Abstract
Purpose
To study the relationship between INPPL1 gene and clinicopathologic characteristics of papillary thyroid carcinoma (PTC).
Patients and methods
INPPL1 expression in PTCs was tested by quantitative real-time reverse transcription PCR. The Cancer Genome Atlas (TCGA) RNA-seq data and our mRNA data were used to analyze and reveal the relationship between INPPL1 and aggressive clinicopathologic characteristics of PTC.
Results
When compared to normal thyroid tissues, INPPL1 was significantly downregulated in PTC tissues, as revealed by our data and TCGA data. INPPL1 underexpression was remarkably related to aggressive clinicopathologic characteristics such as lymph node metastasis (LNM), histological type, tumor size, mulitifocality, and disease stage in TCGA data. Meanwhile, LNM was confirmed to be associated with underexpression of INPPL1 in our data. In addition, logistic analysis clearly showed that underexpression of INPPL1 was an independent factor for LNM in PTC.
Conclusion
INPPL1 may be a novel tumor suppressor gene in PTC, which was significantly correlated with aggressive clinicopathologic characteristics, especially LNM.
Introduction
Thyroid cancer is the most common malignant tumor in the endocrine system and among the neck tumors, whose incidence is increasing globally in recent years. In the USA, ~56,870 new cases were estimated in 2017.Citation1 The prognosis of most thyroid cancer patients is good,Citation2 especially in those with papillary thyroid carcinoma (PTC) which is the most common type of thyroid cancer and accounts for ~80% of all thyroid cancer cases.Citation3 However, PTC is highly metastatic and recurrent after routine treatment, and some patients of PTC have poor prognosis such as disease recurrence and even death.Citation4 Certain clinical and pathological characteristics such as advanced disease stages, extrathyroidal extension, and lymph node metastasis (LNM) have been associated with a poor prognosis of this disease.Citation5 LNM is a major factor for recurrence and mortality,Citation6–Citation8 whose incidence ranges from 20% to 50%Citation9,Citation10 and which leads to recurrence and secondary surgery.Citation11–Citation13 The occurrence and development of thyroid carcinoma are mainly affected by genomic variation, including activation of oncogene and silencing of tumor suppressor gene. It has been proved that BRAF mutation promotes the occurrence and development of thyroid carcinoma by abnormal activation of MAPK pathway,Citation14 and that the mutation of TERT promoterCitation15 and PIK 3CA geneCitation16 also plays an important role. Although great progress has been made in gene research, the pathogenesis and many features of thyroid cancer are still unknown. Therefore, searching for new potential molecular markers and elucidating their molecular mechanisms in the development of thyroid cancer become necessary.
Inositol phosphatase 1 (INPPL1), which is located on chromosome 11, encodes an SH2-containing 5′-inositol phosphatase (SHIP2), a member of the inositol 5-phosphatase family, which is involved in the regulation of insulin function. SHIP2 also plays a role in the regulation of EGFR turnover and actin remodeling.Citation17,Citation18 SHIP2 dephosphorylates 5-phosphate of phosphatidylinositol-3,4,5-trisphosphate and plays important roles in regulating the PI3K/Akt pathway in physiology and disease. SHIP2 is widely expressed in human type II diabetes mellitus and multiple dysplasia, but its role in human cancer remains unclear. In recent years, it has been reported that SHIP2 has both tumor-promoting and antitumor functions in human tumors, which largely depend on the cell model.Citation19 In the glioblastoma cell line 1321 N1, which does not express PTEN, downregulation of SHIP2 expression promotes cell proliferation by reducing the expression of key regulatory proteins (such as p27) in cell cycle and is involved in the migration by controlling phosphatidylinositol 4,5-bisphosphate in the cell membrane.Citation19,Citation20 SHIP2 is frequently downregulated in gastric cancer, and its underexpression promotes the development and proliferation of gastric cancer by activating PI3K/AKT signal.Citation21 However, high expression of SHIP2 was found in breast cancer, hepatocellular carcinoma, non-small cell lung cancer, and colorectal cancer, which was associated with poor survival.Citation22–Citation26 It can be seen that INPPL1 gene plays different roles in different tumors. The expression and role of INPPL1 gene in thyroid carcinoma have not been reported. It is important to study the expression and biological function of human INPPL1 gene in thyroid carcinoma to understand the occurrence and development of thyroid carcinoma.
As next generation sequence has developed, our previous study performed whole transcriptome sequencing of 19 pairs of primary thyroid cancer samples with matched adjacent normal thyroid tissues.Citation27 The present study found by application of bioinformatics that human INPPL1 expression is significantly downregulated in PTC tumors. Thus, we confirm this finding using quantitative real-time reverse transcription PCR (qRT-PCR) in 49 PTC samples. Also, we investigate the relationship between INPPL1 expression and the clinicopathologic characteristics in PTC using The Cancer Genome Atlas (TCGA) data and our data. Furthermore, the relationship between INPPL1 expression and LNM in PTC was assessed using logistic regression analysis. The role of INPPL1 gene in PTC has been discussed in this study.
Patients and methods
Patients and samples
Fresh paired samples including PTC tissues and noncancerous tissues from 49 PTC patients were collected. The samples after resection were immediately snap-frozen in liquid nitrogen and subsequently stored at −80°C before RNA extraction. Final histological diagnosis of all the samples was confirmed as PTC by two pathologists. The study was conducted with the approval of Ethics Committee of The First Affiliated Hospital of Wenzhou Medical University and the written informed consent was received from the patients.
RNA extraction and qRT-PCR
Total RNA was extracted from 49 paired samples using TRIzol reagent according to the manufacturer’s protocol (Thermo Fisher Scientific), and the ReverTra Ace qPCR RT Kit (Toyobo) was used for cDNA. qRT-PCR was conducted by Thunderbird SYBR qPCR Mix (Toyobo) on the Roche 480 System (Hoffman-La Roche Ltd). Each sample was in triplicate. GAPDH was used as an internal control. The primer sequences for INPPL1 were as follows: INPPL1, 5′-AGCTGCCCACGCTCAAACCAA-3′ (forward) and 5′-AGGTCAGGAACTGTTGGGCCGT-3′ (reverse).
TCGA data
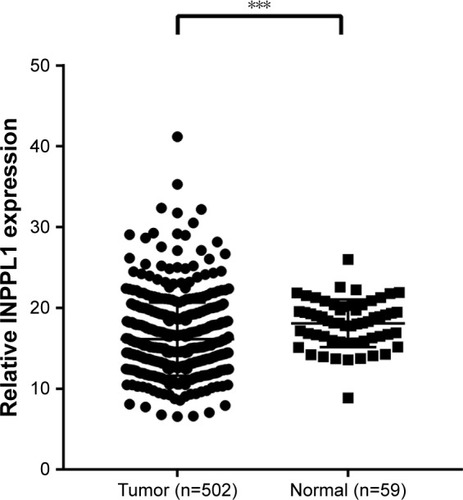
Thyroid cancer RNA-seq data and corresponding clinical information were downloaded from the TCGA database. INPPL1 expression data were available for 502 PTC cancer samples compared with 59 normal thyroid samples.
Statistical analysis
The normally distributed data were expressed as mean±SD and were evaluated by Student’s t-test. Categorical variables were expressed as percentage and evaluated by chi-squared test. Logistic regression analysis was conducted to estimate the ORs of certain factors. Variables with P<0.05 in the univariate analysis were used in a multivariate analysis. All P-values were two sided, and P-value <0.05 was considered statistically significant with SPSS. GraphPad Prism Version 6.0 was used for the graphs.
Results
INPPL1 was underexpressed in PTC
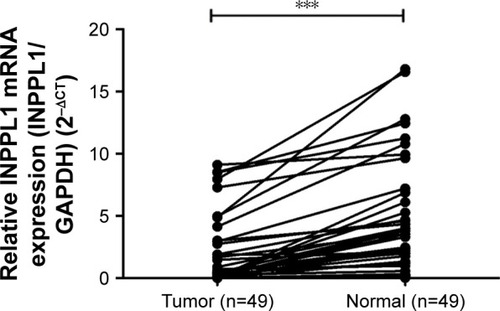
Completing the whole transcriptome sequencing of 19 pairs of tumor and paracancerous normal tissues, we found that the expression of INPPL1 in PTC tumor tissues was significantly lower than that in paracancerous normal tissues (). In order to validate the data of whole transcriptome resequencing, we began to assess INPPL1 mRNA expression in 49 samples of PTC tissues and noncancerous samples by qRT-PCR. As shown in , INPPL1 mRNA expression was remarkably downregulated in tumors against that in the adjacent noncancerous tissues (P<0.001). The same trend was further validated in the TCGA cohort, which included 502 PTC samples and 59 normal thyroid tissues. Accordingly, INPPL1 mRNA expression was significantly lower in PTC tissues than that in normal tissues (P<0.001; ). These results revealed that INPPL1 gene may be a potential tumor suppressor gene in PTC patients.
Table 1 The expression of INPPL1 gene in 19 cases of thyroid papillary carcinoma was lower than that in normal tissue by whole transcriptome sequencing
Figure 1 The mRNA expression of INPPL1 in our local cohort (n=49).
Abbreviations: CT, cycle threshold; PTC, papillary thyroid carcinoma; qRT-PCR, quantitative real-time reverse transcriptase PCR.
Relationship between INPPL1 expression and clinicopathologic characteristics in PTC
In order to explore whether low INPPL1 expression was associated with tumorigenesis and progression of PTC, we studied the relationship of INPPL1 with clinicopathologic characteristics. We divided the 502 PTC patients into low INPPL1 expression group (n=252) and high INPPL1 expression (n=250) group on the basis of the median value according to the INPPL1 expression level in the TCGA cohort. Results showed that INPPL1 underexpression was related to histological type (P=0.001), tumor size (P=0.046), clinical stage (P=0.007), LNM (P=0.001), and multifocality (P=0.019), as shown in . However, age, gender, and distant metastasis were not found to have significant associations with INPPL1 expression (P≥0.05). At the same time, the results of our validation cohort were consistent with the TCGA finding that low INPPL1 expression corresponded to more LNM (P=0.038; ). These findings supported INPPL1 gene as a tumor suppressor gene associated with PTC.
Table 2 The relationship between INPPL1 expression and clinicopathologic features in the TCGA cohort
Table 3 The relationship between INPPL1 expression and clinicopathologic features in our cohort
INPPL1 underexpression was an independent indicator for LNM in PTC
Further study was conducted to find the relationship of INPPL1 expression with LNM. Univariate logistic regression analysis in TCGA data revealed that the relative variables for LNM were histological type (OR=2.383, 95% CI=1.544–3.680, P<0.001), age (OR=0.62, 95% CI=0.427–0.899, P=0.012), gender (OR=1.551, 95% CI=1.022–2.353, P=0.039), tumor size (OR=2.525, 95% CI=1.652–3.858, P<0.001), and INPPL1 expression (OR=0.335, 95% CI=0.242–0.520, P<0.001), as shown in . Multivariate logistic regression analysis in TCGA data also revealed that histological subtype (OR=2.281, 95% CI=1.440–3.613, P<0.001), age (OR=0.577, 95% CI=0.385–0.865, P=0.008), gender (OR=1.549, 95% CI=1.010–2.509, P=0.045), tumor size (OR=2.44, 95% CI=1.555–3.830, P<0.001), and INPPL1 expression (OR=0.360, 95% CI=0.241–0.540, P<0.001) were independent indicators for LNM (). Meanwhile, multivariate logistic regression analysis from our validation cohort also suggested that low INPPL1 expression aggravated the LNM risk of PTC patients (OR=0.156, 95% CI=0.033–0.742, P=0.020; ), which was consistent with the TCGA findings. In general, INPPL1 underexpression can indicate the high risk of LNM independently in PTC.
Table 4 Univariate logistic regression analysis for the risk of lymph node metastasis in TCGA cohort
Table 5 Multivariate logistic regression analysis for the risk of lymph node metastasis in TCGA cohort
Table 6 Multivariate logistic regression analysis for the risk of lymph node metastasis in our cohort
Discussion
PTC is the most common endocrine malignant tumor, and its incidence is increasing year by year.Citation1 Although the prognosis of most thyroid cancer patients is favorable,Citation2 some PTCs are characterized by capsular invasion and lymph node (the incidence being 20%–50%Citation9,Citation10) and distant metastasis, which lead to the possibility of disease recurrence and secondary surgery.Citation11–Citation13 PTCs show different biological behaviors during tumorigenesis due to genomic variations. It is insufficient to make individualized treatment strategies and assess the risk of each PTC patient based only on the current clinical and pathological parameters. Finding new molecular biomarkers to predict the clinical progress and metastasis of PTCs is urgent. So far, indicators to assess the status of LNM are still lacking.
High-throughput sequencing of variable gene variations has been widely used in the study of molecular mechanism of cancer. In our study, transcriptome sequencing was performed in 19 pairs of PTC tumors and adjacent normal tissues, and the results showed that the expression of INPPL1 in PTC tumor tissues was lower than that in adjacent normal thyroid tissues. This finding might suggest its possible role as a tumor suppressor in thyroid cancer.
INPPL1 encodes SHIP2, which is involved in the regulation of insulin function, EGFR turnover, and actin remodeling.Citation17,Citation18 INPPL1 may be a promising therapeutic target for not only type 2 diabetes, but also cancer, neurode-generative diseases, and atherosclerosis.Citation28 Among cancers, high expression of INPPL1 was found in breast cancer, hepatocellular carcinoma, non-small cell lung cancer, and colorectal cancer, where it is associated with poor survival.Citation22-26 INPPL1 supports the metastatic growth in breast cancer and it is a valuable biomarker for breast cancer. By interacting with c-CBL, INPPL1 can prevent the conversion of EGFR and enhance the Akt activation induced by EGF, thus promoting the proliferation and metastasis of breast cancer cells.Citation22,Citation29 In ER-negative breast cancer stem cells, INPPL1 activates Akt and JNK and upregulates epithelial mesenchymal transition markers and vimentin.Citation30 INPPL1 expression contributes to the malignant potential of colorectal cancer by enhancing chemoresistance, cell migration, and cell invasion.Citation31 However, overexpression of INPPL1 in glioblastoma cells inhibits Akt activation and leads to cell cycle arrest and migration.Citation19,Citation20 INPPL1 is often downregulated in gastric cancer, and the decreased INPPL1 expression promotes the development and proliferation of gastric cancer by activating PI3K/AKT signal.Citation21 It can be seen that INPPL1 plays different roles in different tumors.
In this study, we first reported the underexpression of INPPL1 in PTC and it was associated with aggressive clinicopathologic characteristics, especially LNM. INPPL1 mRNA expression detected by qRT-PCR was remarkably downregulated in PTC tissues against that in noncancerous tissues. This result was consistent with our finding of whole transcriptome sequencing. TCGA cohort analysis also confirmed this finding, which is similar to the expression of INPPL1 in gastric cancer.Citation21 Clinicopathologic feature analysis in TCGA cohort showed that underexpression of NINPPL1 was remarkably related to aggressive clinicopathologic characteristics, including tumor size, multifocality, advanced disease stage, and LNM. In our cohort, LNM was confirmed to be associated with the underexpression of INPPL1, which was consistent with TCGA data. Other features have not shown statistical correlation, which may be due to the limited number of cases. Moreover, logistic regression analysis indicated that the underexpression of INPPL1 was an independent factor for LNM in PTCs. This seems to suggest that the INPPL1 gene may inhibit the migration of thyroid cancer, as it does in glioblastoma.Citation20 All these findings support INPPL1 as a tumor suppressor gene associated with PTC and further study must to be conducted.
Our study still contains certain limitations. First, the cellular molecular mechanisms of INPPL1 in the progression of PTC need to be further investigated. Furthermore, the relationship between INPPL1 and prognosis of PTC in large samples needs to be studied.
Conclusion
INPPL1 was generally underexpressed in PTC. Low INPPL1 expression indicates high risk of LNM in PTC. INPPL1 may be a potential tumor suppressor gene in PTC, and it is worth studying further.
Acknowledgments
The Major Science and Technology Projects of Zhejiang Province (2015C03052) supported this work.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2017CA Cancer J Clin201767173028055103
- LeboulleuxSRubinoCBaudinEPrognostic factors for persistent or recurrent disease of papillary thyroid carcinoma with neck lymph node metastases and/or tumor extension beyond the thyroid capsule at initial diagnosisJ Clin Endocrinol Metab200590105723572916030160
- MorrisLGTuttleRMDaviesLChanging trends in the incidence of thyroid cancer in the United StatesJAMA Otolaryngol Head Neck Surg2016142770971127078686
- BurnsWRZeigerMADifferentiated thyroid cancerSemin Oncol201037655756621167375
- ItoYMiyauchiAPrognostic factors and therapeutic strategies for differentiated carcinomas of the thyroidEndocr J200956217719218703852
- KimSKKwonAYBackKPredictive factors of lymph node metastasis in follicular variant of papillary thyroid carcinomaAnn Surg Oncol20172492617262328685355
- LundgrenCIHallPDickmanPWZedeniusJClinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case–control studyCancer2006106352453116369995
- ZidanJKarenDSteinMPure versus follicular variant of papillary thyroid carcinoma: clinical features, prognostic factors, treatment, and survivalCancer20039751181118512599223
- MustafaMKuwertTWeberKRegional lymph node involvement in T1 papillary thyroid carcinoma: a bicentric prospective SPECT/CT studyEur J Nucl Med Mol Imaging20103781462146620358197
- SchneiderDFChenHNew developments in the diagnosis and treatment of thyroid cancerCA Cancer J Clin2013636373394
- LeeYCNaSYParkGCOccult lymph node metastasis and risk of regional recurrence in papillary thyroid cancer after bilateral prophylactic central neck dissection: a multi-institutional studySurgery2017161246547127574773
- LeeYMSungTYKimWBRisk factors for recurrence in patients with papillary thyroid carcinoma undergoing modified radical neck dissectionBr J Surg201610381020102527121346
- LiuFHKuoSFHsuehCChaoTCLinJDPostoperative recurrence of papillary thyroid carcinoma with lymph node metastasisJ Surg Oncol2015112214915426175314
- XingMBRAF mutation in thyroid cancerEndocr Relat Cancer200512224526215947100
- XingMLiuRLiuXBRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrenceJ Clin Oncol201432252718272625024077
- HouPLiuDShanYLiuZGenetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancerClin Cancer Res20071341161117017317825
- BackersKBleroDPaternotteNZhangJErneuxCThe termination of PI3K signalling by SHIP1 and SHIP2 inositol 5-phosphatasesAdv Enzyme Regul200343152812791379
- RaaijmakersJHDeneubourgLRehmannHThe PI3K effector Arap3 interacts with the PI(3,4,5)P3 phosphatase SHIP2 in a SAM domain-dependent mannerCell Signal20071961249125717314030
- Elong EdimoWSchurmansSRogerPPErneuxCSHIP2 signaling in normal and pathological situations: its impact on cell proliferationAdv Biol Regul20145414215124091101
- Elong EdimoWGhoshSDeruaRSHIP2 controls plasma membrane PI(4,5)P2 thereby participating in the control of cell migration in 1321 N1 glioblastoma cellsJ Cell Sci201612961101111426826186
- YeYGeYMXiaoMMSuppression of SHIP2 contributes to tumorigenesis and proliferation of gastric cancer cells via activation of AktJ Gastroenterol201651323024026201869
- PrasadNKTandonMBadveSSnyderPWNakshatriHPhospho-inositol phosphatase SHIP2 promotes cancer development and metastasis coupled with alterations in EGF receptor turnoverCarcinogenesis2008291253417893231
- FuMFanWPuXElevated expression of SHIP2 correlates with poor prognosis in non-small cell lung cancerInt J Clin Exp Pathol20136102185219124133597
- FuMGuXNiHHigh expression of inositol polyphosphate phosphatase-like 1 associates with unfavorable survival in hepatocellular carcinomaInt J Clin Exp Pathol20136112515252224228114
- PrasadNKTandonMHandaAHigh expression of obesity-linked phosphatase SHIP2 in invasive breast cancer correlates with reduced disease-free survivalTumour Biol200829533034119065064
- YangJFuMDingYHigh SHIP2 expression indicates poor survival in colorectal cancerDis Markers20142014218968725525286
- WangQXChenEDCaiYFA panel of four genes accurately differentiates benign from malignant thyroid nodulesJ Exp Clin Cancer Res201635116927793213
- SuwaAKuramaTShimokawaTSHIP2 and its involvement in various diseasesExpert Opin Ther Targets201014772773720536411
- PrasadNKSHIP2 phosphoinositol phosphatase positively regulates EGFR-Akt pathway, CXCR4 expression, and cell migration in MDA-MB-231 breast cancer cellsInt J Oncol20093419710519082482
- FuCHLinRJYuJA novel oncogenic role of inositol phosphatase SHIP2 in ER-negative breast cancer stem cells: involvement of JNK/vimentin activationStem Cells20143282048206024802135
- HoekstraEdasAMWillemsenMLipid phosphatase SHIP2 functions as oncogene in colorectal cancer by regulating PKB activationOncotarget2016745735257354027716613