Abstract
Background
TRIM37 is an ubiquitin E3 ligase. Growing evidence has demonstrated the high value of TRIM37 as a potential biomarker for diagnosis of certain cancers. However, the biological function of TRIM37 in lung cancer is still unknown.
Materials and methods
In order to gain a deep insight into the function of TRIM37 in lung cancer cells, in the present study lentiviral vector was used to mediate RNA interference and overexpression of TRIM37 in lung cancer cells (H292, H358, and H1299). In addition, a specific AKT inhibitor LY294002 was utilized to examine the correlation between the expression of TRIM37 and AKT.
Results
TRIM37 acts as a positive regulator of cell proliferation in lung cancer cells. Moreover, cell apoptosis analyses showed the antiapoptosis function of TRIM37, which was mainly dependent on the regulation of BCL2 and BAX. Our results also indicated that AKT might be a target of TRIM37 in lung cancer cells.
Conclusion
This research not only helps in understanding the molecular mechanisms of TRIM37 in detail but also provides evidence to develop novel biomarkers for lung cancer diagnosis.
Introduction
Lung cancer is one of the most commonly diagnosed cancers worldwide and has been ranked as the top killer cancer all over the world.Citation1,Citation2 Though the traditional treatment (resection and radiotherapy) can contribute to fighting against the recurrence of lung cancer, the outcome usually remains poor. Therefore, gaining a deep insight into the molecular mechanism of lung cancer would help to develop new biomarkers and therapy for this disease.
TRIM37 is one of the members of TRIM family, which is mapped on chromosome 17q22-23.Citation3 TRIM37 serves as an ubiquitin E3 ligase and plays an essential role in oncogenesis. A previous report has shown that TRIM37 is upregulated in human colorectal cancer (CRC) and contributes to the development of CRC.Citation4 Moreover, the expression of TRIM37 is significantly upregulated in pancreatic cancerous tissues, which indicates the oncogenic role of TRIM37 in pancreatic cancer.Citation5 In addition, high expression of TRIM37 is observed in osteosarcoma.Citation6 Growing evidence has indicated that TRIM37 participates in the tumorigenesis of several cancers. However, the biological function of TRIM37 in lung cancer is less well understood.
BCL2 mediates the intrinsic apoptosis pathway. A previous report has shown that BCL2 is also essential for cell cycle progression.Citation7 The dysregulation of BCL2 not only damages normal development but also contributes to tumor progression.Citation8 The high level of BCL2 suppresses apoptosis in human lung cancer cells, which serves as the antiapoptotic factor in this cancer.Citation9 In addition, BAX belongs to the BCL2 family, which functions as proapoptotic marker.Citation10 Therefore, the occurrence of cell apoptosis depends on the ratio of BAX/BCL2. Dysregulation of the ratio of BAX/BCL2 may lead to carcinogenesis. Taken together, BCL2 family members have potential value as critical targets in lung cancer treatment.Citation11,Citation12
The AKT pathway plays an essential role in various biological functions, which is commonly abnormal in human cancer.Citation13 The activity of AKT protein relies on its phosphorylation. A previous report has indicated that the AKT phosphorylation (p-AKT) on Thr308 correlates with the activity of AKT protein in human non-small-cell lung cancer.Citation14 The PI3K/AKT pathway is involved in cell cycle and apoptosis of lung cancer cells.Citation15 Some evidence has demonstrated that the major function of TRIM37 in glioma cells is the inactivation of PI3K/AKT signaling pathway.Citation16 Therefore, further studies are needed to examine the relationship between TRIM37 and AKT in lung cancer cells.
In the present study, we systematically analyzed the function of TRIM37 and its possible targets in lung cancer cells. RNA interference was used to knockdown the expression of TRIM37 in lung cancer cells, while lentiviral vector was used to mediate overexpression of TRIM37. Our data provide not only a deep insight into the molecular mechanisms of TRIM37 but also evidence to develop novel biomarkers for lung cancer.
Materials and methods
Tissue specimens and cell culture
A total of 30 pairs of lung cancer tissues and adjacent noncancerous tissues were obtained from patients at the First Affiliated Hospital of Soochow University. Samples were snap-frozen in liquid nitrogen and stored at −80°C for further analysis. All patients provided written informed consent. This research was approved by the independent ethics committee of the First Affiliated Hospital of Soochow University and was carried out in accordance with the Declaration of Helsinki.
All the cell lines (A549, H1299, H1975, H358, H292, and 16HBE) used in this study were obtained from the cell bank of Shanghai Institute for Biological Sciences (Shanghai, People’s Republic of China). FBS (10%; Thermo Fisher Scientific, Waltham, MA, USA) was mixed into all culture media along with 2 mM L-glutamine and 1% penicillin/streptomycin (Solarbio, Beijing, People’s Republic of China). Cells were grown in DMEM (TrueLine, Nashville, TN, USA) and were incubated in a 5% CO2 atmosphere at 37°C.
Immunohistochemistry
In brief, all tissue samples were fixed on 10% formalin and embedded with paraffin. A set of xylene baths and graded alcohols were used to deparaffinize and rehydrate samples. Antigen retrieval was carried out through steam heating for 15 minutes in 0.01 M citrate buffer at pH 6.0, and slides were rinsed three times with PBS (0.02 M) after natural cooling. Then, the activity of endogenous peroxidase was suppressed using Tris-buffered saline with Tween 20 containing 3% hydrogen peroxide for 10 minutes. The sections were incubated with a primary antibody (TRIM37; Novus, St Charles, MO, USA) overnight at 4°C followed by biotin-labeled secondary antibody (Longislandbio, Shanghai, People’s Republic of China) at room temperature for half an hour. Then, hematoxylin was used for nuclear counterstaining after slides were reacted with diaminobenzidine substrate.
RNA isolation and real-time PCR
Total RNA from all samples was extracted using TRIzol reagent (Thermo Fisher Scientific). After that, cDNA synthesis kit (Thermo Fisher Scientific) was used to synthesize cDNA according to the instruction of the manufacturer. The conditions of real-time PCR were as follows: 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 45 seconds. The expression was normalized to that of GAPDH. The relative gene expression was calculated by the 2−ΔΔCt method. All data represented the average of three replicates. The primer sequences used in this study are listed in the Supplementary materials.
Lentiviral vector-mediated RNA interference and overexpression of TRIM37
Lentiviral plasmids (pLKO.1) containing three shRNAs directed to various regions of human TRIM37 (NM_015294.5) and a negative control shRNA (siNC) were synthesized (Major, Shanghai, People’s Republic of China), respectively. Lentiviral plasmid (pLVX-puro) containing the full length of human TRIM37 cDNA sequence and the mock plasmid served as the negative control (oeNC). All of them were transiently transfected into lung cancer cells as indicated above using Lipofectamine 2000 (Thermo Fisher Scientific) according to the instruction of the manufacturer. Experiments were performed 48 hours after transfection. Detailed sequence information about siTRIM37s is provided in .
Western blot
Whole-protein lysates were extracted from indicated cells (H292, H358, and H1299) using RIPA lysis buffer (JRDUN, Shanghai, People’s Republic of China) with EDTA-free protease inhibitor cocktail (Roche, Basel, Switzerland). The protein concentration was estimated using an enhanced BCA protein assay kit (Thermo Fisher Scientific). An equal amount of total protein (25 µg) was fractionated on a 10% SDS-PAGE gel. Then, the gel was transferred to a nitrocellulose membrane (EMD Millipore, Billerica, MA, USA) overnight. After being blocked with 5% nonfat dry milk for 1 hour at room temperature, the membrane was probed at 4°C overnight with primary antibodies followed by secondary antibody anti-mouse IgG (1:1,000; Beyotime, Haimen, People’s Republic of China) for 1 hour at 37°C. An enhanced chemiluminescence system (Tanon, Shanghai, People’s Republic of China) was used for detecting protein expression value. The detailed information of primary antibodies is provided in .
Cell proliferation assay
Cell Counting Kit-8 (CCK-8) assay (SAB, College Park, MD, USA) was performed to examine cell proliferation according to the protocol of the manufacturer. Briefly, cells transfected as indicated were seeded in 96-well plates and cultured for 0, 24, 48, and 72 hours. CCK-8 solution (1:10) was mixed to each well and incubated for 1 hour. A microplate reader (Pulangxin, Beijing, People’s Republic of China) was used to measure the OD values at a wavelength of 450 nm. Independent triplicate experiments were carried out for each time point.
Cell apoptosis assay
In brief, H292, H358, and H1299 cells were collected and stained with Annexin V-fluorescein isothiocyanate apoptosis detection kit (Beyotime) according to the instructions of the manufacturer at 48 hours after viral infection. A flow cytometer (BD, Franklin Lakes, NJ, USA) was used to determine cell apoptosis.
Migration assay
Briefly, the indicated TRIM37 siRNA cells were serum-starved for 24 hours followed by seeding in the upper chamber, while the medium supplemented with 30% FBS (Thermo Fisher Scientific) was placed in the lower chamber. After 24 hours of incubation, cells on the upper side of the filters were removed and the remaining cells were fixed in 4% formaldehyde and stained with 0.01% crystal violet (Solarbio). Then, cells in the lower chamber were stained with crystal violet and counted under a microscope at ×200 magnification (Caikon, Shanghai, People’s Republic of China). All the procedures were performed using Boyden chambers (Costar; Corning, Corning, NY, USA).
Invasion assay
This assay was also performed using Boyden chambers containing a polycarbonate filter coated with Matrigel on the upper surface. The procedure was performed as indicated above.
Xenograft model
This assay was carried out according to the institute’s guidelines for animal experiments and was approved by the independent ethics committee of the First Affiliated Hospital of Soochow University, People’s Republic of China, and all animals were treated in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines. An equal number of siNC- and siTRIM37-transfected H358 cells (n=2×106) were subcutaneously injected into the right flank of 4- to 6-week-old nude mice (n=26 for each group; Shanghai Laboratory Animal Company, Shanghai, People’s Republic of China). The length and width of the tumor were examined every 3 days for 33 days after injection. The volume of the tumor was counted as length×(width 2/2). Six weeks after injection, six mice from the siNC- and siTRIM37-injected groups were sacrificed by cervical dislocation and tumor tissues were fixed in 4% formalin for further analysis. A total of 20 nude mice in each injected group as indicated were used to measure mouse survival rate. Mouse survival was monitored daily for a total of 100 days.
Statistical analysis
GraphPad Prism software Version 7.0 (GraphPad Software, Inc., La Jolla, CA, USA) was utilized for statistical analyses. Data were expressed as mean ± SD of at least three samples. Statistical significance was determined by one-way ANOVA for multiple comparisons. A P-value <0.05 was considered to indicate statistical significance.
Results
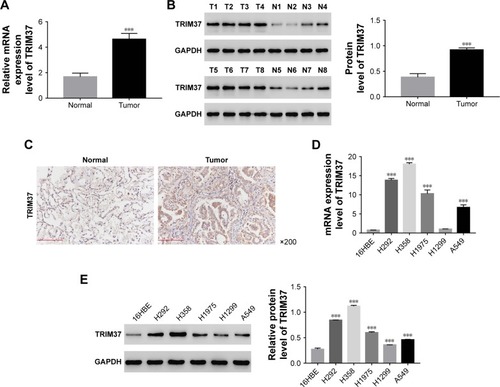
The level of TRIM37 was upregulated in lung cancer tissues and cell lines
The mRNA expression of TRIM37 was examined by quantitative real-time PCR in 30 pairs of lung cancer samples and matched para-cancerous tissues. In addition, Western blot was utilized to examine the protein level of TRIM37 in eight pairs of lung cancer samples and matched normal tissues. As shown in , both the mRNA and protein levels of TRIM37 in lung cancer tissues were significantly upregulated as compared with normal tissues. Moreover, immunohistochemistry was used to further investigate the protein level of TRIM37 in lung cancer and normal tissues. The results also suggested that TRIM37 was upregulated in tumor tissues (). Then, the mRNA and protein expression level of TRIM37 was detected in five lung cancer cell lines, including H292, H358, H1299, A549, and H1975, using a human bronchial epithelial cell line (16HBE) as normal control. As shown in , the mRNA and protein expression levels of TRIM37 were significantly upregulated in lung cancer cell lines, especially in H292 and H358 cells, compared with 16HBE cells. On the other hand, the H1299 cells showed relatively lower level of TRIM37 than other lung cancer cell lines. Based on these results, H292, H358, and H1299 cell lines were chosen for further study.
Figure 1 Expression of TRIM37 in lung cancer tissues and cell lines.
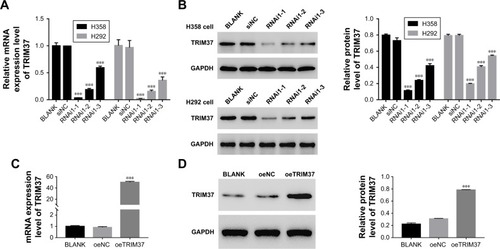
Knockdown and overexpression of TRIM37 in lung cancer cells
To silence the expression of TRIM37, three shRNAs targeting human TRIM37 gene (siTRIM37; RNAi1-1, RNAi1-2, RNAi1-3) and a negative control shRNA (siNC) were synthesized and transfected to H292 and H358 cell lines, respectively. The untreated cells acted as blank control (BLANK). As shown in , all the TRIM37-siRNAs were able to strongly reduce endogenous TRIM37. Cells transfected with RNAi1-1 and RNAi1-2 showed lower TRIM37 level than RNAi1-3. Therefore, RNAi1-1 and RNAi1-2 were chosen for further study. In addition, H1299 cells were transfected with a plasmid overexpressing TRIM37 (oeTRIM37) and a mock plasmid (oeNC), respectively. As shown by the TRIM37 mRNA and protein levels in , a remarkable overexpression of TRIM37 was observed in oeTRIM37-transfected cells. Hence, the oeTRIM37 cells were chosen for the following TRIM37 overexpression analysis.
Figure 2 Knockdown and overexpression of TRIM37 in lung cancer cell lines.
Abbreviation: BLANK, blank control.
Suppression of TRIM37 inhibited cell development and metastasis
The function of TRIM37 in the proliferation of lung cancer cells was examined through CCK-8 assays. siNC, RNAi1-1, and RNAi1-2 were transfected to H358 and H292 cells, respectively. As shown in , there was no obvious difference between BLANK and siNC groups in cell proliferation of the two lung cancer cell lines. However, the cell growth rate of the two cell lines in siTRIM37 group was significantly decreased at 24, 48, and 72 hours compared with siNC group. Thus, these data suggested that TRIM37 is a positive regulator of cell proliferation of lung cancer cells. Next, Annexin V-FITC/propidium iodide staining assay was performed to investigate the effects of TRIM37 on cell apoptosis of lung cancer cells. As shown in , the cell apoptosis ratio of the two cell lines was obviously higher in siTRIM37 group than in siNC group. These result indicated the antiapoptosis function of TRIM37 in lung cancer cells.
Figure 3 Knockdown of TRIM37 inhibited cell proliferation and apoptosis.
Abbreviation: BLANK, blank control.
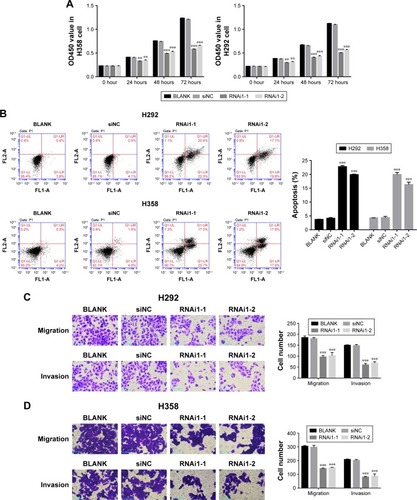
Moreover, we also examined the effect of siTRIM37 on lung cancer cells migration and invasion. As shown in , both the RNAi1-1 and RNAi1-2 significantly inhibited the migration and invasion ability of H292 or H358 cells. Thus, these data suggested a role for TRIM37 in the promotion of lung cancer cells metastasis.
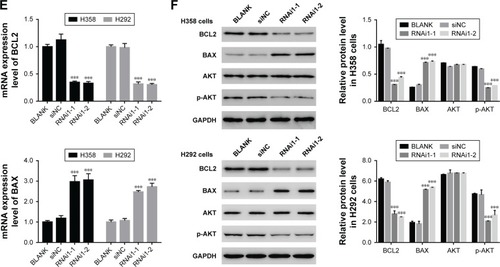
Knockdown of TRIM37 affected the activity of BCL2, BAX, and AKT
As shown in , the mRNA and protein expression level of BCL2 was remarkably downregulated in TRIM37 siRNA cells, which was contrary to the expression pattern of BAX. These results were consistent with our expectation. Moreover, the AKT level showed no obvious difference among all transfected cells as indicated above. However, the protein level of p-AKT was highly decreased in siTRIM37 cells, which indicated that the activity of AKT was deeply suppressed when TRIM37 was knocked down. These results might reflect the interaction between AKT and TRIM37 in lung cancer cells.
The function of TRIM37 was inhibited by a specific PI3K/AKT inhibitor LY294002
A specific AKT inhibitor LY294002 (10 µM) was used to further determine the relationship between TRIM37 and AKT. As shown in , the cell proliferation rate of oeTRIM37 was significantly upregulated as compared with that of oeNC cells. However, a remarkable decrease in cell proliferation rate was observed after the cells were treated with LY294002. Moreover, the cell apoptosis rate was significantly inhibited by oeTRIM37 in lung cancer cells. However, this suppression effect of TRIM37 was partly reduced after oeTRIM37 cells were treated with LY294002 (). Additionally, the cell migration and invasion ability analysis also showed similar results ().
Figure 4 The function of TRIM37 was inhibited by a specific AKT inhibitor LY294002.
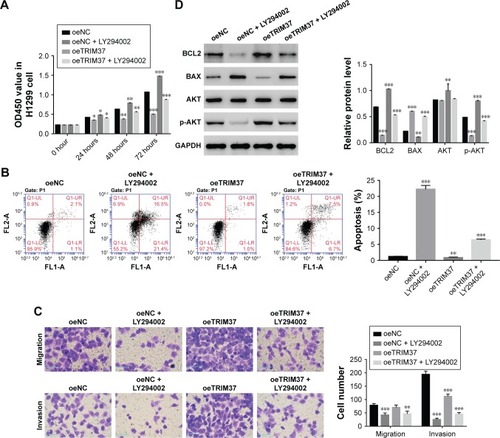
Western blot was performed to analyze the protein level of BCL2, BAX, AKT, and p-AKT in oeNC, oeNC + LY294002, oeTRIM37, and oeTRIM37 + LY294002 cells, respectively. As shown in , the BCL2 level was highly increased in oeTRIM37 cells as compared with oeNC cells. However, the protein level of BCL2 was significantly downregulated after the oeNC and oeTRIM37 cells were treated with LY294002. The protein level of BAX showed the opposite expression pattern to BCL2. Moreover, the AKT level showed no significant difference among the cells. However, the protein level of p-AKT was deeply inhibited after the cells were treated with LY294002, which suppressed the activity of AKT. Taken together, these results demonstrated that AKT might be a target of TRIM37 in lung cancer cells.
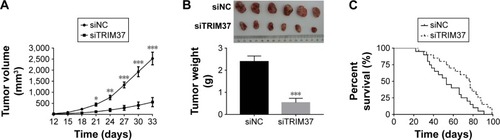
Knockdown of TRIM37 inhibited tumorigenicity of lung cancer cells in vivo
In order to investigate the function of TRIM37 in tumorigenicity in vivo, an equal number of H358 cells transfected with siRNA and siTRIM37 were hypodermically injected into nude mice (n=6 for each group) and tumor formation was determined every 3 days for 33 days (starting at day 12). As shown in , although both the injected cells were capable of developing tumors, it was easily identified that the TRIM37-siRNA cells significantly suppressed the tumor growth rate. The volume and weight of TRIM37-siRNA tumors showed highly significant reduction as compared with that of siNC tumors. In addition, the siTRIM37-transfected cells contributed to prolong the survival length of nude mice ().
Figure 5 Knockdown of TRIM37 in lung cancer cells inhibited tumor growth in vivo.
Discussion
Lung cancer is a highly invasive, rapidly metastasizing, and prevalent cancer all over the world.Citation1 Much attention has been paid to explore the effective methods for its treatment. Although the tumorigenic mechanism of lung cancer is still not fully understood, some factors have been found to have high value as biomarkers for diagnosing it at an early stage.
In this study, we systematically analyzed the function of a positive tumor regulator TRIM37 in lung cancer cells. To obtain reliable results, RNA interference was used to silence the expression of TRIM37. On the other hand, lentiviral vector was used to mediate overexpression of TRIM37. The results from those two analyses were consistent, which showed that our results were reliable.
The CCK-8 assay suggested TRIM37 as a positive regulator of cell proliferation of lung cancer cells. A previous report indicated that TRIM37 mediated cell proliferation in pediatric osteosarcoma.Citation6 In addition, TRIM44 and TRIM59 have been reported to promote proliferation of lung cancer cells.Citation17,Citation18 Therefore, we suspected that all the TRIM proteins contribute to proliferation of lung cancer cells.
A previous study showed that knockdown of TRIM9 induced apoptosis of lung cancer cells through mediating BCL2 and other related proteins.Citation19 In the present study, the cell apoptosis results indicated the antiapoptosis function of TRIM37 in lung cancer cells. Moreover, our results showed that the protein level of a pair of cell apoptosis factors (BCL2 and BAX) was deeply influenced when TRIM37 was knocked down or overexpressed in lung cancer cells, which indicates that TRIM37 can serve as an apoptotic marker of primary lung cancer.Citation20 Hence, we suspected that the antiapoptosis function of TRIM37 was mainly through the regulation of BCL2 and BAX. Moreover, we also evaluated the function of TRIM37 in cell migration and invasion in lung cancer cells. Our results indicated that the level of TRIM37 was positively correlated with the ability of migration and invasion of lung cancer cells. Previous reports have demonstrated that TRIM37 promoted cell metastasis in pancreatic cancer cells.Citation5,Citation21 Our results also insisted this conclusion, which suggested that TRIM37 is a positive regulator of cell metastasis.
It has been reported that TRIM11 functions in lung cancer cells via enhancing the activity of PI3K/AKT.Citation22 Previous reports have demonstrated that the oncogenes TRIM22 and TRIM44 contribute to the activity of P13/AKT in lung cancer cells.Citation23,Citation24 Moreover, knockdown of TRIM37 in glioma cells led to the inactivation of PI3K/AKT signaling pathway.Citation16 In this study, our results indicated that TRIM37 could activate PI3K/AKT. Therefore, we conclude that TRIM37 may promote lung cancer cell development through PI3K/AKT signaling pathway.
Moreover, some evidence has suggested that the activity of AKT is vital for retaining BAX in the cytoplasm.Citation25,Citation26 In addition, the AKT protein also functions to upregulate the expression of BCL2.Citation27,Citation28 Therefore, we suggest that TRIM37/AKT/BCL2 pathway might be a novel target for regulating cell apoptosis in lung cancer cells.
Conclusion
In the present study, we examined the effect of TRIM37 knockdown and overexpression in lung cancer cells. Our results not only help in better understanding of TRIM37 in lung cancer but also provide evidence that TRIM37 might serve as a novel biomarker in clinical application.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No 81401943). Shumin Dong and Xueqin Pang are co-first authors for this study.
Supplementary materials
Primer sequence information
Homo sapiens TRIM37, transcript variant 2, mRNA
NCBI Reference Sequence: NM_001005207.4
Primer F: 5′ TGGACTTACTCGCAAATG 3′
Primer R: 5′ ATCTGGTGGTGACAAATC 3′
Pos: 1645-1874 C
Amplified product: size: 230 bps, product GC: 39%
H. sapiens BCL2, apoptosis regulator, transcript variant alpha, mRNA
NCBI Reference Sequence: NM_000633.2
Primer F: 5′ GCAGTGTGGTCTCCGAATGTC 3′
Primer R: 5′ CATTGCCTCTCCTCACGTTCC 3′
Pos: 2277-2521 C
Amplified product: size: 245 bps, product GC: 56%
H. sapiens BAX, apoptosis regulator, transcript variant 1, mRNA
NCBI Reference Sequence: NM_001291428.1
Primer F: 5′ CTGAGCGAGTGTCTCAAG 3′
Primer R: 5′ CAGCCCATGATGGTTCTG 3′
Pos: 244-485 C
Amplified product: size: 242 bps, product GC: 57%
H. sapiens GAPDH, transcript variant 1, mRNA
NCBI Reference Sequence: NM_001256799.2
Primer F: 5′ AATCCCATCACCATCTTC 3′
Primer R: 5′ AGGCTGTTGTCATACTTC 3′
Pos: 436-653
Amplified product: size: 218 bps
Table S1 Homo sapiens TRIM37 (NM_001005207.4) RNAi targeting locus information
Table S2 The primary antibodies information
Disclosure
The authors report no conflicts of interest in this work.
References
- Lemjabbar-AlaouiHHassanOUYangYWBuchananPLung cancer: Biology and treatment optionsBiochim Biophys Acta20151856218921026297204
- AlbergAJBrockMVFordJGSametJMSpivackSDDiagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice GuidelinesEpidemiology of Lung Cancer20161435
- KallijärviJLahtinenUHämäläinenRLipsanen-NymanMPalvimoJJLehesjokiAETRIM37 defective in mulibrey nanism is a novel RING finger ubiquitin E3 ligaseExp Cell Res2005308114615515885686
- HuCEGanJTRIM37 promotes epithelial-mesenchymal transition in colorectal cancerMol Med Rep20171531057106228098873
- JiangJTianSYuCChenMSunCTRIM37 promoted the growth and migration of the pancreatic cancer cellsTumour Biol20163722629263426395261
- TaoYXinMChengHTRIM37 promotes tumor cell proliferation and drug resistance in pediatric osteosarcomaOncol Lett20171466365637229163677
- ViantCGuiaSHennessyRJCell cycle progression dictates the requirement for BCL2 in natural killer cell survivalJ Exp Med2017214249151028057804
- CuiJPlaczekWJPost-Transcriptional Regulation of Anti-Apoptotic BCL2 Family MembersInt J Mol Sci2018191E30829361709
- SunSYYuePZhouJYOverexpression of BCL2 blocks TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in human lung cancer cellsBiochem Biophys Res Commun2001280378879711162590
- GessnerCLiebersUKuhnHBAX and p16INK4A are independent positive prognostic markers for advanced tumour stage of nonsmall cell lung cancerEur Respir J200219113414011843312
- HanBParkDLiRSmall-Molecule Bcl2 BH4 Antagonist for Lung Cancer TherapyCancer Cell201527685286326004684
- ChenMJWuDWWangGCWangYCChenCYLeeHMicroRNA-630 may confer favorable cisplatin-based chemotherapy and clinical outcomes in non-small cell lung cancer by targeting Bcl-2Oncotarget2018917137581376729568392
- YoshizawaAFukuokaJShimizuSOverexpression of phospho-eIF4E is associated with survival through AKT pathway in non-small cell lung cancerClin Cancer Res201016124024820008839
- VincentEEElderDJThomasECAkt phosphorylation on Thr308 but not on Ser473 correlates with Akt protein kinase activity in human non-small cell lung cancerBr J Cancer2011104111755176121505451
- XuXZhangYQuDJiangTLiSOsthole induces G2/M arrest and apoptosis in lung cancer A549 cells by modulating PI3K/Akt pathwayJ Exp Clin Cancer Res2011303321447176
- TangSLGaoYLWen-ZhongHKnockdown of TRIM37 suppresses the proliferation, migration and invasion of glioma cells through the inactivation of PI3K/Akt signaling pathwayBiomed Pharmacother201899596429324313
- XingYMengQChenXTRIM44 promotes proliferation and metastasis in non-small cell lung cancer via mTOR signaling pathwayOncotarget2016721304793049127058415
- ZhanWHanTZhangCTRIM59 Promotes the Proliferation and Migration of Non-Small Cell Lung Cancer Cells by Upregulating Cell Cycle Related ProteinsPLoS One20151011e014259626599082
- WangXShuYShiHLvXZouHShiWTRIM9 is up-regulated in human lung cancer and involved in cell proliferation and apoptosisInt J Clin Exp Med2016961046110469
- PorebskaIWyrodekEKosackaMAdamiakJJankowskaRHarłozińska-SzmyrkaAApoptotic markers p53, Bcl-2 and Bax in primary lung cancerIn Vivo200620559960417091766
- JiangJYuCChenMTianSSunCOver-expression of TRIM37 promotes cell migration and metastasis in hepatocellular carcinoma by activating Wnt/β-catenin signalingBiochem Biophys Res Commun201546441120112726208456
- WangXShiWShiHTRIM11 overexpression promotes proliferation, migration and invasion of lung cancer cellsJ Exp Clin Cancer Res201635110027329103
- LiuLZhouXMYangFFTRIM22 confers poor prognosis and promotes epithelial-mesenchymal transition through regulation of AKT/GSK3β/β-catenin signaling in non-small cell lung cancerOncotarget2017837620696208028977927
- XingYMengQChenXWangXTRIM44 promotes proliferation and metastasis in non-small cell lung cancer via mTOR signaling pathwayOncotarget2016721304793049127058415
- TsurutaFMasuyamaNGotohYThe phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax translocation to mitochondriaJ Biol Chem200227716140401404711842081
- YamaguchiHWangHGThe protein kinase PKB/Akt regulates cell survival and apoptosis by inhibiting Bax conformational changeOncogene200120537779778611753656
- MarinovMZiogasAPardoOEAKT/mTOR pathway activation and BCL-2 family proteins modulate the sensitivity of human small cell lung cancer cells to RAD001Clin Cancer Res20091541277128719228731
- PugazhenthiSNesterovaASableCAkt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding proteinJ Biol Chem200027515107611076610753867
