. The dose of the OARs was set to meet the following constraints: lung dose <15 Gy, V20 <30%; spinal cord, maximum dose <45 Gy; heart, V30 <40%; and esophagus, maximum dose <66 Gy.
Esophageal contours, grouping, and dosimetric data
For each patient, the esophagus was delineated using the external esophageal contour from the cricoid cartilage to the gastroesophageal junction on the window of the planning CT scan (window width: 250–350 Hu; window level: 30–50 Hu). The esophagus was further divided into four subsites according to the seventh edition of the American Joint Committee on Cancer/Union for International Cancer Control (UICC) TNM staging system for esophageal carcinoma as follows: 1) the cervical esophagus was contoured from the cricoid cartilage to the sternal notch plane; 2) the upper thoracic esophagus was contoured from the sternal notch plane to the lower edge of the azygos vein; 3) the middle thoracic esophagus was contoured from the lower edge of the azygos vein to the lower pulmonary veins; and 4) the lower thoracic esophagus was contoured from the lower pulmonary veins to the gastroesophageal junction (). Patients were classified into four subgroups according to the subsite of the maximum mean dose, defined as the cervical esophagus group, upper thoracic esophagus group, middle thoracic esophagus group, and lower thoracic esophagus group, respectively.
Figure 1 Sagittal section illustrating delineation of the cervical esophagus (pink), the upper thoracic esophagus (purple), the mid-thoracic esophagus (orange), and the lower thoracic esophagus (yellow).
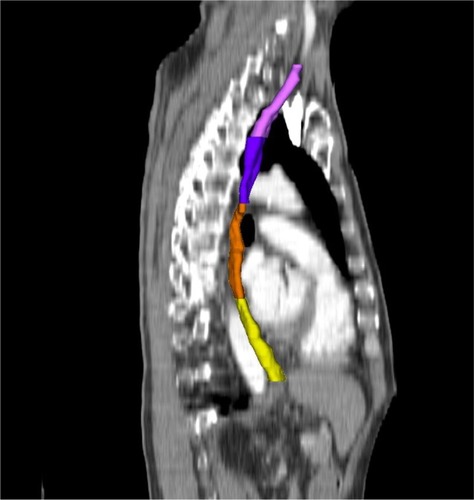
For consistency, the esophagus was redelineated in all patients by the same radiation therapist. DVHs were then generated. The esophageal dosimetric parameters of the entire esophagus and subsites were extracted from the dose data set in the treatment-planning system including volume, mean dose, and the percentage of the volume of esophagus receiving 10 Gy (V10) to 60 Gy (V60) ().
Toxicity scoring
The end point was defined as AE grade ≥2 (AE2+) during or at 3 months after RT treatment. AE was assessed weekly according to CTCAE V5.0. Toxicities were recorded as maximum grade at any point during treatment or in the follow-up period.
Statistical analyses
Patient characteristics as well as clinical and dosimetric variables were summarized by descriptive statistics. One-way ANOVA with least significant difference was performed for analysis among the four subgroups. Univariate analyses were used to identify the significant risk factors among age (dichotomized by 60), Karnofsky Performance Status score (dichotomized by 90), gender, smoking history, pathology, tumor stage (dichotomized by T3), lymph node stage (dichotomized by N3), chemotherapy, maximum irradiated position, and dosimetric parameters, including mean dose and volumetric metrics. Because of the large intervariable correlation within the dosimetric variables, only one of the dosimetric variables was included in the pairs for each multivariable analysis. The Akaike information criterion (AIC) and Bayesian information criterion (BIC) were used for multivariable model selection, which balanced the goodness of fit of the model. The preferred model was chosen as the one resulting in the lowest AIC and BIC values. P<0.05 was considered as statistically significant. Statistical programming was performed using SPSS 24.0 software.
Results
Patient characteristics and clinical and dosimetric variables are summarized in . Of 193 patients treated with (chemo)radiotherapy, 90% received a platinum-containing regimen. AE was scored as grade 1 in 92 (47.7%) patients, as grade 2 in 44 (22.8%) patients, and as grade 3 in nine (4.7%) patients. No grade 4 or 5 AE was observed. The incidence of AE2+ in the cervical esophagus group, upper thoracic esophagus group, middle thoracic esophagus group, and lower thoracic esophagus group was 47.4% (9/19), 34.0% (18/53), 24.2% (22/91), and 13.3% (4/30), respectively. Baseline dosimetric characteristics were comparable between the four subgroups ().
Table 1 Patient characteristics and clinical and dosimetric variables (N=193)
Table 2 Dosimetric variables in four subgroups
Univariate logistic regression analysis revealed a significant increase in females, those with N3 stage, and those receiving concurrent chemotherapy. There was no statistical difference between the IMRT and VMAT (P>0.05). All dosimetric parameters, including mean dose, all relative volumetric variables, and maximum radiation positions, were significant (P<0.05; ). N stage was not analyzed in the multivariate logistic regression model because it was highly associated with dosimetric parameters, which in turn correlated with esophagitis. Regarding the Spearman self-correlation matrix for the dosimetric variables, all variables were significantly correlated. An ANOVA analysis did not show any associations between gender, CCRT, position parameter, and dosimetric variables.
Table 3 Univariate logistic regression analysis of factors associated with the development of grade ≥2 AE
Each multivariate logistic regression model contained four independent variables: gender, CCRT, position parameter, and one of the dosimetric variables including mean dose and V10–V60. Gender, CCRT, maximum radiation position, and all the dosimetric variables demonstrated a significant correlation with AE2+ in all test models (). Goodness of fit was estimated by AIC and BIC values. The model with mean dose showed lower AIC and BIC values than models with other dosimetric variables (). We decided to include mean dose in the modeling procedure. The regression ORs for the optimal model indicated that patients had an increased risk of AE2+ with female (OR=2.47, P=0.014), with increasing mean dose (OR=1.33, P<0.001), when they received CCRT (OR=3.67, P=0.015), or with the maximum radiation position in the upper part of the esophagus (OR=1.65, P=0.016; ).
Table 4 AIC of the models
Table 5 Multivariate logistic regression analysis of factors associated with the development of grade ≥2 AE
Discussion
We constructed a multivariate model for AE2+ in patients with advanced-stage NSCLC treated with intensity-modulated (chemo)radiotherapy. Gender, CCRT, maximum radiation position, and mean dose were significantly associated with AE2+. Patients may be more sensitive to AE in the upper part of the esophagus in comparison with that in the lower part of the esophagus, which may contribute to a high risk of AE in patients with cervical lymph node metastases.
Research has shown that higher grade AE is the consequence of the accumulative effect of both chemotherapy and RT.Citation4,Citation5,Citation24 Meta-analysis of Gong et alCitation25 showed that patients receiving CCRT have an approximately fivefold increase in the risk of AE compared with those receiving sequential chemoradiotherapy (SCRT). The reason may be that cisplatin hampers the repair of DNA damage caused by the irradiation in esophageal epithelial cells. In some IMRT-based studies, CCRT was reported to result in a 27%–38% rate of grade 2 AE and an 8%–22% rate of grade 3 AE.Citation19,Citation24,Citation26,Citation27 In our study, the incidences of grade 2 and 3 AEs were 23.7% and 5.1%, respectively. The possible reason for the lower incidence is that the median RT dose was mostly 66 Gy in the other studies, whereas it was 60 Gy in ours.
Increased nodal stage has been identified as a predictor of AE. A relevant study pointed out that it is most likely a surrogate for larger tumor volumes and radiation fields.Citation28 Most studies have shown that N3 is closely related to the incidence of AE.Citation6,Citation7 However, Uterlinde et al suggested that nodal stage is not correlated with tumor volume but that involved mediastinal nodes have more clinical significance.Citation24 Therefore, N2–3 was selected for our analysis because of its increased correlation with mediastinal lymph nodes compared with N3. Gender was reported only occasionally in related studies.Citation29,Citation30 We found that female was statistically associated with esophagitis.
Various dosimetric parameters have shown a strong correlation with AE.Citation11–Citation16,Citation31,Citation32 However, these results were mostly based on 3D-CRT. The hypothesis that the toxicity prediction models of 3D-CRT are not suitable for predicting toxicity after IMRT was supported. In our study, the mean dose showed a better goodness of fit in the multivariate model. The relevant study by Wijsman et alCitation26 also demonstrated that the mean dose was a good predictor of AE.
To the best of our knowledge, this is the second study suggesting the position parameter as related to AE. Pan et alCitation20 proposed that the upper part of the esophagus is more sensitive to injuries because of the different distributions of the sensory axons.Citation33 We divided the esophagus into four sub-sites according to physiological and anatomical structures. We found that the risk of AE in the upper esophagus was higher compared with that in the lower esophagus. Another possible reason is that the physiological structures of different parts of the esophagus are different. The epidermis of the upper esophageal mucous layer consists of squamous epithelial cells, and the muscularis consists of striated muscle. The epidermis of the lower esophageal mucous layer consists of columnar epithelial cells, and the muscularis consists of smooth muscle. The middle esophagus is a mixture of two different tissues above.Citation34 This conclusion also suggests the importance of personalized sparing of esophageal dose. The esophagus is adjacent to the OARs, including lungs, spinal cord, and heart in the RT plan of NSCLC patients. In some cases, such as where the radiation field is large or the shape of the tumor is irregular, it is often difficult to simultaneously limit the dose of the whole esophagus and all other OARs. The esophagus may even be exposed to over radiation to meet the PTV-planning constraints. However, we found that the irradiation field often does not cover the entire esophagus in patients with advanced-stage NSCLC. A total of 80.3% of the cases had at least one part of the esophagus exposed to little or no radiation in this study (V10=0 Gy). If we can limit only the part of the esophagus covered in the radiation field, the concept of personalized treatment can be achieved. This finding also suggests that there may be differences in the optimal sparing dose for different parts of the esophagus. Moreover, patients with cervical lymph node metastases should be aware of the high risk of AE.
There are still many deficiencies in this study. First, single-center research may lead to regional bias. The model still requires internal and external validation. Second, the evaluation of AE was mainly reported by patients, which may result in deviation of the incidence of esophagitis. Furthermore, the CTCAE score is essentially variable. There is currently a lack of more objective and rigorous criteria to evaluate normal tissue complications. Third, using DVH parameters may result in loss of spatial dosimetric information and may also ignore the movements of esophagus. Currently available studies have attempted to evaluate the effectiveness of other 3D dosimetric parameters (such as the surface dose of esophagus).Citation35
Conclusion
The most relevant prognostic variables in the multivariable model in patients with advanced-stage NSCLC undergoing intensity-modulated (chemo)radiotherapy are gender, concurrent chemotherapy, maximum radiation position, and mean dose. The sensitivity of AE varies in different parts of the esophagus. Validation in a large independent patient cohort is still warranted.
Ethics approval and consent to participate
We declare that this study was approved by the institutional review board at Medical Science Research Ethics Committee of The First Hospital of China Medical University (reference number: [2018]-148). Patients were required to provide written informed consent to participate in this research.
Availability of data and materials
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
Abbreviations
| CCRT | = | concurrent chemoradiotherapy |
| AE | = | acute esophagitis |
| NSCLC | = | non-small-cell lung cancer |
| RTOG | = | Radiation Therapy Oncology Group |
| CTCAE | = | Common Toxicity Criteria for Adverse Events |
| 3D-CRT | = | three-dimensional conformal radiotherapy |
| IMRT | = | intensity-modulated radiotherapy |
| OARs | = | organs at risk |
| UICC | = | Union for International Cancer Control |
| GTV | = | gross tumor volume |
| CTV | = | clinical target volume |
| ITV | = | internal target volume |
| PTV | = | planning target volume |
| DVHs | = | dose–volume histograms |
| SCRT | = | sequential chemoradiotherapy. |
Supplementary materials
Figure S1 Dose-volume histogram for four subgroups as the cervical esophagus (A), the upper thoracic esophagus (B), the mid-thoracic esophagus (C), and the lower thoracic esophagus (D).
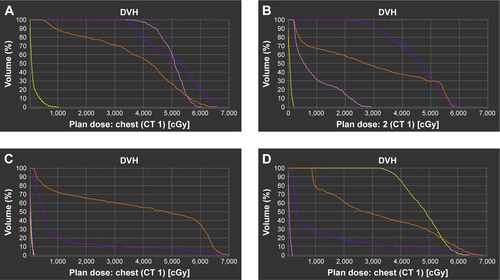
Table S1 Results of multivariate analyses
Disclosure
The authors report no conflicts of interest in this work.
References
- CurranWJPaulusRLangerCJSequential vs. concurrent chemo-radiation for stage III non-small-cell lung cancer: randomized phase III trial RTOG 9410J Natl Cancer Inst2011103191452146021903745
- PalmaDASenanSOberijeCPredicting esophagitis after chemoradiation therapy for non-small cell lung cancer: an individual patient data meta-analysisInt J Radiat Oncol Biol Phys201387469069624035329
- GovaertSLTroostEGSchuurbiersOCTreatment outcome and toxicity of intensity-modulated (chemo) radiotherapy in stage III non-small cell lung cancer patientsRadiat Oncol2012715022958781
- CaglarHBOthusMAllenAMEsophagus in-field: a new predictor for esophagitisRadiother Oncol2010971485320832884
- BradleyJDeasyJOBentzenSEl-NaqaIDosimetric correlates for acute esophagitis in patients treated with radio therapy for lung carcinomaInt J Radiat Oncol Biol Phys20045841106111315001251
- ZhangZCXuJLiBSClinical and dosimetric risk factors of acute esophagitis in patients treated with 3-dimensional conformal radiotherapy for non-small-cell lung cancerAm J Clin Oncol201033327127519823071
- AhnSJKahnDZhouSDosimetric and clinical predictors for radiation-induced esophageal injuryInt J Radiat Oncol Biol Phys200561233534715667951
- MaguirePDSibleyGSZhouSMClinical and dosimetric predictors of radiation-induced esophageal toxicityInt J Radiat Oncol Biol Phys19994519710310477012
- HirotaSTsujinoKEndoMDosimetric predictors of radiation esophagitis in patients treated for non-small-cell lung cancer with carboplatin/paclitaxel/radiotherapyInt J Radiat Oncol Biol Phys200151229129511567801
- De RuyckKSabbeNOberijeCDevelopment of a multicomponent prediction model for acute esophagitis in lung cancerpatients receiving chemoradiotherapyInt J Radiat Oncol Biol Phys201181253754421605946
- ChapetOKongFMLeeJSHaymanJATen HakenRKNormal tissue complication probability modeling for acute esophagitis in patients treated with conformal radiation therapy for non-small cell lung cancerRadiother Oncol200577217618116256230
- ZhuJZhangZCLiBSAnalysis of acute radiation-induced esophagitis in non-small-cell lung cancer patients using the Lyman NTCP modelRadiother Oncol201097344945421067834
- ZhangZXuJZhouTRisk factors of radiation-induced acute esophagitis in non-small cell lung cancer patients treated with concomitant chemoradiotherapyRadiat Oncol201495424528546
- KimTHChoKHPyoHRDose-volumetric parameters of acute esophageal toxicity in patients with lung cancer treated with three-dimensional conformal radiotherapyInt J Radiat Oncol Biol Phys2005624995100215990000
- EtizDBaymanEAkcayMSahinBBalCDosimetric and clinical predictors of acute esophagitis in lung cancer patients in Turkey treated with radiotherapyAsian Pac J Cancer Prev20131474223422823991980
- BelderbosJHeemsbergenWHoogemanMPengelKRossiMLebesqueJAcute esophageal toxicity in non-small cell lung cancer patients after high dose conformal radiotherapyRadiother Oncol200575215716415890421
- CoxJDStetzJPajakTFToxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC)Int J Radiat Oncol Biol Phys1995315134113467713792
- KwintMUyterlindeWNijkampJAcute esophagus toxicity in lung cancer patients after intensity modulated radiation therapy and concurrent chemotherapyInt J Radiat Oncol Biol Phys2012842e223e22822560551
- BeetzISchilstraCvan LuijkPExternal validation of three dimensional conformal radiotherapy based NTCP models for patient-rated xerostomia and sticky saliva among patients treated with intensity modulated radiotherapyRadiother Oncol201210519410022169766
- PanYBrinkCKnapMAcute esophagitis for patients with local-regional advanced non small cell lung cancer treated with concurrent chemoradiotherapyRadiother Oncol2016118346547026803187
- NielsenTBHansenOSchytteTBrinkCInhomogeneous dose escalation increases expected local control for NSCLC patients with lymph node involvement without increased mean lung doseActa Oncol201453111912523627917
- VeldemanLMadaniIHulstaertFDe MeerleerGMareelMDe NeveWEvidence behind use of intensity-modulated radiotherapy: a systematic review of comparative clinical studiesLancet Oncol20089436737518374290
- ChristianJABedfordJLWebbSBradaMComparison of inverse-planned three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for non-small-cell lung cancerInt J Radiat Oncol Biol Phys200767373574117187941
- UyterlindeWChenCKwintMPrognostic parameters for acute esophagus toxicity in intensity modulated radiotherapy and concurrent chemotherapy for locally advanced non-small cell lung cancerRadiother Oncol2013107339239723647749
- GongBJiangNYanGPredictors for severe acute esophagitis in lung cancer patients treated with chemoradiotherapy: a systematic reviewCurr Med Res Opin2016321018
- WijsmanRDankersFTroostEGMultivariable normal-tissue complication modeling of acute esophageal toxicity in advanced stage non-small cell lung cancer patients treated with intensity-modulated (chemo-)radiotherapyRadiother Oncol20151171495426341608
- Werner-WasikMYorkeEDeasyJNamJMarksLBRadiation dose-volume effects in the esophagusInt J Radiat Oncol Biol Phys2010763 SupplS86S9320171523
- Dehing-OberijeCde RuysscherDPetitSDevelopment, external validation and clinical usefulness of a practical prediction model for radiation-induced dysphagia in lung cancer patientsRadiother Oncol201097345546121084125
- De RuyckKSabbeNOberijeCDevelopment of a multicomponent prediction model for acute esophagitis in lung cancer patients receiving chemoradiotherapyInt J Radiat Oncol Biol Phys201181253754421605946
- TakedaKNemotoKSaitoHOgawaYTakaiYYamadaSDosimetric correlations of acute esophagitis in lung cancer patients treated with radiotherapyInt J Radiat Oncol Biol Phys200562362662915936536
- Dehing-OberijeCDe RuysscherDPetitSDevelopment, external validation and clinical usefulness of a practical prediction model for radiation-induced dysphagia in lung cancer patientsRadiother Oncol201097345546121084125
- RoseJRodriguesGYaremkoBLockMD’SouzaDD’SouzaDSystematic review of dose-volume parameters in the prediction of esophagitis in thoracic radiotherapyRadiother Oncol200991328228718950881
- CollmanPITremblayLDiamantNEThe central vagal efferent supply to the esophagus and lower esophageal sphincter of the catGastroenterology19931045143014388387041
- QueJThe initial establishment and epithelial morphogenesis of the esophagus: a new model of tracheal-esophageal separation and transition of simple columnar into stratified squamous epithelium in the developing esophagusWiley Interdiscip Rev Dev Biol20154441943025727889
- DankersFWijsmanRTroostEGMonshouwerRBussinkJHoffmannALEsophageal wall dose-surface maps do not improve the predictive performance of a multivariable NTCP model for acute esophageal toxicity in advanced stage NSCLC patients treated with intensity-modulated (chemo-)radiotherapyPhys Med Biol20176293668368128379845
