Abstract
Background
EGFR-tyrosine kinase inhibitors (EGFR-TKIs) including afatinib, dacomitinib, erlotinib, gefitinib, and osimertinib have proven efficacy in terms of progression-free survival (PFS) in patients with non-small-cell lung cancer (NSCLC) harboring EGFR mutations. However, an overall view for comparing efficacy and toxicity on a meta-level is lacking. This study compared efficacy and toxicity of first-line treatment with five different EGFR-TKIs by conducting a network meta-analysis (NMA).
Methods
A systematic review was performed, aiming to find eligible literature. Data of PFS, overall survival (OS), objective response rate (ORR), and adverse events were extracted. An NMA based on Bayesian statistics was established to synthesize the efficacy and toxicity of all treatments.
Results
Thirteen randomized controlled trials, including data from 3,539 patients with EGFR-mutated NSCLC, were analyzed. Rank probabilities showed that osimertinib had a potentially better efficacy in terms of PFS and OS compared to all other TKIs. For ORR, afatinib and osimertinib showed a trend of superiority compared to the other four TKIs. Furthermore, there was a high risk of diarrhea and rash for patients treated with afatinib or dacomitinib as well as a moderate risk for treatment with erlotinib, gefitinib, and osimertinib.
Conclusion
Our study showed a favorable efficacy of osimertinib in terms of PFS and OS compared to all other EGFR-TKIs in patients with NSCLC harboring activating EGFR mutations. Furthermore, gefitinib, erlotinib, and osimertinib were associated with fewer toxicities compared to the other TKIs. Therefore, osimertinib is indicated as a preferable first-line TKI in patients with activating EGFR-mutated NSCLC.
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide.Citation1 Of all lung cancer cases, 80–85% are non-small-cell lung cancers (NSCLC), and the majority of these cases are in advanced or metastatic stage (III or IV) at the time of diagnosis.Citation2,Citation3 Among these patients with NSCLC, a substantial number are harboring activating EGFR mutations, ranging from 10% in Europe to 38.4% in Asia.Citation4,Citation5 During the past years, targeted therapies including tyrosine kinase inhibitors (TKIs) have been developed and have become standard first-line treatment for patients with EGFR mutation-positive NSCLC.Citation6–Citation8 Various trials showed higher response rates and improved progression-free survival (PFS) for first-line treatment with afatinib, erlotinib, and gefitinib compared to platinum-based doublet therapy in patients with activating EGFR-mutated (exon 19 deletion or exon 21 L858R mutation) NSCLC.Citation9–Citation18 Recently, in head-to-head trials, dacomitinib and osimertinib showed a significant longer PFS compared to standard EGFR-TKIs, while dacomitinib, a second-generation EGFR-TKI, had a better efficacy compared to gefitinib, and osimertinib showed a more favorable PFS compared to standard EGFR-TKI (gefitinib or erlotinib).Citation19,Citation20 Different EGFR-TKIs are available for the treatment of patients with EGFR mutation-positive NSCLC. However, since sufficient data from head-to-head trials of all these EGFR-TKIs are lacking, evidence of relative efficacy and toxicity of these first-line TKIs is also scarce. Therefore, a network meta-analysis (NMA) was performed to compare the efficacy and toxicity of these TKIs as first-line treatment for patients with EGFR mutation-positive NSCLC. In traditional meta-analyses, the same intervention is compared to the same comparator in all included studies. NMA combines direct comparisons of interventions within randomized controlled trials (RCTs) with indirect comparisons across RCTs in multiple pairwise comparisons across a range of interventions. A greater share of available evidence is produced by using the NMA method compared to traditional meta-analysis. The NMA method enables judicious estimation of the relative treatment effect for comparative effectiveness purposes.Citation21 Previously published NMAs did not show significant differences between EGFR-TKIs.Citation22–Citation26 New data for several (new) TKIs are available (ARCHER1050 and FLAURA trials),Citation19,Citation20 which may lead to new insights into the relative efficacy and toxicity of the EGFR-TKIs.
This study aimed to compare the efficacy and toxicity of first-line treatment with gefitinib, erlotinib, afatinib, dacomitinib, and osimertinib for patients with activating EGFR-mutated (exon 19 deletion or exon 21 L858R mutation) NSCLC by conducting an NMA of all available evidence in the literature.
Materials and methods
Search strategy and selection criteria
An electronic search of the PubMed, EMBASE, and Cochrane Library databases was conducted in order to find eligible studies for the NMA, following PRISMA guidelines.Citation27 Eligible studies were Phase IIB/III RCTs that compared the efficacy and toxicity of a single TKI to another TKI or to standard chemotherapy as first-line treatments in patients with stage IIIB/IV NSCLC harboring EGFR mutations and in patients who were not eligible for surgery or radiotherapy. Standard chemotherapy was defined as platinum-based doublet therapy.
Papers published from 1 January, 2010 up to and including 1 November, 2016 were included. Literature was reviewed by two reviewers (MSH and CAUG) and discrepancies were discussed. The selection of studies was based on inclusion and exclusion criteria. Details of the search strategy can be found in Appendix A. Reference lists of published systematic reviews and meta-analyses were checked to ensure that no studies were overlooked. In February 2018, the literature search was manually updated to ensure that no relevant studies were missing, as new trials have been published in the previous 2 years.
Data extraction and quality assessment
Information on study design, number of participants, patient characteristics, interventions, comparators, objective response rate (ORR) (complete or partial response according to RECIST v1.1), PFS (time from randomization until disease progression according to RECIST v1.1 or death from any cause), overall survival (OS) (time from randomization until death from any cause), and adverse events (AEs) were extracted. Toxicity was scored according to the Common Toxicity Criteria (CTC).Citation28 Absolute numbers of AEs were extracted and ORs were calculated. Diarrhea and rash (CTC grade 3 or higher) were included in the analyses of this study because these are the most common TKI-related AEs. Other AEs were not included in the final analysis because they are less impacting and are known to be relatively homogenous across all EGFR-TKIs.Citation29,Citation30 Data extraction was verified by the second reviewer (CAUG). For studies with more than one publication, the data were compared between publications. The most updated results were included in this study. Extracted data can be found in Appendix B.
Quality and risk of bias of the RCTs were assessed by using the Cochrane Collaboration’s tool for assessing risk of bias.Citation31
Statistical analyses
We performed a Bayesian fixed-effects NMA in WinBUGS 1.4 by using an adapted version of WinBUGS code from Dias et alCitation32 (Appendix C). Due to the limited number of trials in each specific TKI group, a fixed-effects framework was deemed appropriate for the NMA.Citation33–Citation35 The outcomes of PFS, OS, ORR, and AEs within trials were linked in a network.
To obtain the HR of treatment a vs b, the following formula was used for all comparisons: HRa,b = (e(∂b-∂a)), and chemotherapy was used as the reference treatment in the network (∂ chemo = 0). All other ∂’s were calculated based on direct and indirect evidence from the RCTs. The NMA also enabled us to estimate the probability of being the best treatment and to rank the treatments based on these probabilities. Brooks–Gelman–Rubin diagnostic with WinBUGS was used to assess convergence, which enabled the determination of the number of burn-in simulations that should be discarded before calculating the converged results.Citation36
The FLAURA trial compared osimertinib with gefitinib or erlotinib. In this trial, no separate HRs of osimertinib vs gefitinib or osimertinib vs erlotinib were reported. Therefore, we assumed that the HRs of PFS and OS were the same for osimertinib vs gefitinib as they were for osimertinib vs erlotinib.
Results
Identification of studies and study quality
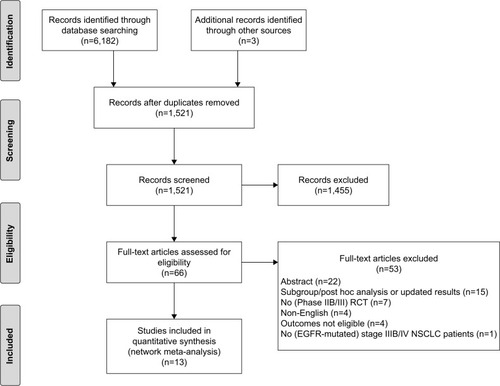
Electronic search in the databases resulted in 6,182 records, from which 4,664 internal and external duplicates were excluded. Three additional records were included after a manual update of the literature search. After screening the titles and abstracts of the remaining 1,521 records, 66 abstracts and manuscripts were eligible for full-text reading. After this, 53 records were excluded and 13 unique RCTs were included in the analyses. The flow chart is presented in .
Figure 1 Flow chart of the selection of studies.
Abbreviations: RCT, randomized controlled trial; NSCLC, non-small-cell lung cancer.
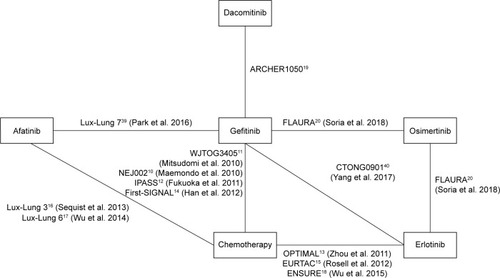
The patient characteristics of the 13 RCTs are summarized in . Eight of the 13 RCTs studied gefitinib (NEJ002, WJTOG3405, IPASS, First-SIGNAL, Lux-Lung 6, CTONG0901, ARCHER1050, and FLAURA).Citation9–Citation12,Citation14,Citation19,Citation20,Citation37–Citation41 Four RCTs studied erlotinib (OPTIMAL, EURTAC, ENSURE, and CTONG0901),Citation13,Citation15,Citation18,Citation40,Citation42 three studied afatinib (Lux-Lung 3, Lux-Lung 6, and Lux-Lung 7),Citation16,Citation17,Citation39,Citation43,Citation44 and one trial was included in the analyses for both dacomitinib and osimertinib (the ARCHER1050 and FLAURA study, respectively).Citation19,Citation20 Due to the heterogeneous study population of the IPASS and First-SIGNAL trials, we only included the results of the patients with activating EGFR-mutated (exon 19 deletion or exon 21 L858R mutation) NSCLC. A total of 3,539 patients with EGFR mutation-positive NSCLC were available for analyses, 2,691 of whom were randomly assigned to a TKI-arm and 848 of whom received platinum-based doublet therapy. The HRs for PFS and OS, as reported in the trials, are presented in . All 13 RCTs were classified as having acceptable quality and low risk of bias, according to the Cochrane Collaboration’s tool (Appendix D).
Table 1 Characteristics of included studies regarding TKIs
Table 2 HRs for PFS and OS of randomized studies in patients with EGFR-mutated advanced NSCLC treated with TKIs
Network meta-analysis
shows the complete network, which comprised 13 RCTs that studied a TKI compared to another TKI or chemotherapy in patients with EGFR-mutated NSCLC. We simulated three different chains, which produced 60,000 iterations each. Due to a burn-in period, 30,000 iterations were discarded in each chain; the results were based on a total sample of 90,000 iterations. Brooks–Gelman–Rubin plots showed convergence of the parameters.
, , and Figures S1–S6 in Appendix E present the NMA results for PFS, OS, ORR, and AEs (diarrhea and rash). Osimertinib showed a significantly better PFS and OS compared to all other TKIs. It also had the highest probability of 99% and 85%, thus showing the longest PFS and OS, respectively, as compared to other TKIs. Dacomitinib also showed a significantly improved PFS compared to gefitinib, erlotinib, and afatinib. Furthermore, afatinib and osimertinib performed best in terms of ORR compared to all other drugs with a probability of 46% for both drugs. However, the distribution of probabilities of being the best did not differ significantly on ORR ().
Table 3 Treatment comparisons for PFS, OS (HRs [95% CI]), ORR, diarrhea, and rash (ORs [95% CI])
Figure 3 Distribution of probabilities of being the best for outcomes and two major toxicities, classified by drugs.
Note: *P<0.0001.
Abbreviations: ORR, objective response rate; OS, overall survival; PFS, progression- free survival.
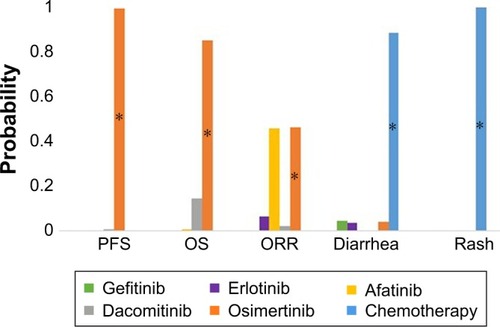
Diarrhea occurred significantly more often in patients treated with afatinib or dacomitinib. Gefitinib, erlotinib, and osimertinib showed a mild risk of diarrhea and chemotherapy had a low risk, with probabilities of being the best for diarrhea, with 7%, 6%, 15%, and 72%, respectively. Regarding rash, occurrence was high among patients treated with afatinib or dacomitinib and moderate among patients treated with gefitinib, erlotinib, or osimertinib. The risk of rash was low for chemotherapy with 99% probability of being the best treatment.
Discussion
In patients with EGFR-mutated NSCLC, TKIs have shown superior efficacy compared to platinum-based doublet therapy.Citation10–Citation18 Now that we have at least five different EGFR-TKIs, the relative efficacy and toxicity of these TKIs become important to help physicians choose the optimal drug for treatment. In contrast to meta-analysis, which only estimates the relative effect of the same interventions with the same comparators, an NMA combines direct evidence within RCTs with indirect evidence across RCTs to estimate the relative effect of multiple pairwise comparisons. In this way, the relative efficacy of a whole set of treatments for a disease can be synthesized.Citation21 Previous NMAs tried to provide relative evidence on the efficacy of EGFR-TKIs by using only three or four TKIs, or by including both first- and second-line TKIs in the network. These studies did not show significant differences between EGFR-TKIs in terms of efficacy and toxicity. Since a number of head-to-head trials between these drugs and data from new EGFR-TKIs are now available, we performed an NMA with five different TKIs (afatinib, dacomitinib, erlotinib, gefitinib, and osimertinib) to estimate their relative efficacy and toxicity as first-line treatment in patients with EGFR-mutated (exon 19 deletion or exon 21 L858R mutation) NSCLC. The results of the NMA indicated that osimertinib was significantly more effective on PFS compared to all other drugs. Dacomitinib proved to be the second best TKI effective on PFS with a significantly better PFS compared to gefitinib, erlotinib, and afatinib. Osimertinib also showed a significantly better efficacy in terms of OS compared to all other TKIs. Furthermore, AEs (diarrhea and rash) occurred more often in patients treated with afatinib or dacomitinib, compared to the other treatments. Due to the limited number of trials per treatment arm, a fixed-effect NMA was considered appropriate because heterogeneity could not be appropriately assessed.Citation33–Citation35
To our knowledge, this is the first study that performed an NMA to compare the results between five first-line EGFR-TKIs. Previous NMA studies failed to show significant differences between EGFR-TKIs.Citation22–Citation26 By including additional evidence from new RCTsCitation19,Citation20,Citation40 and updating results in the network, new results were produced, namely significant efficacy differences between the TKIs.
An important assumption in our study was that all included studies were generally similar, both clinically and methodologically. All 13 studies included only patients with activating EGFR mutations, with the percentage of males ranging from 11% to 47%, the median age range being 56–65 years, and the percentage of adenocarcinoma histology type ranging between 90% and 100% across the studies, which contributed to the homogeneity of the study population. Additionally, efficacy of EGFR-TKIs could be different when it was provided as second- or third-line treatment. A previous study showed that chemotherapy might change the proportion of tumor cells with EGFR mutations within the primary tumor.Citation45 Treatment with a TKI after platinum-based doublet therapy would thus probably affect the efficacy by inducing resistance mechanisms. Therefore, only first-line TKI treatments were included in our analyses in order to avoid such bias and to improve homogeneity.
For our analysis, the most common NMA method was used and, consequently, proportional hazards were assumed.Citation32 Since in eleven of the 13 trials the proportional hazard assumption could be checked, the assumption was not violated.
The length of follow-up differed among the included studies. As HRs may depend on the follow-up period, findings may vary when HRs are estimated at a different followup period. Due to a lack of patient-level data, correction for the different lengths of follow-up in an NMA is not possible. Insight into the long-term direction of HRs can be obtained with a longer follow-up duration, although this will also induce selection bias.Citation46
Although osimertinib showed a significant better OS compared to all drugs, gefitinib, erlotinib, and afatinib did not reveal a significant effect on OS compared to chemotherapy, which was similar to the individual studies. Some individual studies even showed OS results which were in favor of chemotherapy due to high proportions of crossover in the chemotherapy arms.Citation9,Citation11,Citation13–Citation15 The minimum proportion of crossover in the chemotherapy arm was 59.3% in the WJTOG3405 studyCitation11 and the maximum was 94.6% in the NEJ002 study.Citation10 A much smaller proportion of patients in whom TKI was initiated received chemotherapy as subsequent treatment.Citation10,Citation11,Citation13–Citation17 A recent study suggested that patients who received chemotherapy or TKI after first-line TKI or first-line chemotherapy had a longer OS than patients who only received first-line therapy.Citation42 The imbalanced subsequent treatments of the TKI and chemotherapy arms may have resulted in no significant OS differences between TKIs and chemotherapy. Therefore, it is questionable whether OS is an appropriate outcome measure in studies with substantial crossover.
Since final OS data were not available during our study period, the OS data of the FLAURA study were based on an interim analysis. Although this analysis did not show a formal statistical significance for OS, osimertinib seems to show a potential survival benefit compared to standard TKI.Citation20 An update of our NMA is desirable when final OS data of the FLAURA trial become available.
Conclusion
Our study showed that osimertinib is the most favorable EGFR-TKI in terms of PFS and OS. With regard to AEs, afatinib and dacomitinib had a higher risk of diarrhea and rash. Gefitinib, erlotinib, and osimertinib showed a mild risk of AEs. Thus, regarding its high efficacy and mild toxicity pattern, osimertinib is indicated as the most favorable first-line TKI in patients with activating EGFR-mutated (exon 19 deletion or exon 21 L858R mutation) NSCLC.
Disclosure
Carin A Uyl-de Groot reports grants received from Boehringer-Ingelheim, Janssen-Cilag B.V., Genzyme, Astellas Pharma B.V., Sanofi Netherlands, Roche Netherlands B.V., AstraZeneca Netherlands, Amgen Europe B.V., Gilead Sciences, Merck, and Bayer, outside the submitted work. The other authors report no conflicts of interest in this work.
References
- World Health OrganizationFact sheet cancer Available from: http://www.who.int/mediacentre/factsheets/fs297/en/Accessed September, 2017
- SunSSchillerJHSpinolaMMinnaJDNew molecularly targeted therapies for lung cancerJ Clin Invest2007117102740275017909619
- AlvarezMRomanESantosESRaezLENew targets for non-small-cell lung cancer therapyExpert Rev Anticancer Ther20077101423143717944567
- KernerGSSchuuringESietsmaJCommon and rare EGFR and KRAS mutations in a Dutch non-small-cell lung cancer population and their clinical outcomePLoS One201387e7034623922984
- ZhangYLYuanJQWangKFThe prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysisOncotarget2016748789857899327738317
- LynchTJBellDWSordellaRActivating mutations in the epidermal growth factor receptor underlying responsiveness of Non–Small-Cell lung cancer to gefitinibN Engl J Med2004350212129213915118073
- PaezJGEGFR mutations in lung cancer: correlation with clinical response to gefitinib therapyScience200430456761497150015118125
- PaoWMillerVZakowskiMEGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinibProc Natl Acad Sci U S A200410136133061331115329413
- MokTSWuYLThongprasertSGefitinib or carboplatin-paclitaxel in pulmonary adenocarcinomaN Engl J Med20093611094795719692680
- MaemondoMInoueAKobayashiKGefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFRN Engl J Med2010362252380238820573926
- MitsudomiTMoritaSYatabeYGefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trialLancet Oncol201011212112820022809
- FukuokaMWuYLThongprasertSBiomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS)J Clin Oncol201129212866287421670455
- ZhouCWuY-LChenGErlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (optimal, CTONG-0802): a multicentre, open-label, randomised, phase 3 studyLancet Oncol201112873574221783417
- HanJ-YParkKKimS-WFirst-SIGNAL: first-line single-agent Iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lungJ Clin Oncol201230101122112822370314
- RosellRCarcerenyEGervaisRErlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trialLancet Oncol201213323924622285168
- SequistLVYangJCYamamotoNPhase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutationsJ Clin Oncol201331273327333423816960
- WuY-LZhouCHuC-PAfatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trialLancet Oncol201415221322224439929
- WuYLZhouCLiamCKFirst-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ensure studyAnn Oncol20152691883188926105600
- WuYLChengYZhouXDacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (Archer 1050): a randomised, open-label, phase 3 trialLancet Oncol201718111454146628958502
- SoriaJ-COheYVansteenkisteJOsimertinib in untreated EGFR-mutated advanced non–small-cell lung cancerN Engl J Med2018378211312529151359
- JansenJPFleurenceRDevineBInterpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on indirect treatment comparisons good research practices: Part 1Value Health201114441742821669366
- LiangWWuXFangWNetwork meta-analysis of erlotinib, gefitinib, afatinib and icotinib in patients with advanced non-small-cell lung cancer harboring EGFR mutationsPLoS One201492e8524524533047
- PopatSMokTYangJC-HAfatinib in the treatment of EGFR mutation-positive NSCLC – a network meta-analysisLung Cancer201485223023824929780
- ZhangYShengJYangYOptimized selection of three major EGFR-TKIs in advanced EGFR-positive non-small cell lung cancer: a network meta-analysisOncotarget2016715200932010826933807
- BatsonSMitchellSAWindischRDamonteEMunkVReguartNTyrosine kinase inhibitor combination therapy in first-line treatment of non-small-cell lung cancer: systematic review and network meta-analysisOncoTargets and Therapy201710102473248228503070
- ZhangYZhangZHuangXTherapeutic efficacy comparison of 5 major EGFR-TKIs in advanced EGFR-positive Non–Small-cell lung cancer: a network meta-analysis based on head-to-head trialsClin Lung Cancer2017185e333e34028462807
- MoherDLiberatiATetzlaffJAltmanDGThe PRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementPLoS Med200967e100009719621072
- U.S. Department of Health and Human ServicesCommon terminology criteria for adverse events (CTCAE) version 5.0Bethesda, MDNational Cancer Institute2017
- CalifanoRTariqNComptonSExpert consensus on the management of adverse events from EGFR tyrosine kinase inhibitors in the UKDrugs201575121335134826187773
- HirshVManaging treatment-related adverse events associated with EGFR tyrosine kinase inhibitors in advanced non-small-cell lung cancerCurrent Oncology201118312613821655159
- HigginsJPTAltmanDGGotzschePCThe Cochrane collaboration’s tool for assessing risk of bias in randomised trialsBMJ20113432d592822008217
- DiasSWeltonNJSuttonAJAdesAEA generalized linear modelling framework for pairwise and network meta-analysis of randomized controlled trialsSheffieldScHARR, University of Sheffield2011198
- van Beurden-TanCHYFrankenMGBlommesteinHMUyl-de GrootCASonneveldPSystematic literature review and network meta-analysis of treatment outcomes in relapsed and/or refractory multiple myelomaJ Clin Oncol201735121312131928240968
- WeiselKDoyenCDimopoulosMA systematic literature review and network meta-analysis of treatments for patients with untreated multiple myeloma not eligible for stem cell transplantationLeuk Lymphoma201758115316127124703
- HigginsJPTGreenSCochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 updated March 2011LondonThe Cochrane Collaboration2011
- BrooksSPGelmanAGeneral methods for monitoring convergence of iterative simulationsJ Comput Graph Stat19987434455
- InoueAKobayashiKMaemondoMUpdated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002)Ann Oncol2013241545922967997
- YoshiokaHMitsudomiTMoritaSFinal overall survival results of WJTOG 3405, a randomized phase 3 trial comparing gefitinib (G) with cisplatin plus docetaxel (CD) as the first-line treatment for patients with non-small cell lung cancer (NSCLC) harboring mutations of the epidermal growth factor receptor (EGFR)J Clin Oncol20143215_suppl8117
- ParkKTanE-HO’ByrneKAfatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trialLancet Oncol201617557758927083334
- YangJJZhouQYanHHA phase III randomised controlled trial of erlotinib vs gefitinib in advanced non-small cell lung cancer with EGFR mutationsBr J Cancer2017116556857428103612
- MokTChengYZhouXDacomitinib (daco) versus gefitinib (GEF) for first-line treatment of advanced NSCLC (Archer 1050): final overall survival (OS) analysisJ Clin Oncol20183615_suppl9004
- ZhouCWuYLChenGFinal overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (optimal, CTONG-0802)Ann Oncol20152691877188326141208
- YangJC-HWuY-LSchulerMAfatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trialsLancet Oncol201516214115125589191
- IngelheimBOverall survival data from LUX-Lung 7 head-to-head trial of afatinib versus gefitinib presented at ESMO 2016 Available from: https://www.boehringer-ingelheim.com/press-release/overall-survival-data-lux-lung-7-head-head-trial2016Accessed April, 2017
- BaiHWangZChenKInfluence of chemotherapy on EGFR mutation status among patients with non-small-cell lung cancerJ Clin Oncol201230253077308322826274
- HernánMAThe hazards of hazard ratiosEpidemiology2010211131520010207