Abstract
Background
Apatinib is a newly approved antitumor drug (molecular targeted agent/small molecular kinase inhibitor) for advanced hepatocellular carcinoma (HCC) treatment. However, current oral administration of apatinib could induce the even distribution of drugs in the body and cause the concentration of apatinib in the HCC location to be limited or insufficient. Therefore, it is urgent to develop novel formulations of apatinib to improve its efficiency.
Materials and methods
Apatinib was prepared to form a stable and even dispersion with cyclodextrin (a clathrate complex/inclusion complex named Apa-Cyc). A temperature-sensitive phase-change hydrogel of apatinib (named Apa-Gel) was prepared using apatinib–cyclodextrin and poloxamer 407. Apa-Gel was injected into HCC tissues in nude mice to examine the long-term antitumor effect.
Results
Apa-Gel can transform from liquid to hydrogel at near body temperature and maintain slow release of apatinib in HCC tumor tissues. When injected subcutaneously, one-time administration of Apa-Gel has a long-acting antitumor effect on the subcutaneous growth or epithelial–mesenchymal transition process of HCC cells.
Conclusion
This novel slow-releasing system allows apatinib to be released effectively on the long term and facilitates tissue attachment, thereby preserving the efficiency of apatinib over the long term.
Introduction
Oral administration of molecular targeted agents (multitarget protein kinase inhibitors) continues to be the primary choice for advanced hepatocellular carcinoma (HCC) chemotherapeutic treatment (antitumor agent treatment).Citation1–Citation5 Sorafenib (BAY 43-9006) was the first approved molecular targeted agent and remains one of the foremost choices for advanced HCC treatment.Citation6,Citation7 Recently, some other molecular targeted agents, eg, apatinib or regorafenib (BAY 73-4506), were approved for the treatment of advanced HCC.Citation8–Citation10 Application of molecular targeted agents could prolong the survival and improve the life quality of patients suffering from advanced HCC.Citation8–Citation10 However, current oral administration of these drugs is still not satisfactory. Oral administration of these drugs could induce the even distribution of drugs in the entire body and cause the concentration of drugs in the HCC location to be limited or insufficient. Moreover, high daily doses (over 800 mg) result in a range of side effects and heavy financial burden.Citation11 Our aim is to develop novel approaches for increasing the efficacy and safety of molecular targeted agents.
Apatinib is a newly approved antitumor drug (molecular targeted agent/small molecular kinase inhibitor) for advanced HCC treatment.Citation12 As a result of the structure of apatinib, it is hydrophilic in nature but not easily soluble. The current strategy is to prepare apatinib as apatinib mesylate (AiTan®, apatinib mesylate tablets).Citation13 Although preparation of apatinib as apatinib mesylate tablets could possibly improve the solubility of apatinib, ionized apatinib (positive ion) may not improve transmembrane conductance in the human gastrointestinal system after oral administration. Therefore, it is valuable to develop novel pharmaceutical formulations for apatinib. In this work, apatinib was prepared to achieve a stable and even dispersion with cyclodextrin. A temperature-sensitive phase-change hydrogel (Apa-Gel) was prepared using apatinib–cyclodextrin and poloxamer 407. Apa-Gel was injected into HCC tissues in nude mice.
Materials and methods
Cell culture and agents
MHCC97-H cells (a highly aggressive HCC cell line) were purchased from Type Culture Collection of the Chinese Academy of Sciences (Shanghai, People’s Republic of China), an organization possessing typical biological samples of the Chinese government. The protocol, methods, and usage of cell line in cell-based experiments were approved by the Ethics Committee, Henan Medical College. MHCC97-H cells were cultured in DMEM (Thermo Fisher Scientific Corporation, Waltham, MA, USA) with 10% FBS (Thermo Fisher Scientific Corporation) in 5% CO2 at 37°C. Apatinib mesylate (Cat. No S2221) was purchased from Selleck Corporation (Houston, TX, USA). Cyclodextrin was purchased from Sinopharm Chemical Reagent Beijing Corporation (Beijing, People’s Republic of China).
Preparation of apatinib formulations
Apatinib mesylate was first prepared into apatinib. For the apatinib suspension preparation (named Control), 20 mg of apatinib was simply mixed with 10 mL of double-distilled H2O (ddH2O). For the apatinib solution preparation, 20 mg of apatinib was dissolved in a solution of 100 µL dimethyl sulfoxide, 200 µL polyethylene glycol 400 (PEG 400), and 200 µL Tween 80. Next, the solutions were carefully and slowly diluted by PBS to a total volume of 10 mL, accompanied with ultrasonic or churning treatment. The apatinib concentration in the diluted solution (named Apa-Sol) was ~2 mg/mL. For the apatinib–cyclodextrin clathrate complex/inclusion complex preparation, apatinib and cyclodextrin were mixed to form an apatinib–cyclodextrin inclusion complex (named Apa-Cyc), following the methods provided by Giglio et al and Kim et al.Citation14,Citation15 Next, these formulations were scanned by a multifunctional microplate reader to examine the OD values under a series of wavelengths. For temperature-sensitive phase-change hydrogel of apatinib preparation, poloxamer 407 (FREDA Corporation, Jinan City, People’s Republic of China) was added to the apatinib– cyclodextrin inclusion complex formulation (Apa-Cyc). Moreover, these formulations were extracted with acetonitrile (ACN), and the apatinib in each formulation was examined by liquid chromatography mass spectrometry/mass spectrometry (LC-MS/MS) methods as described by Feng et al, Feng et al, and Xie et al.Citation16–Citation18
Releasing or clearance of apatinib
All animal experiment protocols were approved by the Institutional Animal Care and Use Committee of Henan Medical College. All animal studies were carried out in accordance with the UK Animals (Scientific Procedures) Act of 1986 and associated guidelines. MHCC97-H cells were cultured and seeded into nude mice to prepare the subcutaneous tumor model. When the tumor volumes reached 1,200–1,500 mm3, the apatinib formulations (Solvent Control, Apa-Cyc, Apa-Gel, and Apa-Sol) were injected into subcutaneous tumors formed by MHCC97-H cells. Total volumes of formulations were 50 µL for each tumor, and formulations were administered once by intratumor injection. At the indicated time points, mice were harvested, and tumor tissues were collected. Collected tumor tissues were weighed and lysated, and apatinib sustaining in tumor tissues was extracted with ACN for LC-MS/MS examination. The LC-MS/MS experiments were performed, and the sustaining curves of apatinib in HCC tumor tissues were obtained through calculations following the methods provided by Xie et al, Feng et al, Li et al, and Wu et al.Citation18–Citation21
In vivo antitumor effects of apatinib
MHCC97-H cells were cultured and injected into nude mice (5×106 cells per animal) to form subcutaneous tumor models.Citation22,Citation23 To examine the antitumor effect of oral administration of apatinib mesylate suspension to mimic the apatinib clinical application or tail vein injection of Apa-Cyc prepared in this present work, nude mice 4–6 weeks of age were purchased from Si-Bei-Fu Corporation (Beijing, People’s Republic of China). Four to five days after MHCC97-H cells were injected into nude mice, the mice received oral administration of the indicated dose of apatinib mesylate to mimic the clinical application of apatinib or tail vein injection of the indicated dose of Apa-Cyc every 2 days. After 25 days of treatment (12 treatments), tumors were harvested. Tumor weight was measured by precision balances, and tumor volumes were measured as length × width × width/2.
Next, to examine the long-acting antitumor effect of Apa-Gel on HCC cells’ subcutaneous growth, MHCC97-H cells were injected into nude mice to form subcutaneous tumors. After 1–2 weeks of growth, when tumors reached 1,000–1,200 mm3 volume, mice were randomly divided into four groups as follows: 1) solvent control group (mice with intratumors injected with 50 µL solvent control); 2) mice with intratumors injected with 50 µL Apa-Sol; 3) mice with intratumors injected with 50 µL Apa-Cyc; and 4) mice with intratumors injected with 50 µL Apa-Gel. After 14–20 days of growth, tumors were harvested. Tumor weight was measured by precision balances and tumor volumes were measured as length × width × width/2.Citation24,Citation25
Quantitative polymerase chain reactions
MHCC97-H cells were injected into nude mice to form subcutaneous tumors. After 1–2 weeks of growth, when tumors reached 1,000–1,200 mm3 volume, the mice were randomly divided into four groups as follows: 1) untreated group; 2) mice with intratumors injected with Apa-Sol; 3) mice with intratumors injected with Apa-Cyc; and 4) mice with intratumors injected with Apa-Gel. After 14–20 days growth, tumors were harvested, and total RNA samples were extracted with the PARISTM Kit (Applied Biosystems, Foster City, CA, USA) and reverse transcribed to cDNA with a Multiscribe™ Reverse Transcriptase Kit (Applied Biosystems) according to the manufacturer’s instructions for quantitative polymerase chain reactions (qPCRs). The mRNA levels of E-cadherin, N-cadherin, and vimentin were examined by qPCR, referring to the methods described by Kang et al.Citation26 The sequences of the primers used are presented in .
Table 1 The primers used in this work
Statistical analysis
Statistical analysis was carried out using Bonferroni’s correction with or without two-way analysis of variance using SPSS Statistics software (IBM Corporation, Armonk, NY, USA). The half-life (t1/2 value) of apatinib in MHCC97-H was calculated with Origin software (Version No 6.1; OriginLab Corporation, Northampton, MA, USA). A P-value <0.05 was considered statistically significant between groups.
Results
Preparation of apatinib formulations
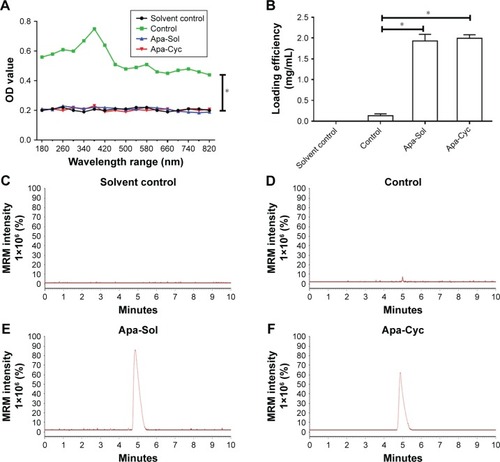
To reveal the characteristics of the apatinib formulations, the samples were examined by either multifunctional microplate reader or LC-MS/MS methods. As shown in , apatinib simply mixed in ddH2O was a suspension containing tiny drug particles and had much higher absorbance value measured under a series of wavelengths than the Solvent Control group, Control group, Apa-Sol group, or Apa-Cyc group. Next, the apatinib suspension (Control group) was filtered with a 0.22 µm pore size filter to extract undissolved apatinib particles and used as the control. The concentrations of apatinib in the formulations were determined. As shown in , the concentrations of apatinib in Apa-Sol and Apa-Cyc were 1.93±0.16 and 1.99±0.08 mg/mL, respectively, and after multiple filtrations (0.22 µm aperture), apatinib sustaining in the apatinib suspension was found to be rare (0.03±0.02 mg/mL). The results identifying apatinib in formulations from LC-MS/MS are shown in . Therefore, we successfully prepared the formulations of apatinib and determined the features and apatinib concentrations in the formulations.
Table 2 The loading efficiency of apatinib formulations
Figure 1 Preparation of apatinib formulations.
Abbreviations: ACN, acetonitrile; Apa-Cyc, apatinib–cyclodextrin inclusion complex; Apa-Sol, apatinib solution; ddH2O, double-distilled H2O; LC-MS/MS, liquid chromatography mass spectrometry/mass spectrometry; MRM, multi reaction monitoring.
The antitumor effect of apatinib formulations
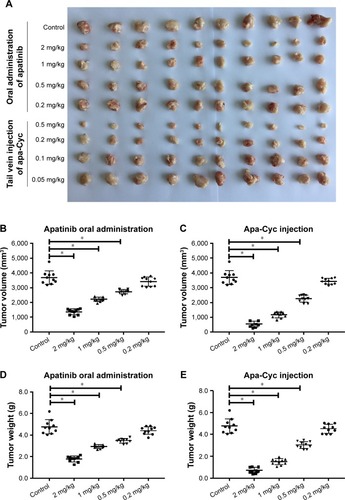
To reveal whether Apa-Cyc could be used in HCC treatment, MHCC97-H cells were seeded into nude mice to form subcutaneous tumors. Mice received apatinib treatment by 1) oral administration of apatinib mesylate to mimic the clinical application of apatinib or 2) injection of Apa-Cyc through the tail vein. Results are shown in . Oral administration of a 2 mg/kg dose of apatinib significantly reduced the tumor volumes or tumor weights of MHCC97-H cells. The antitumor effects of 0.5 and 1.0 mg/kg doses of apatinib were weaker than that of the 2 mg/kg dose (). Oral administration of a 0.2 mg/kg dose of apatinib did not significantly inhibit the subcutaneous growth of MHCC97-H cells in nude mice (). Injection of 0.5, 0.2, and 0.1 mg/kg doses of Apa-Cyc through the tail vein inhibited the subcutaneous growth of MHCC97-H cells in nude mice (). The antitumor effects of 0.1 and 0.2 mg/kg doses of Apa-Cyc were much lower than those of the 0.5 mg/kg dose of Apa-Cyc, and injection of a 0.05 mg/kg dose of Apa-Cyc did not significantly inhibit the subcutaneous growth of MHCC97-H cells in nude mice (). Moreover, the antitumor effect of the injection of a 0.5 mg/kg dose of Apa-Cyc was more effective than the oral administration of a 2 mg/kg dose of apatinib. The inhibition rate of the oral administration of a 2 mg/kg dose of apatinib on the subcutaneous growth of MHCC97-H cells in nude mice was 64.19%±7.22%, whereas the inhibition rate of the injection of a 0.5 mg/kg dose of Apa-Cyc on subcutaneous growth of MHCC97-H cells in nude mice was 87.93%±3.54%. Therefore, the formulation of Apa-Cyc not only achieved injective administration of apatinib but also improved the efficiency of apatinib treatment. Also, the injection of a smaller concentration of Apa-Cyc achieved a therapeutic effect similar to that of the oral administration of a much higher dose of the apatinib.
Figure 2 Antitumor effect of apatinib formulations on the subcutaneous growth of MHCC97-H cells.
Abbreviation: Apa-Cyc, apatinib–cyclodextrin inclusion complex.
Intratumor injection of Apa-Gel and the long-term sustaining of apatinib in HCC tumor tissues
As mentioned above, we successfully prepared a cyclodextrin inclusion complex of apatinib, which enabled to inject apatinib far more effectively for treating advanced HCC. Next, a temperature-sensitive phase-change hydrogel of apatinib (named Apa-Gel) was prepared by using apatinib–cyclodextrin and poloxamer 407. As shown in , Apa-Gel achieved phase-change features and transformed from liquid to hydro-gel. The phase-transition temperature of Apa-gel containing 7.5%, 10%, 12.5%, 15%, 17.5%, and 20% poloxamer 407 was 41.38°C±0.18°C, 39.21°C±0.44°C, 37.05°C±0.22°C, 32.11°C±0.27°C, 25.50°C±0.33°C, and 19.83°C±0.54°C, respectively. Therefore, the phase-transition temperature of Apa-Gel which contains 12.5% poloxamer 407 was nearest to body temperature (37.05°C±0.22°C), so we chose 12.5% poloxamer 407 for further steps of the experiment.
Table 3 Phase-transition temperature of Apa-Gel containing different poloxamer 407 concentrations
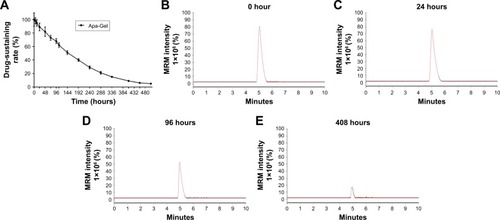
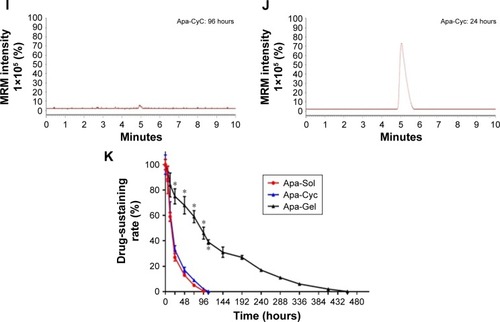
To identify whether Apa-Gel could achieve the long-term sustaining of apatinib, the in vitro release of Apa-Gel was determined by LC-MS/MS experiments. As shown in , Apa-Gel achieved the slow-releasing of apatinib. Apatinib could sustain in Apa-Gel for more than 400 hours. The t1/2 value of apatinib releasing from Apa-Gel was 164.57±7.82 hours. Therefore, the formulation of Apa-Gel achieved long-term releasing of apatinib.
Figure 3 In vitro release of Apa-Gel.
Abbreviations: ACN, acetonitrile; Apa-Gel, a temperature-sensitive phase-change hydrogel of apatinib; ddH2O, double-distilled H2O; LC-MS/MS, liquid chromatograph mass spectrometry/mass spectrometry; MRM, multi reaction monitoring.
Intratumor injection of Apa-Gel and the long-acting antitumor effect of apatinib
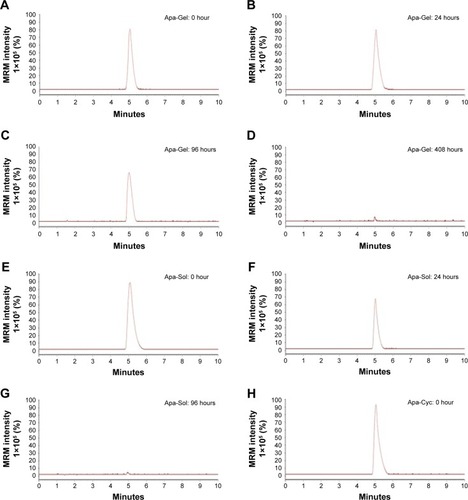
To further examine the long-sustaining feature of Apa-Gel, MHCC97-H cells were seeded into nude mice to form subcutaneous tumors, and apatinib formulations were injected into the subcutaneous tumors. Next, tumor tissues were harvested at indicated time points for LC-MS/MS analysis to examine the sustaining of apatinib in tumor tissues. As shown in and , after Apa-Sol and Apa-Cyc injections, apatinib was almost completely cleared from the tumor tissues at 48 hours. The t1/2 values of apatinib in the Apa-Sol and Apa-Cyc groups were 16.55±1.66 and 18.65±2.07 hours, respectively. Compared to Apa-Sol and Apa-Cyc, injections of Apa-Gel achieved long-term sustaining in tumor tissues (t1/2 value was 99.16±7.67 hours), and apatinib could still be detected in tumor tissues at the 408-hour time point.
Table 4 Half-life of apatinib in HCC tissues injected with apatinib formulations
Figure 4 In vivo release of apatinib formulations in HCC tumor tissues.
Abbreviations: ACN, acetonitrile; Apa-Gel, a temperature-sensitive phase-change hydrogel of apatinib; Apa-Sol, apatinib solution; Apa-Cyc, apatinib–cyclodextrin inclusion complex; LC-MS/MS, liquid chromatography mass spectrometry/mass spectometry.
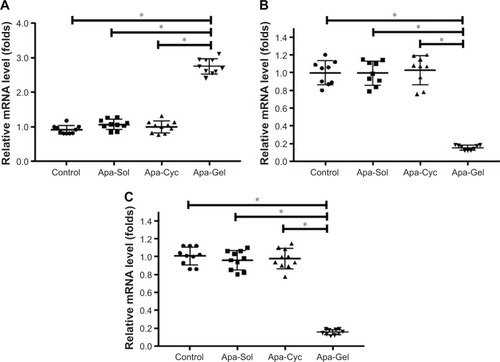
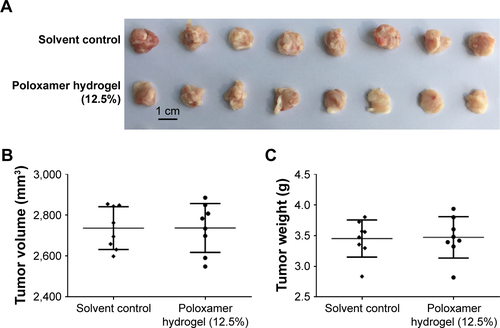
To further examine the potential application of Apa-Gel in HCC treatment, we examined whether Apa-Gel could exert a long-term antitumor effect on HCC tissues. Polox-amer hydrogel itself did not inhibit the subcutaneous growth of MHCC-97H cells in nude mice (). Results are shown in . One intratumor injection of Apa-Gel, but not Apa-Sol or Apa-Cyc, inhibited the subcutaneous growth of MHCC97-H cells in nude mice compared with that in the untreated control group. Moreover, to further examine the antitumor effect of Apa-Gel on MHCC97-H cells, qPCR experiments were performed. As shown in , one administration of Apa-Gel, but not Apa-Sol or Apa-Cyc, significantly enhanced the mRNA level of E-cadherin, an epithelial indicator () and decreased the mRNA level of N-cadherin and vimentin, both of which are mesenchymal indicators (). Therefore, intratumor injection of Apa-Gel exerted a long-acting antitumor effect on HCC tumors and inhibited activation of the epithelial– mesenchymal transition process of MHCC97-H cells in subcutaneous tumors.
Figure 5 Long-acting antitumor effect of Apa-Gel formulations on the subcutaneous growth of MHCC97-H cells.
Abbreviations: Apa-Gel, a temperature-sensitive phase-change hydrogel of apatinib; Apa-Sol, apatinib solution; Apa-Cyc, apatinib–cyclodextrin inclusion complex.
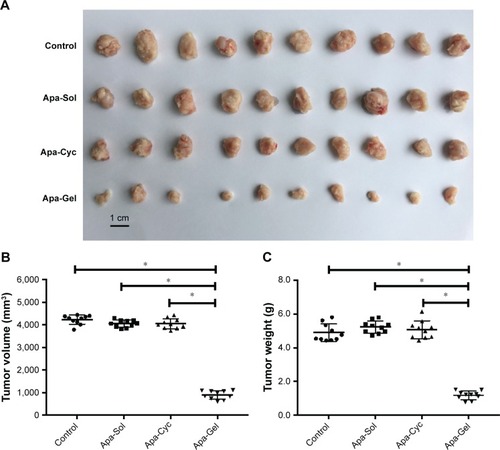
Figure 6 Long-acting antitumor effect of Apa-Gel formulations on the EMT process of MHCC97-H cells.
Abbreviations: Apa-Cyc, apatinib–cyclodextrin inclusion complex; Apa-Gel, a temperature-sensitive phase-change hydrogel of apatinib; Apa-Sol, apatinib solution; EMT, epithelial–mesenchymal transition; qPCR, quantitative polymerase chain reaction.
Discussion
Despite many achievements in research related to advanced HCC treatment in recent years, there are limited options for drug treatment in advanced HCC.Citation27 Sorafenib, the molecular targeted agent/small molecular protein kinase inhibitor, remains the only first-line choice for advanced HCC treatment.Citation28 Although sorafenib has been widely used to prolong survival and improve the daily life quality of patients with advanced HCC, there are many inadequacies: the antitumor efficiency of sorafenib in clinical application exhibits individual differences among patients, whereas sorafenib resistance often occurs during treatment in some patients who are initially sensitive to sorafenib.Citation29,Citation30 To solve these problems and achieve better therapeutic effects, a variety of new molecular targeted drugs, including regorafenib or apatinib, have been approved for clinical treatment of advanced HCC. Although these strategies, which rely on research and development of new drugs, could be innovative at the source and produce new drugs, there are still some problems: 1) the mechanism of drug resistance occurring during HCC treatment is not completely clear, and simply relying on the development new drugs is risky and costly and 2) sorafenib, apatinib, and regorafenib, which are all inhibitors of the VEGFR (vascular endothelial growth factor receptor) and MAPK (mitogen-activated protein kinase) signaling pathways, have similar core and chemical properties, and it is difficult to make an epoch-making breakthrough. These problems result in higher demands on researchers.
Apatinib is a recently approved small molecular protein kinase inhibitor used in treating advanced HCC. Clinically, patients are prescribed oral AiTan®, apatinib mesylate tablets, and there are no reports of other apatinib formulations for injections.Citation13 Because the greatest proportion of HCC cases in People’s Republic of China consists of HBV/HCV-related tumors, patients also often suffer from gastrointestinal-digestive tract dysfunctions caused by cirrhosis,Citation31–Citation34 which seriously affect the patient’s absorption and bioavailability of orally administered apatinib. In addition, apatinib is chemically hydrophilic but has poor solubility. Although apatinib is often prepared to form a mesylate salt to improve its solubility, the ionized apatinib is not easily absorbed by the human intestinal tract. Therefore, exploring and establishing more effective strategies of administration and pharmaceutical formulation could help to improve the effectiveness of molecular targeted therapies. Cyclodextrin is a generic term for a series of linear oligosaccharides derived from cyclodextrin glucosyltransferase produced by Bacillus, which have a lumen that can form inclusion complexes and molecular assembly systems with many organic and inorganic molecules, which, in turn, form stable dispersions in aqueous/parent solutions.Citation35–Citation38 In the present work, an inclusion complex of Apa-Cyc was prepared, and this inclusion complex was stable in nature, achieving a stable dispersion of apatinib in an aqueous solution after inclusion of cyclodextrin. Injection of Apa-Cyc achieved a better antitumor effect than oral administration of apatinib. This is important for the treatment of advanced HCC with apatinib, as clinical oral administration of apatinib with daily doses up to 850 mg can represent a great economic burden to patients and have potential side effects that should not be underestimated. Preparation of the cyclodextrin inclusion complex to achieve apatinib injection can improve the efficacy of drugs and relieve the financial burden and the side effects of apatinib experienced by patients.
Moreover, the Apa-Cyc inclusion complex helps to improve the chemical properties of apatinib and enable the preparation of the insoluble apatinib into a stable aqueous solution, which also facilitates the pharmaceutical preparation of apatinib. Application of Apa-Cyc in clinical treatment could also avoid the potential stimulation or toxic/side effects of organic solvents (eg, PEG or Tween) in an apatinib solution (eg, Apa-Sol) when injecting an apatinib solution. Based on this, a temperature-sensitive phase-change hydrogel of apatinib (named Apa-Gel) was prepared with apatinib– cyclodextrin and poloxamer 407. Apa-Gel was injected into HCC tissues in nude mice to examine the long-term antitumor effect. Apa-Gel is a liquid medicament at room temperature, and it can be converted into a hydrogel at body temperature to avoid the quick clearance of drug from tumor tissues being injected into the tissue. HCC cells in the tissue gradually degrade and dissolve the hydrogel (Apa-Gel), releasing the drug from the hydrogel, thus achieving long-term sustaining of apatinib in tumor tissues. In addition, Apa-Gel is liquid at room temperature and becomes hydrogel when its temperature is close to body temperature, which not only contributes to more effective drug administration but also offers more choices for transcatheter arterial chemoembolization (TACE) when treating advanced HCC.Citation39,Citation40 Current TACE strategy is that chemotherapeutic drugs, such as adriamycin, are directly mixed with the lipiodol and then deposited in the tumor tissues via tumor blood vessels. There are disadvantages to this treatment strategy: 1) adriamycin has excellent water solubility properties, and other drugs, such as lobaplatin, also contain metal ions. However, these drugs are not miscible with lipiodol and cannot exert the best synergistic effect, and 2) embolization agents, such as gel sponges, cannot be loaded with these drugs. In the present work, Apa-Gel, a liquid at room temperature, was found to enter into the tumor tissue and be converted into a gel. Such a hydrogel can not only achieve long-term release of the drug in the tumor tissue but also block the tumor blood vessel and exert an embolism function.
Conclusion
This study prepared formulations of apatinib to improve the efficiency of apatinib, a newly approved and potentially highly effective molecular targeted agent for treating advanced HCC. A novel slow-releasing system prepared with apatinib–cyclodextrin-poloxamer allows apatinib to be slowly released, facilitates tissue attachment, and thereby preserves the long-acting efficiency of apatinib.
Author contributions
All authors made substantial contributions to the design and conception, acquisition, analysis, or interpretation of data. All authors took part in either drafting or revising the manuscript. All authors also gave final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplementary material
Figure S1 Poloxamer 407 hydrogel did not inhibit subcutaneous growth of MHCC97-H in nude mice.
Disclosure
The authors report no conflicts of interest in this work.
References
- ChowPKHGandhiMTanSBAsia-Pacific Hepatocellular Carcinoma Trials Group SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma J Clin Oncol 2018 36 1913 1921 29498924
- ReissKAYuSMamtaniR Starting dose of sorafenib for the treatment of hepatocellular carcinoma: a retrospective, multi-institutional study J Clin Oncol 2017 35 31 3575 3581 28872925
- El-KhoueiryABSangroBYauT Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial Lancet 2017 389 10088 2492 2502 28434648
- FengFJiangQJiaH Which is the best combination of TACE and Sorafenib for advanced hepatocellular carcinoma treatment? A systematic review and network meta-analysis Pharmacol Res 2018 135 89 101 29959032
- FornerAReigMBruixJ Hepatocellular carcinoma Lancet 2018 391 10127 1301 1314 29307467
- KabirTDGandaCBrownRM A microRNA-7/growth arrest specific 6/TYRO3 axis regulates the growth and invasiveness of sorafenib-resistant cells in human hepatocellular carcinoma Hepatology 2018 67 1 216 231 28833396
- PetrouP Value-Based pricing and the end of pharmaceutical pricing as we know it? A case study on sorafenib and axitinib Pharmacol Res 2017 124 160 163 28579443
- TianYLiuZZhangL Apatinib-loaded lipid nanobubbles combined with ultrasound-targeted nanobubble destruction for synergistic treatment of HepG2 cells in vitro Onco Targets Ther 2018 11 4785 4795 30127626
- GourleyC Apatinib and etoposide: surprising efficacy of an oral combination Lancet Oncol 2018 19 9 1146 1147 30082171
- BruixJQinSMerleP Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial Lancet 2017 389 10064 56 66 27932229
- RaufiATironaMT Prospect of the use of checkpoint inhibitors in hepatocellular cancer treatments Cancer Manag Res 2017 9 19 27 28223846
- WangYGouQXuRChenXZhouZ Efficacy and safety of sorafenib versus apatinib in the treatment of intermediate and advanced hepatocellular carcinoma: a comparative retrospective study Onco Targets Ther 2018 11 3407 3413 29928132
- LanCYWangYXiongY Apatinib combined with oral etopo-side in patients with platinum-resistant or platinum-refractory ovarian cancer (AEROC): a phase 2, single-arm, prospective study Lancet Oncol 2018 19 9 1239 1246 30082170
- GiglioVVialeMBertoneVMaricIVaccaroneRVecchioG Cyclodextrin polymers as nanocarriers for sorafenib Invest New Drugs 2018 36 3 370 379 29116478
- KimAMccullyCCruzR The plasma and cerebrospinal fluid pharmacokinetics of sorafenib after intravenous administration in non-human primates Invest New Drugs 2012 30 2 524 528 21072558
- FengSQWangGJZhangJW Combined treatment with apatinib and docetaxel in A549 xenograft mice and its cellular pharmacokinetic basis Acta Pharmacol Sin 2018 39 10 1670 1680 29770798
- FengSZhangJWangY Application of liquid chromatography-tandem mass spectrometry to study the effect of docetaxel on pharmacokinetics and tissue distribution of apatinib in mice J Chromatogr B Analyt Technol Biomed Life Sci 2018 1083 198 203
- XieHTianSYuH A new apatinib microcrystal formulation enhances the effect of radiofrequency ablation treatment on hepatocellular carcinoma Onco Targets Ther 2018 11 3257 3265 29910621
- ShaoZLiYDaiW ETS-1 induces Sorafenib-resistance in hepatocellular carcinoma cells via regulating transcription factor activity of PXR Pharmacol Res 2018 135 188 200 30114438
- LiJZhaoJWangH MicroRNA-140-3p enhances the sensitivity of hepatocellular carcinoma cells to sorafenib by targeting pregnenolone X receptor Onco Targets Ther 2018 11 5885 5894 30271172
- WuMZhaoGZhuangX Triclosan treatment decreased the antitumor effect of sorafenib on hepatocellular carcinoma cells Onco Targets Ther 2018 11 2945 2954 29849464
- JiaHYangQWangT Rhamnetin induces sensitization of hepatocellular carcinoma cells to a small molecular kinase inhibitor or chemotherapeutic agents Biochim Biophys Acta 2016 1860 7 1417 1430 27091611
- AnLLiDDChuHX Terfenadine combined with epirubicin impedes the chemo-resistant human non-small cell lung cancer both in vitro and in vivo through EMT and Notch reversal Pharmacol Res 2017 124 105 115 28754458
- FengFJiangQCaoS Pregnane X receptor mediates sorafenib resistance in advanced hepatocellular carcinoma Biochim Biophys Acta Gen Subj 2018 1862 4 1017 1030 29369785
- LiLLiangYKangL Transcriptional regulation of the Warburg effect in cancer by SIX1 Cancer Cell 2018 33 3 368 385 29455928
- KangJKimEKimW Rhamnetin and cirsiliol induce radiosensitization and inhibition of epithelial-mesenchymal transition (EMT) by miR-34a-mediated suppression of Notch-1 expression in non-small cell lung cancer cell lines J Biol Chem 2013 288 38 27343 27357 23902763
- WonJKYuSJHwangCY Protein disulfide isomerase inhibition synergistically enhances the efficacy of sorafenib for hepatocellular carcinoma Hepatology 2017 66 3 855 868 28439950
- ParikhNDMarshallVDSingalAG Survival and cost-effectiveness of sorafenib therapy in advanced hepatocellular carcinoma: An analysis of the SEER-Medicare database Hepatology 2017 65 1 122 133 27770556
- ZhuYJZhengBWangHYChenL New knowledge of the mechanisms of sorafenib resistance in liver cancer Acta Pharmacol Sin 2017 38 5 614 622 28344323
- HouJHongZFengF A novel chemotherapeutic sensitivity-testing system based on collagen gel droplet embedded 3D-culture methods for hepatocellular carcinoma BMC Cancer 2017 17 1 729 29117859
- Polaris Observatory Collaborators Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study Lancet Gastroenterol Hepatol 2018 3 6 383 403 29599078
- NayagamSThurszMSicuriE Requirements for global elimination of hepatitis B: a modelling study Lancet Infect Dis 2016 16 12 1399 1408 27638356
- WangFSFanJGZhangZGaoBWangHY The global burden of liver disease: the major impact of China Hepatology 2014 60 6 2099 2108 25164003
- ZhangSWangFZhangZ Current advances in the elimination of hepatitis B in China by 2030 Front Med 2017 11 4 490 501 29170919
- DiamantisDARamesovaSChatzigiannisCM Exploring the oxidation and iron binding profile of a cyclodextrin encapsulated quercetin complex unveiled a controlled complex dissociation through a chemical stimulus Biochim Biophys Acta Gen Subj 2018 1862 9 1913 1924 29886278
- de RossiMCWetzlerDEBenseñorL Mechanical coupling of microtubule-dependent motor teams during peroxisome transport in Drosophila S2 cells Biochim Biophys Acta Gen Subj 2017 1861 12 3178 3189 28935608
- Martínez-NegroMCaraccioloGPalchettiS Biophysics and protein corona analysis of Janus cyclodextrin-DNA nanocomplexes. Efficient cellular transfection on cancer cells Biochim Biophys Acta Gen Subj 2017 1861 7 1737 1749 28315770
- CostaGAde SouzaSBda Silva TeixeiraLR Tumor cell cholesterol depletion and V-ATPase inhibition as an inhibitory mechanism to prevent cell migration and invasiveness in melanoma Biochim Biophys Acta Gen Subj 2018 1862 3 684 691 29253593
- European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma J Hepatol 2012 56 908 943 22424438
- European Association for Study of Liver; European Organisation for Research and Treatment of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma Eur J Cancer 2012 48 599 641 22424278
