Abstract
Hodgkin lymphoma (HL) represents ~11% of all lymphoma cases. This disease occurs in young adults, but also affects people over 55 years of age. Despite the fact that >80% of all newly diagnosed patients under 60 will achieve a sustained complete response (CR), 5%–10% of HL patients are refractory to initial treatment and 10%–30% of patients will eventually relapse after an initial CR. The treatment recommendation for primary refractory or relapsed HL patients is salvage therapy followed by high-dose chemotherapy and autologous stem cell transplantation. Following this approach, a significant part will still relapse at any moment. Thus, further research and new drugs or combinations are required. Overexpression of COX-2 has been associated with poor prognosis in relapse/refractory HL patients, so it could be a potential therapeutic target in HL. For this purpose, several drugs may have a role: specific COX-2 inhibitors such as celecoxib or other anti-inflammatory drugs such as lenalidomide may further inhibit lipopolysaccharide-mediated induction of COX-2. Moreover, lenalidomide and COX-2 inhibitors (celecoxib) have been tested in solid tumors with encouraging results. We present a case of a young female diagnosed with a heavily pretreated HL nodular sclerosis subtype who, after failing six treatment lines, only achieved clinical and radiological CR after six cycles of lenalidomide/celecoxib that resulted in an event-free survival of 22 months. We explain the rationale of using this chemotherapy regimen and our patient follow-up.
Introduction
Hodgkin lymphoma (HL) represents ~11% of all lymphomas in the US.Citation1 This disease presents with a bimodal distribution (young adults and ≥55-year-old patients). Thanks to advances in HL treatment, >80% of all newly diagnosed patients under 60 years will be cured of their disease. Despite these optimistic results, 5%–10% of HL patients show refractoriness to initial treatment and 10%–30% of patients will eventually relapse after achieving an initial complete response (CR).Citation2
In primary refractory disease, conventional salvage therapy followed by high-dose chemotherapy and autologous stem cell transplantation is the standard of care in fit patients. Nevertheless, the 5-year freedom from failure and overall survival are 31% and 43%, respectively, for these patients, which means that the vast majority will relapse even when intensive chemotherapy is used.Citation2,Citation3 At this point, new drugs such as brentuximab vedotin, anti-PD1, PI3K or mTOR inhibitors have shown their role in monotherapy or as a bridge to allogeneic stem cell transplantation in this subgroup of patients.Citation4–Citation6 However, there are no clear treatment recommendations for patients who are not eligible for intensive regimens or allogeneic stem cell transplantation.
We present a 35-year-old woman with a diagnosis of primary refractory HL nodular sclerosis (NS) subtype, with a continuous pattern of relapses or partial responses and not considered a candidate for intensive chemotherapy or SCT. After several regimens (including radiotherapy), our patient achieved a CR to a combination regimen of lenalidomide and celecoxib. We discuss the rationale behind the use of these new drugs in relapsed HL.
Case presentation
A 35-year-old mentally disabled woman with a psychiatric history of obsessive behavior in childhood and anxiety disorder consulted the dermatology department for generalized pruritus. She was diagnosed with atopic dermatitis which resolved after corticoid-based therapy. In 2005, she was referred to our department because of laterocervical lymphadenopathies and night fever with intense sweating. Body computed tomography (CT) showed supra- and infra-diaphragmatic lymphadenopathies with various abdominal masses, the most relevant being 8×6×8 cm3. A biopsy of a lymphadenopathy revealed NS classic HL. With a final diagnosis of NS HL stage IIIB with an International Prognostic Score 2, she received six cycles of ABVD chemotherapy regimen with partial response and an early relapse in March 2006 (primary refractory to ABVD). This was followed by a salvage chemotherapy regimen (two cycles of ESHAP) with no response. In September 2006, a third chemotherapy regimen (four cycles of GemOx) was initiated with neutropenia and thrombocytopenia grade 4, which led to dose-intensity failures followed by chemotherapy discontinuation and radiotherapy consolidation. One month after the last radiotherapy session, the HL progressed; so, two cycles of IFE regimen were given with stable disease. Considering the basal comorbidities of the patient, we decided to pursue a watch-and-wait policy from 2008 to 2010, treating localized areas of progression with radiotherapy and obtaining transient partial responses (). In October 2010, a new mass appeared in D8–D9, presenting with a medullar compression syndrome. After a biopsy that confirmed HL relapse, it was treated with radiotherapy and six cycles of GemOx, which led to a partial response. However, a new progression occurred in the left cervical and axillary nodes within 1 month, which was observed with a watch-and-wait policy with the consent of the family.
Figure 1 CT scan showing an axillary node transiently controlled with radiotherapy in June 2009.
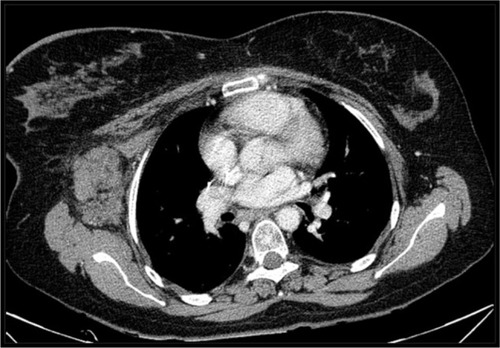
In May 2012, our patient presented a new clinically symptomatic disease progression confirmed by positron emission tomography (PET)/CT, requiring bendamustine 90 mg/m2 and finally reaching disease stability. In August 2014, the HL progressed again with significant B symptoms and worsening clinical status (). At this point, based on our experience of the potential role of COX-2 expression in HLCitation7,Citation8 and that the patient was not a candidate for intensive chemotherapy and brentuximab was still not available in our center, we decided to start an experimental treatment with celecoxib 200 mg every 12 hours and lenalidomide 20 mg (for 3 of every 4 weeks). This regimen was obtained through a compassionate use request. Our patient received this treatment from August 2014 to January 2015 (six cycles) with unremarkable toxicity and excellent tolerance, finally showing a CR for the first time in both the interim after three cycles and the final CT/PET after six cycles (). This response was maintained in a new CT scan in August 2015. After this induction, we started 1 year of celecoxib maintenance (200 mg/12 hours) that had to be stopped because of anemia due to gastrointestinal bleeding in March 2016.
Figure 2 PET/CT scan before lenalidomide/celecoxib (A) and after six cycles of lenalidomide/celecoxib (B).
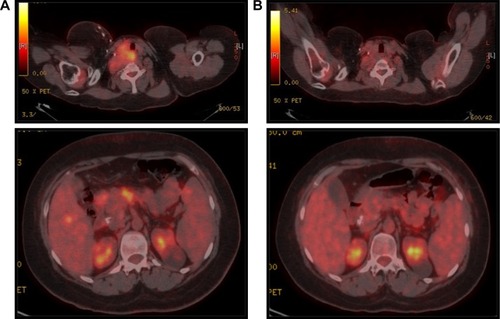
In July 2016, with the most durable response until that moment being 22 months, our patient presented a new disease relapse. CT/PET showed supra- and infra-abdominal adenopathies with associated hepatic and splenic lesions. As two different biopsies of hypermetabolic locations resulted negative and keeping in mind the asymptomatic situation of our patient, we decided to restart celecoxib 200 mg every 12 hours. In this context, the disease progressed to ascites requiring paracentesis. Biopsies were constantly negative until February 2017, when a final biopsy confirmed the persistence of HL. At that point, we decided to start brentuximab vedotin (two cycles). During the second cycle of brentuximab, the patient required intensive care admission due to a sepsis with a fatal outcome.
Discussion
Patients with HL refractory to standard chemotherapy regimens represent a challenge, and even more do those patients who cannot receive intensive chemotherapy regimens or autologous stem cell transplantation. Thus, the management of these patients requires both active and well-tolerated drugs. We present a case in which lenalidomide combined with celecoxib provided a sustained CR in a patient refractory to radiotherapy and six prior chemotherapy lines. After six cycles of lenalidomide 20 mg for 3 of every 4 weeks and celecoxib 200 mg/12 hours, followed by celecoxib maintenance, our patient achieved a CR confirmed by PET/TC that lasted almost 2 years.
Our rationale for using a COX-2 inhibitor (celecoxib) is based on a study published by Mestre et al which concluded that the expression of COX-2 on Reed–Sternberg cells is an independent unfavorable prognostic factor in HL patients.Citation7 This study suggested that COX-2 could be a new potential therapeutic target in these patients with poor prognosis.Citation9 Other authors such as Barisik et al demonstrated a significant relationship between COX-2 expression and mixed cellular and NS HL subtypes with worse prognosis.Citation10 COX-2 inhibitors have already been tested in solid tumors and have been found to significantly increase the overall response rate.Citation11
Celecoxib generates a direct COX-2 inhibition that stops the conversion of arachidonic acid to prostaglandin as well as downregulates antiapoptotic genes, induces proapoptotic molecules and potentiates the formation of the apoptosome and processing of caspase-9 (cox-2–independent mechanism). Recently, celecoxib has been proposed as a good partner of CD19 CAR T-cell therapy in non-Hodgkin lymphomas due to its regulatory effect on apoptosis.Citation12
On the other hand, lenalidomide not only acts through immunomodulatory, antiangiogenic and antiproliferative mechanisms, but also is an anti-inflammatory drug that inhibits lipopolysaccharide-mediated induction of COX-2.Citation13 Due to these properties of lenalidomide and considering how increased neoangiogenesis and impaired immunity critically contribute to HL, Böll et al tested lenalidomide as a single agent in a continuous dosing schedule in 12 HL patients who had relapsed after at least four chemotherapy regimens. They evidenced benefit from the use of lenalidomide in all patients. Despite none showing radiological evidence of progression after two cycles, two of the four responding patients had evidence of progression after 2 or 4 months. Overall response rate (ORR) observed was 50%, including one patient with complete remission.Citation14 Other series and clinical cases have been reported with variable ORR (19%–50%) and a median duration of response of 6 months even with a continuous monthly administration.Citation15,Citation16 Thus, we consider that just six cycles of lenalidomide may not fully justify the long-term remission reported here, giving special relevance to the celecoxib combination and maintenance. Moreover, the disease relapse occurred soon after celecoxib discontinuation.
Considering all these data and the evidence of good results obtained with the lenalidomide/celecoxib combination in several malignancies (prostate cancer,Citation17 sarcomasCitation18 and hematological malignancies such as multiple myelomaCitation19 or blastic plasmacytoid dendritic cell neoplasm),Citation20 we decided to initiate this regimen. After three and six cycles of lenalidomide/celecoxib, we obtained a clinical, metabolic and radiological CR, which was the only and longest obtained one in this patient, and the subsequent relapse occurred 4 months after discontinuing celecoxib. Considering all potential mechanisms of celecoxib-mediated apoptosisCitation12 in the context of a patient with HL with a continuous pattern of relapses, we hypothesize that celecoxib maintenance had a vital role in controlling the disease after the induction therapy.
Conclusion
HL is a disease with poor prognosis when relapse or progression occurs. Despite advances in salvage therapy, patients who are not candidates for intensive chemotherapy, or cannot reach/maintain CR, still have a very poor survival with no specific treatment.
We present preliminary evidence of activity of the combination of lenalidomide and celecoxib in a heavily pretreated HL patient who was not a candidate for intensive chemotherapy approaches. This combination was well tolerated and without significant toxicity. Our patient reached a 22-month CR. However, the precise role of this combination, as well as of celecoxib and lenalidomide in this case, should be confirmed in clinical trials.
Consent statement
Written informed consent has been obtained from the patient’s relative (father) to publish all the case details and images needed.
Acknowledgments
We thank Mr Jonathan McFarland for his contribution to English language revision of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- AnsellSMHodgkin lymphoma: diagnosis and treatmentMayo Clin Proc201590111574158326541251
- AnsellSMLymphomaHHodgkin lymphoma: 2016 update on diagnosis, risk-stratification, and managementAm J Hematol201691443444227001163
- AndréMHenry-AmarMPicoJLComparison of high-dose therapy and autologous stem-cell transplantation with conventional therapy for Hodgkin’s disease induction failure: a case–control study. Société Francaise de Greffe de MoelleJ Clin Oncol199917122222910458237
- ArmandPShippMARibragVProgrammed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failureJ Clin Oncol201634313733373927354476
- YounesAGopalAKSmithSEResults of a pivotal Phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphomaJ Clin Oncol201230182183218922454421
- JohnstonPBInwardsDJColganJPA Phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphomaAm J Hematol201085532032420229590
- MestreFGutierrezARamosRExpression of COX-2 on Reed–Sternberg cells is an independent unfavorable prognostic factor in Hodgkin lymphoma treated with ABVDBlood2012119256072607922547578
- MestreFGutiérrezARodriguezJRadiation therapy overcomes adverse prognostic role of cyclooxygenase-2 expression on Reed–Sternberg cells in early Hodgkin lymphomaInt J Radiat Oncol Biol Phys2015921849025475251
- KohYWParkCYoonDHSuhCHuhJPrognostic significance of COX-2 expression and correlation with Bcl-2 and VEGF expressionAm J Surg Pathol20133781242125123851330
- BarisikNOBozkurtSGumusMExpression and prognostic significance of Cox-2 and p-53 in Hodgkin lymphomas: a retrospective studyDiagn Pathol201051920346139
- ChenJShenPZhangXCZhaoMDZhangXGYangLEfficacy and safety profile of celecoxib for treating advanced cancers: a meta-analysis of 11 randomized clinical trialsClin Ther20143681253126325016505
- DinhTNOneaASJazirehiARCombination of celecoxib (Celebrex®) and CD19 CAR-redirected CTL immunotherapy for the treatment of B-cell non-Hodgkin’s lymphomasAm J Clin Exp Immunol201763274228804691
- FujitaJMestreJRZeldisJBSubbaramaiahKDannenbergAJThalidomide and its analogues inhibit lipopolysaccharide-mediated induction of cyclooxygenase-2Clin Cancer Res20017113349335511705847
- BöllBBorchmannPToppMSLenalidomide in patients with refractory or multiple relapsed Hodgkin lymphomaBr J Haematol2010148348048219863533
- FehnigerTALarsonSTrinkausKA Phase 2 multicenter study of continuous dose lenalidomide in relapsed or refractory classical Hodgkin lymphomaBlood2012120211623
- MandacIKolonicSOLenalidomide induced good clinical response in a patient with multiple relapsed and refractory Hodgkin’s lymphomaJ Hematol Oncol201032020509896
- MarschnerNZaissMLong-term disease stabilization in a patient with castration-resistant metastatic prostate cancer by the addition of lenalidomide to low-dose dexamethasone and celecoxibOnkologie201235527928222868509
- TsaiYCWuCTHongRLResponse of refractory osteosarcoma to thalidomide and celecoxibLancet Oncol200561299799916321769
- PrinceHMMileshkinLRobertsAA multicenter Phase II trial of thalidomide and celecoxib for patients with relapsed and refractory multiple myelomaClin Cancer Res200511155504551416061867
- García-RecioMMartinez-SerraJBentoLLenalidomide, celecoxib, and azacitidine therapy for blastic plasmocytoid dendritic cell neoplasm: a case reportOnco Targets Ther201695507551127660468
