Abstract
Background
As a component of the EIF3 complex, EIF3C is essential for several steps in protein synthesis initiation. Recently, it has been addressed that EIF3C is overexpressed in several human cancers and plays a pivotal role in cell proliferation and tumorigenesis.
Materials and methods
Immunohistochemistry, quantitative real-time PCR (qPCR), and Western blotting assays were employed to determine the expression of EIF3C in osteosarcoma (OsC) tissues obtained from 60 patients. The levels of EIF3C mRNA and protein were assessed by qPCR and Western blotting, respectively. The effect of EIF3C knockdown on OsC cell proliferation was detected by MTT and colony formation assays, respectively. Cell apoptosis induced by EIF3C silencing was analyzed by flow cytometric analysis. PathScan stress and apoptosis signaling antibody array kit was used to analyze the potential effects of EIF3C knockdown on OsC cells.
Results
The levels of EIF3C were high in OsC tissues and cell lines. In addition, EIF3C knockdown by lentivirus-mediated shRNA targeting EIF3C significantly suppressed cell proliferation and colony formation and induced apoptosis in U-2OS cells. Moreover, EIF3C knockdown led to the upregulated expression of CASP3/7, Chk1/2, and SAPK/JNK, indicating that the downregulated expression of EIF3C might be associated with pro-apoptosis of U-2OS cells.
Conclusion
EIF3C may be a promising target for gene therapy of human OsC. However, the precise mechanisms behind the effect of EIF3C on OsC tumorigenesis require further analysis.
Introduction
Osteosarcoma (OsC), also known as “osteogenic sarcoma”, is the most frequent type of primary bone tumor. OsC is the second most leading cause of cancer-related deaths in adolescents and children and accounts for ~20% of all primary bone cancers.Citation1–Citation4 Treatment of OsC includes neoadjuvant and postoperative adjuvant chemotherapy, and although some improvements have been achieved in effectively curing the disease, OsC still remains a devastating disease with poor early diagnosis and multidrug resistance of OsC cells.Citation5 For OsC patients, the 5-year survival rate is, 40%.Citation6,Citation7 Therefore, it is of utmost importance to elucidate the molecular mechanisms underlying the development and progression of OsC, as well as to identify novel therapeutic targets and therapeutic approaches to treat this disease.
Translation is an essentially fundamental process that can be divided into three steps: initiation, elongation, and termination. During the initiation step, the EIF3 complex is responsible for stabilizing the 43S pre-initiation complex by interacting directly with eIF1, eIF2, eIF5, and the 40S ribosomal subunit.Citation8,Citation9 EIF3 is the largest mammalian scaffolding initiation factor and contains 13 subunits that are designated as EIF3A–3M.Citation9 Among these subunits, EIF3C is an essential subunit that allows for the assembly of the EIF3 complex.Citation10,Citation11 Increasing evidence has revealed that alterations in the expression of EIF3C are associated with oncogenic properties;Citation12 for instance, EIF3C was found to be overexpressed in seminomasCitation13 and meningiomas.Citation14 Additionally, it has been demonstrated that EIF3C is critical for proliferation of human colon cancer cells,Citation15 glioma cells,Citation16,Citation17 and breast cancer cells.Citation18 However, little is known about the role of EIF3C in human OsC.
In the present study, we first evaluated the expression of EIF3C in human OsC tissues and cell lines. Next, we used RNA interference technology in OsC U-2OS cells to determine the role of EIF3C in tumor proliferation, colony formation, and apoptosis. Finally, we used the PathScan stress and apoptosis signaling antibody array kit to determine the potential of EIF3C silencing to inhibit tumorigenesis in human OsC.
Materials and methods
Patients and samples
In the present study, 60 patients with OsC treated at the First Affiliated Hospital of Anhui Medical University between 2013 and 2016 were enrolled. The study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Anhui Medical University. All the patients provided written informed consent, and the study was conducted in accordance with the Declaration of Helsinki. Tumor specimens and para-carcinoma tissues (referred to as normal tissues, at least 1.0 cm apart from the visible cancerous tissues) were used for detecting the levels of EIF3C expression by immunohistochemistry (IHC; paraffin-embedded tissues) and quantitative real-time PCR (qPCR; frozen tissues) assays as described below.
Immunohistochemistry
IHC was carried out on formalin-fixed, paraffin-embedded tissues. The slices (5 µm thick) were prepared for incubating sequentially with primary EIF3C antibody (1:500, catalog no PA5-62137; Thermo Fisher Scientific, Waltham, MA, USA). The experimental procedure and immunostaining scoring of EIF3C expression were conducted as previously described.Citation19
Cell lines
Two human OsC cell lines, U-2OS and Soas-2, were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). Human lung fibroblasts (MRC-5 cell line) were provided by Prof Shengquan Zhang (Department of Biochemistry and Molecular Biology, Anhui Medical University) as a gift. Cells were maintained in Roswell Park Memorial Institute 1640 medium (Thermo Fisher Scientific) supplemented with 10% FBS, 2 mM L-glutamine, penicillin (100 U/mL), and streptomycin (100 µg/mL) (Sangon Biotech, Shanghai, China). Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2.
Construction and transfection of shEIF3C lentivirus
For manipulating EIF3C expression, a lentiviral system containing shRNA was used. A candidate siRNA specifically targeting human EIF3C (GenBank Accession No NM_003752) was designed to target the sequence 5′-CCA TCC GTA ATG CCA TGA A-3′. In addition, a scramble sequence that served as the negative control shRNA was included in the study: 5′-TTC TCC GAA CGT GTC ACG T-3′. Stem-loop DNA oligonucleotides were synthesized and cloned into the lentiviral pGV115-GFP vector (GeneChem, Shanghai, China). An EIF3C shRNA-expressing lentivirus (shEIF3C) and scramble control siRNA-expressing lentivi-rus (shCtrl) were prepared using a Lentivector Expression System (GeneChem).
For lentiviral infection, U-2OS cells (1×105) were cultured in six-well plates and infected with shEIF3C or shCtrl lentivirus using a multiplicity of infection of 80. After 72 hours of infection, GFP expression was examined under a fluorescence microscope (Olympus XI-71; Tokyo, Japan). After 120 hours of infection, U-2OS cells were harvested and quantitative reverse transcription PCR (qRT-PCR) and Western blot analysis were performed to determine knockdown efficiency.
RNA extraction and qRT-PCR
Total RNA was extracted from human OsC tissues and cells using the TRIzol method (Thermo Fisher Scientific) under RNase-free conditions and according to the manufacturer’s guidelines. From each sample, 2 µg of total RNA was reverse-transcribed using M-MuLV reverse transcriptase and Oligo (dT) primers (Sangon Biotech) to generate single-stranded cDNA. To quantify EIF3C expression in U-2OS cells infected with shEIF3C lentivirus, qPCR was conducted using SYBR Green Premix Ex Taq (Takara, Dalian, China) at an ABI 7500 system (Thermo Fisher Scientific). The housekeeping gene GAPDH was used as an internal control. One microgram of cDNA was used as a template for PCR. The primers used for qPCR were as follows: GAPDH forward, 5′-TGA CTT CAA CAG CGA CAC CCA-3′ and reverse, 5′-CAC CCT GTT GCT GTA GCC AAA-3′ (product size: 121 bp); and EIF3C forward, 5′-AGA TGA GGA TGA GGA TGA GGA C-3′ and reverse, 5′-GGA ATC GGA AGA TGT GGA ACC-3′ (product size: 175 bp). The expression of EIF3C was normalized to GAPDH expression, and data analysis was conducted using the 2−ΔΔCT method.
Cell proliferation assay
Cell proliferation was measured using a multi-parametric high-content screening approach as described by Liu et al.Citation20 After infection with either shEIF3C or shCtrl lentivirus, U-2OS cells in the logarithmic phase were reseeded at a density of 2×103 cells/well into 96-well plates. Cells were cultured at 37°C with 5% CO2 for 5 days. Subsequently, cells were counted on a daily basis using a Celigo® Cell Imaging Cytometer (Nexcelom Bioscience, Boston, MA, USA). At least 800 cells/well were analyzed for each experiment, and per-sample counting was performed in triplicate.
In addition, cell proliferation was evaluated by MTT assay as described previously.Citation21 Briefly, at 1, 2, 3, 4, or 5 days postinfection, cells were incubated with 20 µL of MTT reagent (5 mg/mL; Gen-View Scientific Inc, Talla-hassee, FL, USA) for 4 hours. Supernatants were removed by centrifugating at 1,800 rpm for 10 minutes at room temperature, and then the formazan product was dissolved using 150 µL/well of dimethyl sulfoxide (Sangon Biotech) for 10 minutes. The absorbance was read at 490 nm with ELx800 Absorbance Microplate Reader (BioTek Instruments, Winooski, VT, USA). All experiments were performed in triplicate.
Apoptosis assay
For the detection of apoptotic cells, an Annexin V-APC apoptosis detection kit (eBioscience, San Diego, CA, USA) was used. Briefly, U-2OS cells were infected with shEIF3C or shCtrl lentivirus, incubated for 96 hours, collected, and washed with PBS. The cells were adjusted to a final density of 1×106/mL in staining buffer, and 100 µL of the cell suspension was incubated with 5 µL APC-conjugated Annexin V for 15 minutes at room temperature in the dark. Cell apoptosis was analyzed by flow cytometry using an FACS Calibur (Becton-Dickinson, Franklin Lakes, NJ, USA). All experiments were performed in triplicate.
Colony formation assay
U-2OS cells transfected with shEIF3C or shCtrl lentivirus were cultured for 2 days until the logarithmic phase was reached. Subsequently, cells were reseeded at a density of 600 cells/well in six-well plates in triplicate and cultured for 2 weeks at 37°C with 5% CO2. Cells were fixed for 60 minutes in 4% paraformaldehyde at room temperature and stained with Giemsa stain (Dingguo Biotech, Shanghai, China) for 20 minutes. After rinsing with distilled H2O, images were taken using a fluorescence microscope (Olympus XI-71) and cell colonies were counted.
Western blotting analysis
To evaluate shEIF3C knockdown efficiency in U-2OS cells, Western blot analysis was employed to determine the levels of EIF3C expression. Briefly, U-2OS cells transfected with shEIF3C or shCtrl lentivirus were cultured for 96 hours, collected, and lysed in ice-cold lysis buffer (Sangon Biotech). Cell lysates were centrifuged at 12,000 rpm for 10 minutes at 4°C, and supernatants were harvested. Protein concentration was determined using the BCA protein assay kit (Sangon Biotech). For each sample, equal amounts of total protein were separated by SDS-PAGE using a 12.5% polyacrylamide gel and transferred to a polyvinylidene fluoride membrane (Sangon Biotech). Membranes were blocked with 5% skimmed milk in Tris-buffered saline containing Tween-20 for 1 hour and incubated overnight at 4°C with anti-EIF3C mouse antibody (catalog no E6283; Sigma-Aldrich Co., St Louis, MO, USA) or anti-GAPDH (catalog no sc-32233; Santa Cruz Biotechnology Inc., Dallas, TX, USA) at 1:2,000 dilution. Membranes were incubated using horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (catalog no sc-2005; Santa Cruz Biotechnology) and visualized using an enhanced chemiluminescence (ECL) reagent (catalog no 32106, Pierce™ ECL Western Blotting Substrate; Pierce Biotech, Inc., Rockford, IL, USA). Gray values of bands were measured using ImageJ software.
PathScan analysis
PathScan analysis was performed using the PathScan® stress and apoptosis signaling antibody array kit (catalog no 12923; Cell Signaling Technology, Danvers, MA, USA) according to the manufacturer’s instructions. Briefly, cells transfected with shEIF3C or shCtrl lentivirus were lysed with 1X Cell Lysis Buffer (catalog no 7018; Cell Signaling Technology). After collection of whole-cell lysates, protein concentration was determined using a BCA protein assay kit (Sangon Biotech). Proteins, at equal concentration, were analyzed by a PathScan sandwich ELISA kit according to the manufacturer’s instructions. The array target map used can be found at the manufacturer’s homepage (http://www.cst-c.com.cn/products/12856. html). Slides were imaged using a ChemiScope5300 Pro Integrated chemiluminescence imaging system (CLiNX Science Instruments, Shanghai, China).
Statistical analyses
Data were analyzed using SPSS software version 16.0, and all experiments were performed in triplicate. Differences between groups were analyzed using Student’s t-test. Categorical data were evaluated by chi-squared test. P<0.05 was considered statistically significant.
Results
Relationship between EIF3C expression and clinicopathological characteristics of patients with OsC
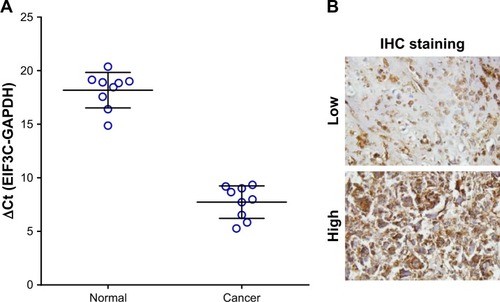
High EIF3C mRNA expression was measured in OsC tissues (), and high EIF3C immunoreactivity was also observed in cancerous tissues with 70% of tumors expressing EIF3C ( and ). However, there was no significant relationship between EIF3C expression and clinicopathological features of patients enrolled in our study, including age, pathological grade, TNM stage, and lymph node metastasis ().
Table 1 Relationship between EIF3C expression and clinico-pathological characteristics of patients with osteosarcoma
Figure 1 Expression of EIF3C in human OsC tissues.
Notes: (A) EIF3C expression levels in OsC tissue were measured by qPCR. (B) EIF3C expression levels in OsC tissue were measured by IHC (original magnification, ×200).
Abbreviations: IHC, immunohistochemistry; OsC, osteosarcoma; qPCR, quantitative real-time PCR.
Lentivirus-mediated siRNA efficiently inhibits EIF3C expression in human OsC U-2OS cells
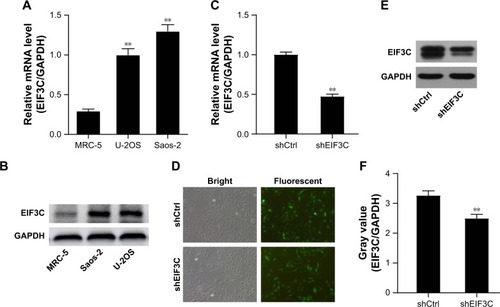
In malignant human OsC cell lines, U-2OS and Saos-2, high expression levels of EIF3C were detected by qPCR () and verified by Western blotting (). To evaluate the effect of knocking down EIF3C expression using lentivirus-mediated siRNA, the OsC cell line U-2OS was transfected with shEIF3C or shCtrl lentivirus. Twenty-four hours after transfecting with shEIF3C or shCtrl lentivirus, cell proliferation was found to be unaffected (). EIF3C mRNA levels were determined using qPCR analysis, which demonstrated that U-2OS cells treated with shEIF3C lentivirus showed an ~52.7% reduction in EIF3C mRNA expression (). Western blot analysis was consistent with the mRNA data, and quantification of the protein bands also showed that shEIF3C lentivirus-treated cells had reduced EIF3C protein levels (). Together, these data demonstrated that EIF3C expression levels were successfully downregulated by shEIF3C lentivirus infection at both the mRNA and protein levels.
Figure 2 Expression of EIF3C in human MRC-5 cells and malignant OsC cell lines and EIF3C knockdown in human malignant OsC cell line U-2OS.
Notes: (A) Expression of EIF3C in human malignant OsC cell lines U-2OS and Saos-2 (**P<0.01). (B) Expression of EIF3C in human fibroblast MRC-5 cells and OsC cell lines U-2OS and Saos-2 analyzed by Western blotting. (C) Successful knockdown of EIF3C at the mRNA level was achieved in U-2OS cells transfected with shEIF3C or shCtrl lentivirus, and the expression was normalized to GAPDH mRNA. Data shown are the mean ± SD of three independent experiments (**P<0.01). (D) Cell proliferation was unaffected after transfection of U-2OS cells with shEIF3C or shCtrl lentivirus. (E) Western blot analysis indicating successful knockdown of EIF3C in U-2OS cells at the protein level after transfection with shEIF3C or shCtrl lentivirus. (F) Gray values of EIF3C relative to GAPDH protein levels were measured from three independent experiments using ImageJ software (**P<0.01).
Abbreviation: OsC, osteosarcoma.
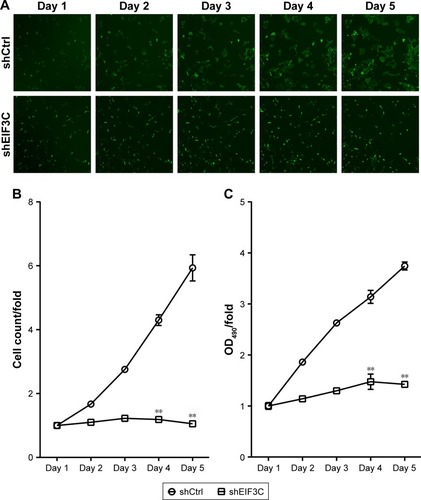
EIF3C knockdown suppresses U-2OS proliferation
To evaluate the role of EIF3C in the proliferation, U-2OS cells were transfected with shEIF3C or shCtrl lentivirus and cell count was continuously monitored over a 5-day period. In cells transfected with shEIF3C, statistically significant proliferation inhibition was observed after 96–120 hours when compared to cells transfected with shCtrl (P<0.01; ). To verify the suppressive effect of shEIF3C knockdown on the proliferation of U-2OS cells, an MTT assay was performed and the OD490/fold ratio was determined 24 hours after plating of the cells. Consistent with the cell counting results, the OD490/fold ratio after 4 and 5 days of proliferation was significantly reduced in EIF3C-knocked down U-2OS cells when compared to controls ().
Figure 3 Knockdown of EIF3C expression inhibits proliferation of human malignant osteosarcoma cell line U-2OS.
Notes: (A) Representative images of U-2OS cells transfected with shEIF3C or shCtrl lentivirus at various time points after the start of lentivirus transfection. (B) Proliferation of U-2OS cells was evaluated by a Celigo® Cell Imaging Cytometer. Data are shown as the fold-change of cell numbers and are expressed as the mean ± SD (**P<0.01). (C) Determination of proliferation of U-2OS cells treated with shEIF3C by MTT assay. The ratio is represented as fold-change of absorbance at 490 nm (OD490/fold). The data represent the mean ± SD (**P<0.01).
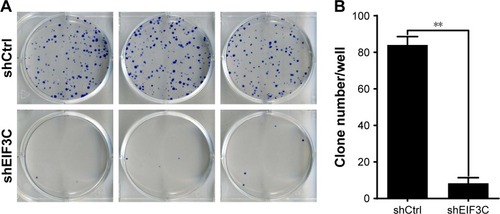
EIF3C knockdown decreases the ability of U-2OS cells to form colonies
To evaluate the colony formation ability of U-2OS cells after EIF3C knockdown, a colony formation assay was performed. The data showed that a reduction in the expression of EIF3C in U-2OS cells significantly impaired the colony formation ability, resulting in añ90.5% reduction in colony formation. An average of 84 colonies was present on the plates of cells that were transfected with shCtrl lentivirus, whereas only eight clones were found on the plates of cells transfected with shEIF3C lentivirus ().
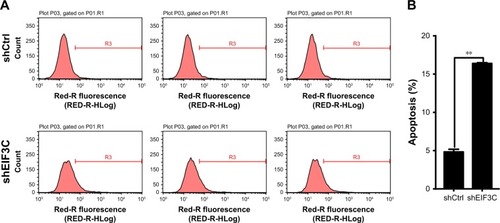
Knockdown of EIF3C in U-2OS cells promotes apoptosis
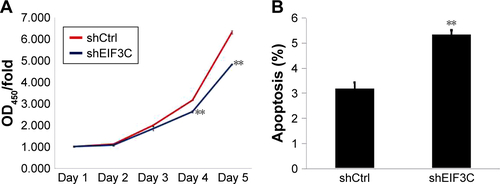
To evaluate whether or not EIF3C expression affects apop-tosis, we used the lentivirus approach and successfully knocked down EIF3C in U-2OS cells. Annexin V-APC staining was used to evaluate cell apoptosis. Apoptosis was observed in 16.39% of U-2OS cells treated with shEIF3C lentivirus; however, in U-2OS cells infected with shCtrl lentivirus, only 4.83% of the cells were apoptotic (). These results suggest that EIF3C knockdown induces apoptosis in U-2OS cells. Meanwhile, we found the EIF3C knockdown in human fibroblast MRC-5 cells also triggered enhanced apoptosis and decreased proliferation, while the proliferation inhibition and apoptosis rates were lower than that in cancerous cells after EIF3C knockdown (Figure S1).
Figure 5 EIF3C knockdown induces apoptosis in U-2OS cells.
Notes: (A) The degree of apoptosis was determined by Annexin V staining and analyzed by flow cytometry. Experiments were performed in triplicate. (B) Quantification of apoptosis rate. Bar graphs indicate the mean ± SD of the percentage of apoptosis from three separate experiments (**P<0.01).
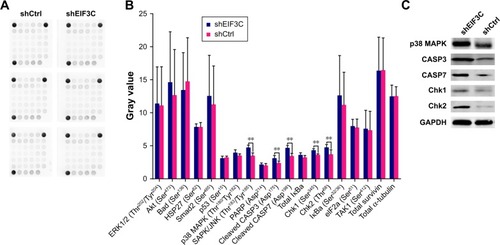
Silencing of EIF3C in U-2OS cells alters profiles of signaling molecules involved in stress and apoptosis
To investigate the signaling pathways in EIF3C-silenced U-2OS cells, we evaluated the profiles of signaling molecules involved in stress and apoptosis using a PathScan stress and apoptosis signaling antibody array kit. Using this kit, 19 signaling molecules involved in the regulation of stress response and apoptosis could be evaluated simultaneously (). The data showed that, compared to shCtrl-treated U-2OS cells, five signaling molecules were significantly enhanced in shEIF3C-treated U-2OS cells, including SAPK/JNK (Thr183/Tyr185), cleaved CASP3 (Asp175), cleaved CASP7 (Asp198 Chk1 (Ser345), and Chk1 (Thr68) (). All differentially expressed molecules were also verified by Western blotting ().
Discussion
Previous studies have implicated a role of the EIF3 complex in functions other than translation and outside its general accepted role as a protein scaffold for the formation of initiation complexes.Citation22 Aberrant EIF3 expression can result in translational dysregulation, which may lead to disease states, including tumorigenesis.Citation23 It has been shown that mutated or inactivated EIF3 subunits are related to developmental defects.Citation24,Citation25 In addition, overexpression of EIF3 is associated with diverse types of cancer, including breast, prostate, and esophageal malignancies.Citation12,Citation23 Studies have demonstrated that in tumor types such as seminoma,Citation13 meningioma,Citation14 colon cancer,Citation15 and glioma,Citation16,Citation17 expression of the EIF3C subunit of the EIF3 complex is upregulated. Recently, Lee et al demonstrated that overexpression of EIF3C augmented exosome secretion and promoted tumorigenesis of human hepatocellular carcinoma.Citation18 These findings imply that EIF3C is closely related to human tumorigenesis;Citation12,Citation17 however, the role of EIF3C in human OsC has not yet been identified.
In the present study, we first detected the levels of EIF3C mRNA by qPCR and protein by IHC in human OsC tissues. Our data showed that the levels of EIF3C expression in cancer tissues were higher than those in normal tissues. Unfortunately, high EIF3C expression in cancer tissues was irrelevant to the clinicopathological characteristics of patients enrolled in our study, including age, pathological grade, TNM stage, and lymph node metastasis.
We then evaluated the expression levels of EIF3C mRNA in two different OsC cell lines and found that EIF3C was highly expressed in both cell lines. To assess the role of EIF3C in the OsC cell line U-2OS, we constructed the EIF3C-siRNA lentiviral vector to efficiently silence EIF3C in U-2OS cells. We found that EIF3C knockdown decreased both proliferation and the ability of U-2OS cells to form colonies. In addition, we verified that silencing EIF3C can induce apoptosis of U-2OS cells.
In eukaryotes, translational regulation is a fundamental process that is responsible for controlling cell development, homeostasis, and stress responses.Citation26 Deregulation of translational control results in initiation and progression of cancer.Citation27,Citation28 A disrupted tumor suppressor mechanism causes apoptosis, which is a frequent event seen in cancer and contributes to tumorigenesis.Citation29 Activation of key oncogenic pathways may alter the overall profiles of increased protein synthesis and specific regulatory networks for cellular transformation.Citation28 As a major controller of translation initiation in protein synthesis, alteration of components in the EIF3C complex such as EIF3C leads to acceleration of protein synthesis involved in tumorigenesis and progression.Citation30 Although EIF3C has been indicated as an important molecule, its mechanism of action in human OsC remains unclear.
To investigate the effect of EIF3C knockdown on shEIF3C-transfected U-2OS cells, we evaluated the stress and apoptosis profiles of various signaling molecules. The data showed that EIF3C knockdown significantly promoted the expression of SAPK/JNK (Thr183/Tyr185), cleaved CASP3 (Asp175), cleaved CASP7 (Asp198), Chk1 (Ser345), and Chk1 (Thr68). SAPKs, also known as JNKs, induce apoptosis by translocating to mitochondria with Bcl-xl interaction response to DNA damage.Citation31 As effector caspases, both CASP3 and CASP7 cleave important cellular substrates to accomplish apoptosis.Citation32 Both caspases are activated during Fas- and mitochondria-induced apoptosis.Citation33,Citation34 Downregulation of CASP3 or CASP7 is frequently seen in several forms of cancer.Citation35–Citation37 Inanami et alCitation38 demonstrated that apoptosis is induced by CASP3 activation, which is required for de novo protein synthesis and operates through SAPK/JNK activation. DNA damage due to failure of repair results in cellular senescence or cell death.Citation39 Phosphorylated Chk1/2 activates replication stress response, which is characterized by stalled replication forksCitation40 and contributes to cancer cells senescence.Citation41 The present study indicates that the increased apoptosis rate of U-2OS cells due to EIF3C knockdown may result in increased activation of SAPK/JNK, which is responsible for accelerating protein synthesis of CASP3/7 and Chk1/2. However, the detailed mechanism of action of pro-apoptosis due to EIF3C knockdown is currently unclear and requires further investigation.
Conclusion
Our study investigated the role of EIF3C in tumorigenesis in vitro. We found that EIF3C is involved in OsC proliferation and survival. Moreover, the inhibitory role of EIF3C knockdown in OsC proliferation was validated by a PathS-can stress and apoptosis signaling antibody array kit, which suggested that the effect of EIF3C knockdown on tumor proliferation and apoptosis may involve the upregulation of SAPK/JNK, which promotes apoptosis by increasing the protein synthesis of CASP3/7 and Chk1/2.
Acknowledgments
This work was funded by Natural Science Foundation of Anhui Province (1608085QH204) and Youth Science Foundation Training Program of The First Affiliated Hospital of Anhui Medical University (2016KJ04).
Supplementary material
Figure S1 Knockdown of EIF3C expression inhibits proliferation and induces apoptosis in MRC-5 fibroblast cells.
Notes: (A) Determination of proliferation of MRC-5 cells treated with shEIF3C by CCK-8 assay. At 1, 2, 3, 4, or 5 days postinfection, cells were incubated with 10% CCK-8 solution for 2 hours. The ratio is represented as fold-change of absorbance at 450 nm (OD450/fold). The data represent the mean ± SD (**P<0.01). (B) Quantification of apoptosis rate. Bar graphs indicate the mean ± SD of the percentage of apoptosis from three independent experiments (**P<0.01).
Abbreviation: CCK-8, Cell Counting Kit-8.
Disclosure
The authors report no conflicts of interest in this work.
References
- AndoKHeymannM-FStresingVMoriKRédiniFHeymannDCurrent therapeutic strategies and novel approaches in osteosarcomaCancers (Basel)20135459161624216993
- ClarkJCDassCRChoongPFA review of clinical and molecular prognostic factors in osteosarcomaJ Cancer Res Clin Oncol2008134328129717965883
- SampoMKoivikkoMTaskinenMIncidence, epidemiology and treatment results of osteosarcoma in Finland – a nationwide population-based studyActa Oncol20115081206121422023116
- SiclariVAQinLTargeting the osteosarcoma cancer stem cellJ Orthop Surg Res2010517820979639
- MirabelloLTroisiRJSavageSAOsteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results programCancer200911571531154319197972
- QureshiAAhmadZAzamMIdreesREpidemiological data for common bone sarcomasAsian Pac J Cancer Prev201011239339520843122
- MeyersPASchwartzCLKrailoMDOsteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall Survival – A report from the children’s Oncology GroupJCO2008264633638
- ValásekLNielsenKHZhangFFeketeCAHinnebuschAGInteractions of eukaryotic translation initiation factor 3 (eIF3) subunit NIP1/c with eIF1 and eIF5 promote preinitiation complex assembly and regulate start codon selectionMol Cell Biol200424219437945515485912
- LeFebvreAKKorneevaNLTrutschlMTranslation initiation factor eIF4G-1 binds to eIF3 through the eIF3e subunitJ Biol Chem200628132229172293216766523
- EmmanuelRWeinsteinSLandesman-MiloDPeerDEIF3c: a potential therapeutic target for cancerCancer Lett2013336115816623623922
- ObayashiELunaRENagataTMolecular landscape of the ribosome pre-initiation complex during mRNA scanning: structural role for eIF3c and its control by eIF5Cell Rep201718112651266328297669
- ZhangLPanXHersheyJWIndividual overexpression of five subunits of human translation initiation factor eIF3 promotes malignant transformation of immortal fibroblast cellsJ Biol Chem200728285790580017170115
- RotheMKoYAlbersPWernertNEukaryotic initiation factor 3 p110 mRNA is overexpressed in testicular seminomasAm J Pathol200015751597160411073819
- ScolesDRYongWHQinYWawrowskyKPulstSMSchwannomin inhibits tumorigenesis through direct interaction with the eukaryotic initiation factor subunit C (eIF3c)Hum Mol Genet20061571059107016497727
- SongNWangYGuXDChenZYShiLBEffect of siRNA-mediated knockdown of eIF3c gene on survival of colon cancer cellsJ Zhejiang Univ Sci B201314645145923733421
- HaoJWangZWangYEukaryotic initiation factor 3C silencing inhibits cell proliferation and promotes apoptosis in human gliomaOncol Rep20153362954296225823503
- HaoJLiangCJiaoBEukaryotic translation initiation factor 3, subunit C is overexpressed and promotes cell proliferation in human glioma U-87 MG cellsOncol Lett2015962525253326137101
- LeeHYChenCKHoCMEIF3C-enhanced exosome secretion promotes angiogenesis and tumorigenesis of human hepatocellular carcinomaOncotarget2018917131931320529568350
- RadhikaKPrayagaAKEstrogen and progesterone hormone receptor status in breast carcinoma: comparison of immunocytochemistry and immunohistochemistryIndian J Cancer201047214815020448377
- LiuHLiangSYangXRNAi-mediated rpL34 knockdown suppresses the growth of human gastric cancer cellsOncol Rep20153452267227226323242
- MoodleySKoorbanallyNAMoodleyTRamjugernathDPillayMThe 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay is a rapid, cheap, screening test for the in vitro anti-tuberculous activity of chalconesJ Microbiol Methods2014104727824978593
- LeeASKranzuschPJCateJHEIF3 targets cell-proliferation messenger RNAs for translational activation or repressionNature2015522755411111425849773
- HersheyJWRegulation of protein synthesis and the role of eIF3 in cancerBraz J Med Biol Res2010431092093020922269
- ChoudhuriAMaitraUEvansTTranslation initiation factor eIF3h targets specific transcripts to polysomes during embryogenesisProc Natl Acad Sci U S A2013110249818982323716667
- CurranSPRuvkunGLifespan regulation by evolutionarily conserved genes essential for viabilityPLoS Genet200734e5617411345
- SilveraDFormentiSCSchneiderRJTranslational control in cancerNat Rev Cancer201010425426620332778
- StumpfCRRuggeroDThe cancerous translation apparatusCurr Opin Genet Dev201121447448321543223
- MavrakisKJWendelHGTranslational control and cancer therapyCell Cycle20087182791279418787409
- LoweSWCeperoEEvanGIntrinsic tumour suppressionNature2004432701530731515549092
- SilveraDArjuRDarvishianFEssential role for eIF4GI over-expression in the pathogenesis of inflammatory breast cancerNat Cell Biol200911790390819525934
- KharbandaSSaxenaSYoshidaKTranslocation of SAPK/ JNK to mitochondria and interaction with Bcl-x(L) in response to DNA damageJ Biol Chem2000275132232710617621
- LiJYuanJCaspases in apoptosis and beyondOncogene200827486194620618931687
- HirataHTakahashiAKobayashiSCaspases are activated in a branched protease cascade and control distinct downstream processes in Fas-induced apoptosisJ Exp Med199818745876009463409
- SleeEAHarteMTKluckRMOrdering the cytochrome c–initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a Caspase-9–dependent mannerJ Cell Biol199914422812929922454
- VolmMMatternJKoomägiRInverse correlation between apoptotic (Fas ligand, caspase-3) and angiogenic factors (VEGF, microves-sel density) in squamous cell lung carcinomasAnticancer Res1999193A1669167110470099
- PalmeriniFDevilardEJarryABirgFXerriLCaspase 7 down-regulation as an immunohistochemical marker of colonic carcinomaHum Pathol200132546146711381362
- SoungYHLeeJWKimHSInactivating mutations of caspase-7 gene in human cancersOncogene200322398048805212970753
- InanamiOTakahashiKKuwabaraMAttenuation of caspase-3-dependent apoptosis by Trolox post-treatment of x-irradiated MOLT-4 cellsInt J Radiation Biol1999752155163
- ZhouBBElledgeSJThe DNA damage response: putting checkpoints in perspectiveNature2000408681143343911100718
- SmithJThoLMXuNGillespieDAThe ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancerAdv Cancer Res20101087311221034966
- LeeHKimYJeongJHRyuJHKimWYATM/CHK/p53 pathway dependent chemopreventive and therapeutic activity on lung cancer by pterostilbenePLoS One2016119e016233527612029