Abstract
The Warburg effect in tumor cells involves the uptake of high levels of glucose, enhanced glycolysis, and the metabolism of pyruvate to lactic acid rather than oxidative phos-phorylation to generate energy under aerobic conditions. This effect is closely related to the occurrence, invasion, metastasis, drug resistance, and poor prognosis of gastric cancer (GC). Current research has further demonstrated that the Warburg effect in GC cells is not only mediated by the glycolysis pathway, but also includes roles for mitochondria, noncoding RNAs, and other proteins that do not directly regulate metabolism. As a result, changes in the glycolysis pathway not only lead to abnormal glucose metabolism, but they also affect mitochondrial functions, cellular processes such as apoptosis and cell cycle regulation, and the metabolism of lipids and amino acids. In this review, we discuss metabolic reprogramming in GC based on glycolysis, a possible link between glucose metabolism, lipid metabolism, and amino acid metabolism, and we clarify the role of mitochondria. We also examine recent studies of metabolic inhibitors in GC.
Introduction
Gastric cancer (GC) is currently one of the most common malignant tumors diagnosed, and it is the second leading cause of mortality due to cancer.Citation1 GC originates in gastric epithelial cells and similar to other types of tumors, it exhibits a Warburg effect. This effect involves a high uptake of glucose, enhanced glycolysis, and the metabolization of a large amount of pyruvate to lactic acid rather than oxidative phosphorylation to provide energy under aerobic conditions.Citation2 The metabolic changes dominated by the Warburg effect have recently been referred to as metabolic reprogramming, and studies of these changes have provided a deeper understanding of tumor cell metabolism. For example, it has been demonstrated that GC cells and normal cells exhibit metabolic differences not only in glucose metabolism, but also in the metabolism of lipids and amino acids. Accordingly, a large number of specific biomarkers of GC have been identified. Metabolic reprogramming of GC has also been recognized as a novel approach for the study of the biological characteristics of GC.
In this review, we focus on a discussion of recent insights into the glycolysis pathway in GC and we summarize the complex relationship that exists between glucose metabolism, lipid metabolism, and amino acid metabolism. The role and impact of mitochondrial function in each of these aspects are also considered.
Helicobacter pylori promotes the genesis of GC by inducing metabolic reprogramming
Infection by Helicobacter pylori (Hp) is the most important primary cause of GC. However, progression from a Hp infection to atrophic gastritis and eventually GC is a long-term process.Citation3 In vitro, Hp-infected gastric epithelial cells have exhibited increased glycolysis and increased expression of Lon protease 1 (Lonp1), a protein that activates the mitochondrial unfolded protein response and maintains mitochondrial function. Correspondingly, knockdown of Lonp1 has been shown to reverse alterations in metabolism that are caused by Hp,Citation4 thereby suggesting that aerobic glycolysis and mitochondrial dysfunction correlate with the genesis of GC. Hp-induced GC is also characterized by higher expression levels of the M2 isoform of pyruvate kinase, PKM2, among other factors that are induced in GC and that affect mitochondrial function.Citation5,Citation6 Cytotoxin-associated gene A (CagA) has been shown to upregulate expression of PKM2 and pyruvate dehydrogenase kinase (PDK1). Moreover, when CagA localizes to mitochondria, it inhibits the activity of sirtuin 3 (SIRT3) and promotes stability of hypoxia-inducible factor 1α (HIF-1α).Citation7 Vacuolating cytotoxin A (VacA) is another Hp protein, and it has been shown to trigger mitochondrial dysfunction, promote mitochondrial division, and reduce mitochondrial DNA (mtDNA) copy number.Citation8–Citation10 Taken together, these findings support a model in which Hp induces GC by promoting glycolysis and mitochondrial dysfunction ().
Table 1 Specific Hp proteins that are associated with metabolic reprogramming in GC
Molecular factors that affect metabolic reprogramming in GC cells
Metabolic reprogramming that occurs in GC mainly involves changes in the following molecular factors: 1) key enzymes in glycolysis, 2) mitochondrial proteins, 3) noncoding RNAs, and 4) proteins that regulate these factors. The changes manifested by these molecules include significant differences in their levels between GC tissues and normal tissues. Moreover, interventions targeting these molecules have been found to not only affect the metabolic profile of GC cells, but also to affect the genesis, proliferation, invasion, and metastasis of GC cells as we describe below.
Changes in key glycolysis enzymes during metabolic reprogramming in GC
The glycolysis profile of GC is unique and is based on the ability of GC cells to maintain their growth under hypoxic conditions, to provide raw materials for cell biosynthesis and cell division, and to maintain intracellular redox homeostasis.Citation11 These functions also correlate with the genesis, proliferation, invasion, and metastasis of GC cells. The following six proteins have been identified as key glyco-lytic enzymes that are affected by metabolic reprogramming in GC ().
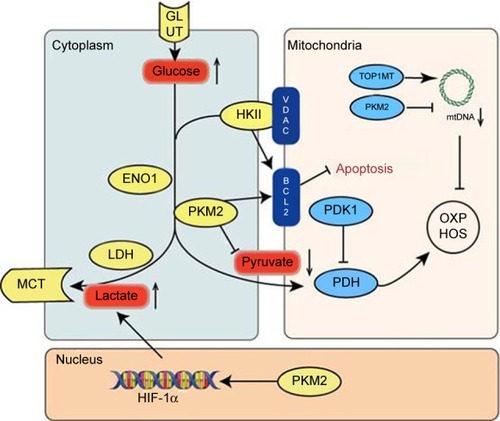
Figure 1 An overview of the pathways mediating upregulation of glycolysis and mitochondrial dysfunction in GC.
Abbreviations: GC, gastric cancer; mtDNA, mitochondrial DNA; OXPHOS, oxidative phosphorylation; PKM2, pyruvate kinase M2.
Hexokinase II
The hexokinase (HK) family of proteins catalyzes the conversion of glucose to glucose 6-phosphate (G6P) as the first rate-limiting step in glycolysis. G6P also participates in the pentose phosphate pathway and the process of gluconeo-genesis. Among the HK family of proteins, HKII has been found to be consistently overexpressed in most tumors, including GC. HKII is also associated with poor prognosis of GC in most cases.Citation12 HKII binds to voltage-dependent anion channels (VDACs) on the outer membrane of mitochondria to obtain ATP that is required for glycolysis.Citation13 However, HKII can also bind to the outer membrane of mitochondria, specifically via the antiapoptotic protein, Bcl-2, and the pro-apoptotic protein, Bax, to inhibit apoptosis.Citation14,Citation15 Thus, HKII is an excellent target for blocking glucose flux.
Pyruvate kinase M2
Pyruvate kinase (PK) is a key enzyme in the final step of glycolysis, which catalyzes the conversion of phosphoenolpyruvate to pyruvate. There are four members of the PK protein family, PKM1, PKM2, PKL, and PKR.Citation16 PKM1 and PKM2 are encoded by PKM, while PKL and PKR are encoded by the PKLR gene.Citation16 In addition, PKM1 exhibits PK activity, yet PKM2 does not. Tumor cells generally express high levels of PKM2 and low levels of PKM1, thereby promoting glycoly-sis and inhibiting mitochondrial oxidative phosphorylation.Citation16 When PKM2 was knocked out in GC cells, the PI3K/AKT/ mTOR pathway and autophagy were inhibited, thereby leading to a decrease in the proliferation and invasive phenotype of GC cells.Citation17,Citation18 PKM2 can also translocate to the nucleus and promote transcription of HIF-1α and Bcl-xl to further enhance glycolysis.Citation19 Moreover, interactions between PKM2, β-catenin, and octamer-binding transcription factor 4 (OCT4) have been shown to maintain the stemness quality of cells.Citation20,Citation21 In mitochondria, PKM2 interacts with and activates Bcl-2 to inhibit apoptosis.Citation22 Correspondingly, overexpression of PKM2 promotes mitochondrial fusion, fewer copies of mtDNA, and the expression and degradation of p53. Over-expression of PKM2 also reduces levels of electron transport chain complex proteins I, III, and V.Citation23 Taken together, these studies indicate that PKM2 promotes glycolysis and contributes to the dysfunction of mitochondria.
Pyruvate dehydrogenase kinase
The PDK family of proteins includes four isoforms. Many studies have recently focused on PDK1, which is generally expressed at high levels in tumors and is associated with tumor proliferation, metastasis, and poor prognosis.Citation24 PDK1 inhibits the activity of pyruvate dehydrogenase (PDH) to promote the metabolization of pyruvate to lactic acid, and it helps regulate the AKT/NF-κB pathway.Citation6 The ability of PDK to inhibit PDH activity also leads to a decrease in the level of acetyl-CoA to influence the de novo synthesis of lipids.Citation25
Enolase
Enolase (ENO1) catalyzes the conversion of phosphoglycerol to phosphoenolpyruvate in glycolysis and is highly expressed in GC. Knockdown of ENO1 has been shown to inhibit gly-colysis and increase the sensitivity of GC cells to cisplatin. Conversely, overexpression of ENO1 enhances the proliferation and metastasis of GC cells.Citation26,Citation27 In a proteomic analysis, ENO1 was found to be closely related to heat shock protein beta-1 (also known as Hsp27), while it has also been found to affect the regulation of anti-stress pathways.Citation28
Glucose transporter
As implied by their name, glucose transporters (GLUTs) 1–4 are responsible for the transport of glucose into cells, and in GC, where GLUT1 and GLUT4 are highly expressed. When GLUT1 was knocked out in GC cells in vitro, metabolic reprogramming was significantly reversed and apoptosis was triggered.Citation29 Levels of HK2 and PKM2 also declined in the absence of GLUT1.Citation29 Conversely, upregulation of GLUT4 by p38 mitogen-activated protein kinase affects myocyte enhancer factor 2α and promotes glycolysis.Citation30
Lactate dehydrogenase
Lactate dehydrogenase (LDH) catalyzes the conversion of pyruvate to lactic acid and is a key enzyme in the metabolic reprogramming of tumors. LDH is highly expressed in GC and promotes glycolysis. In GC, the transcription factor, fork head/winged-helix 1, upregulates the M isoform of LDH, LDHA.Citation31 Meanwhile, downregulation of LDHA by OCT4 has been associated with a good prognosis in GC.Citation32
Association between mitochondria and metabolic reprogramming in GC
Mitochondria are the energy factories for cells and their functions are often compromised in tumors. While dysfunctional mitochondria have been recently shown to promote tumor survival, the underlining mechanism(s) remain to be elucidated.Citation33 Here, we describe three aspects of mitochondrial function in GC.
MtDNA
Low levels of mtDNA have been shown to have an important effect on the biogenesis and homeostasis of mitochondria.Citation34
In 62.5% of GC patients, mutations in mitochondrial genes have been identified. A decrease in mtDNA copy number has also been observed in 54.8% of GC patients, especially patients affected by advanced GC.Citation15,Citation35 Furthermore, it has recently been reported that curcumin suppresses the growth of GC by increasing the production of ROS to mediate depletion of DNA polymerase gamma. These changes affect mitochondrial oxidative phosphorylation and inhibit cell biosynthesis.Citation36
Mitochondrial topoisomerase I
Replication of mtDNA requires topoisomerases, except for mitochondrial topoisomerase I (TOP1MT). The latter exerts a negative effect on the regulation on mtDNA replication, yet plays an important role in the formation and maintenance of D-LOOP regions of mtDNA and in maintaining mitochondrial homeostasis.Citation37,Citation38 Thus, low levels of TOP1MT are generally expressed in GC. In the absence of TOP1MT, oxidative stress and mitochondrial autophagy are induced,Citation37 glycolysis is promoted, the epithelial–mesenchymal transition (EMT) is accelerated, and the proliferation, invasion, and metastasis of GC cells are enhanced.Citation39 Expression of LDHA and glycolysis are also increased in the absence of TOP1MT, thereby indicating that TOP1MT affects cell proliferation by modulating glycolysis.Citation39 In clinical studies of GC, TOP1MT has been associated with poor prognosis.
SIRT3
SIRT3 localizes to mitochondria and is mainly involved in regulating oxidative stress. However, SIRT3 has also been shown to upregulate manganese-dependent superoxide dismutase, inhibit mitochondrial ROS levels, regulate the unfolded protein response of mitochondria, and maintain mitochondrial homeostasis.Citation7,Citation40,Citation41 Thus, SIRT3 is considered a tumor suppressor gene. However, there are conflicting data regarding the role of SIRT3 in GC. For example, expression of SIRT3 in GC tissues has been found to be lower in some studies, and higher in others, than in normal tissues.Citation42 Similarly, overexpression of SIRT3 has been associated with a significant reduction in cell proliferation,Citation43 and conversely, has also been associated with enhanced cell proliferation.Citation42 When SIRT3 was overexpressed in GC cells, promotion of ATP production and glycolysis were observed.Citation42 Moreover, SIRT3 has been shown to acetylate LDHA to enhance its LDH activity.Citation42 In the clinic, patients with high levels of SIRT3 expression have been associated with good prognosis.Citation44 Thus, the role of SIRT3 in GC remains an active area of research, and inconsistencies among reported results remain to be resolved.
Despite advances in characterizing mitochondrial factors and their roles in metabolic reprogramming in GC, there have been very few studies that have examined the relationship between mitochondria and metabolic reprogramming. However, as described above, the localization of glycolysis-related proteins to mitochondria can affect the functions of mitochondria, while mitochondrial proteins can also affect glycolysis. Thus, it appears that mitochondria are tightly linked with metabolic reprogramming that occurs, and this complex relationship remains to be thoroughly characterized.
The effect of other molecules on metabolic reprogramming in GC
There are other types of molecules which can regulate glycolysis and mitochondrial function in GC, and we introduce some of these molecules below. These representative molecules demonstrate how metabolic reprogramming can correlate with the EMT, cell cycle, and other cellular processes.
Snail
Snail is a key molecule in the EMT process and also regulates glycolysis. When Snail is overexpressed in GC cells, glucose intake is increased while activity of fructose bisphosphatase 1 is inhibited.Citation45 In GC, overexpression of Snail has been shown to correlate with metastasis and poor prognosis.Citation46
Hypoxia-inducible factor 1α
HIF-1α is a key factor for cell survival under hypoxic conditions. In GC, HIF-1α is highly expressed and is closely related to poor prognosis and drug resistance.Citation47 The level of HIF-1α expression in GC metastases has also been found to be higher than that in primary tumors, and it appears to promote GC metastasis by inhibiting integrin 5α.Citation48
Thymidine phosphorylase
Thymidine phosphorylase (TP) catalyzes the conversion of thymidine to thymine and 2-deoxy-D-ribose 1-phosphate. In GC, TP activates the NF-κB pathway to promote angio-genesis, lymphangiogenesis, and metastasis.Citation49 Under conditions of nutritional deprivation, thymidine provides a carbon source for glycolysis, and a high level of thymidine catabolism has been observed in GC.Citation49
Monocarboxylic acid transporter
The monocarboxylic acid transporter (MCT) family of proteins has 14 members. In GC, MCT1, MCT2, and especially MCT4, are highly expressed. These proton pumps transport monocarboxylic acids, pyruvic acid, lactic acid, ketone bodies, and other substances to maintain high glucose flux. They also play an important role in cell survival under highly acidic conditions in tumor cells.Citation50 Moreover, expression of MCT4 has also been associated with advanced tumor size-lymph nodes-metastases staging of GC.Citation51
p53
p53 is a tumor suppressor protein which plays an important role in cell cycle arrest, apoptosis, DNA repair, and glycolysis.Citation52 Currently, it is widely accepted that wild-type (WT) p53 inhibits glycolysis and promotes oxidative phosphorylation. For example, WT p53 downregulates GLUT1/4 and GLUT3 by inhibiting NF-κB signaling, thereby reducing the intracellular flux of glucose.Citation53,Citation54 WT p53 can also inhibit expression of HKII at the transcriptional level and upregulate the protein, parkin,Citation55 to induce ubiquitination of PKM2 and inhibit glycolysis.Citation56 In addition, WT p53 is able to inhibit glycolysis by upregulating TP53-induced glycolysis and the apoptosis regulator, TIGAR.Citation54 In humans, p53 mutations have been detected in .50% of cancer patients.Citation57 Mutant p53 proteins have also been shown to mediate functions that oppose those of WT p53. For example, mutant p53 proteins have been shown to promote glycolysis in tumors via upregulation of GLUT1, GLUT4, and HKII.Citation58 In GC, mutant p53 inhibits TIGAR, thereby enhancing glycolysis, and it correlates with poor prognosis.Citation59,Citation60
Effect of noncoding RNAs on metabolic reprogramming in GC
Noncoding RNAs, including microRNAs and long noncoding RNAs (lncRNAs), mediate complex regulatory effects on the structure, function, and processes of cells.Citation61 It has been reported that microRNAs and lncRNAs regulate key molecules involved in the metabolism of glucose to influence the proliferation, invasion, and metastasis of GC.Citation62,Citation63
Effect of microRNAs on metabolic reprogramming in GC
Various microRNAs have been shown to regulate glycolysis in GC. For example, Mir-22 upregulates ENO1.Citation26 Mir-186 inhibits the activity of HIF-1α, thereby leading to an increase in glucose uptake, lactate production, ATP/ADP, and a decrease in NAD+/NADH.Citation64 Mir-let-7a inhibits GC progression by inhibiting PKM2,Citation65 while Mir-133b inhibits polypyrimidine tract-binding protein 1 to reduce levels of PKM2.Citation66 Mir-181b reduces glucose intake and increases ATP production via inhibition of HKII expression.Citation67 Furthermore, Mir-448 maintains glycolysis levels by inhibiting KDM2B,Citation68 Mir-148 enhances glycolysis by upregulating GLUT1,Citation69 Mir-375 inhibits expression of PDK1,Citation70 and Mir-128b binds the 3′ UTR of PDK1 to inhibit expression of PDK1.Citation71
Effects of lncRNAs on metabolic reprogramming in GC
Various lncRNAs have also been shown to regulate glycolysis in GC. For example, the lncRNA, MACC1-AS1, has been shown to enhance MET-related transcription factor 1 (MACC1), activate the AMPK-LIN28 pathway, enhance glycolysis and the antioxidant capacity of GC cells, and play a cancer-promoting role in GC.Citation72 Correspondingly, MACC1 is associated with poor prognosis in GC. Recent studies have further shown that MACC1 contributes to resistance to trastuzumab in GC via activation of the PI3K/ AKT signaling pathway.Citation73 Under conditions of nutritional deprivation, AMPK upregulates levels of MCAA1, while levels of HKII, PDK1, and LDH are also upregulated under these conditions.Citation74 Lnc00152 promotes GC by inhibiting miR-139-5p, although miR-139-5p is able to inhibit glycolysis by upregulating AMP-activated protein kinase 1α (PRKAA1).Citation75 Finally, lncTINCR is a molecular sponge of miR-375, and it promotes expression of PDK1 and proliferation and metastasis in GC.Citation76
The effects of lipid metabolism and amino acid metabolism on metabolic reprogramming in GC
Intracellular pathways for the metabolism of lipids, amino acids, and glucose are needed for life, and they are closely linked with each other. Correspondingly, in cancer cells, abnormal glucose metabolism is often accompanied by changes in the metabolism of lipids and amino acids.
Effect of lipid metabolism on metabolic reprogramming in GC
In GC, levels of lipids and triglycerides generally increase.Citation77 In a metabolomic analysis of GC cells, low levels of 3-hydroxybutanoic acid (the end product of fatty acid β-oxidation) and inhibition of cholesterol synthesis were detected.Citation28 A metabolic analysis of a rat GC model also showed that oxidative stress-related pathways, fatty acid degradation, and amino acid metabolism were all blocked.Citation78
Endoscopic explorations have revealed a novel ultra-structure for GC, which includes a white opaque substance (WOS). Histology studies further revealed that the interior of this WOS consists of a large number of lipid drops. This novel structure is considered to be a hallmark of GC, and its formation is consistent with observations that lipid degradation is inhibited and lipid synthesis is enhanced in GC. The latter processes also contribute to an accumulation of lipids intracellularly.Citation79,Citation80 Meanwhile, if lipid metabolism is compromised, low levels of mitochondrial oxidative phos-phorylation are observed, and this may be related to poor prognosis in GC.Citation81 Correspondingly, when a ketogenic diet consisting of average protein content, low levels of carbohydrates, and enriched omega-3 fatty acid and medium-chain triglyceride content was fed to a nude mouse GC metastasis model, reduced glucose uptake and significant inhibition of GC growth were observed.Citation82
The effect of amino acid metabolism on metabolic reprogramming in GC
Metabolomic analyses have shown that the concentration of leucine, a ketogenic amino acid, is significantly reduced in GC, while levels of glycine, phenylalanine, and arginine are significantly increased.Citation28 In addition, the amino acid transporter gene, LAT1, has been shown to be upregulated in GC cell membranes.Citation83 In GC cells subjected to glucose deprivation, nonessential amino acids can prevent activation of the mitochondrial apoptosis pathway by maintaining mitochondrial homeostasis and a stable mtDNA copy number.Citation84,Citation85 The ability of PKM2 to act as a sensor of amino acids has also recently been demonstrated. In particular, phenylalanine, alanine, tryptophan, methionine, valine, and proline are able to inhibit it, and histidine and serine are able to activate PKM2.Citation86,Citation87 These findings suggest that amino acids can influence glucose metabolism in a more complex manner than previously anticipated, and a further study of this relationship is needed.
Novel metabolic inhibitors for treatment of GC
There are many new drugs that have been designed to target the glycolysis pathway, and many of these induce cell apoptosis. Below, we describe various metabolic inhibitors that have been developed to target four of the six glycolysis targets listed in Section “Molecular factors that affect metabolic reprogramming in GC cells”, as well as HIF-1α.
Targeting of key glycolysis enzymes
Targeting HKII
HKII binds to VDACs or Bcl-2 in combination with Bax on the outer membrane of mitochondria to obtain ATP for glycolysis, or to inhibit apoptosis, respectively. Both 3-BrPA, an inhibitor of HKII, and SCT, an inhibitor of phosphate fructose kinase,Citation13,Citation88 downregulate Bcl-2 and upregulate Bax, to inhibit glycolysis and enhance mitochondria-associated apoptosis.Citation89,Citation90 Meanwhile, 2-deoxy-D-glucose has been shown to competitively combine with HKII to block glucose flux.Citation91 Glycolysis activity is also reduced when baicalein inhibits HKII, PDK1, and LDHA, and baicalein has been shown to reduce the resistance of stomach adenocarcinoma cells to fluorouracil.Citation92 Furthermore, licorice chalcone A is a novel HKII inhibitor, as well as an inhibitor of the AKT pathway,Citation93 which has been shown to reduce glucose consumption and lactate production in GC cells, with the latter condition inducing apoptosis.
Targeting PKM2
As described above, PKM2 has various roles in a cell. Thus, inhibitors that target this protein exhibit various effects. For example, inhibition of PKM2 in GC cells by LY294002 induces apoptosis.Citation94 Meanwhile, pantoprazole (PPZ), a third-generation proton pump inhibitor and a newly identified PKM2 inhibitor, inhibits the Akt/GSK-β/β-catenin pathway and reverses sensitivity to chemotherapy in GC cells.Citation95,Citation96 PPZ has also been shown to reverse chemotherapy resistance in SGC7901 cells by downregulating V-ATPases/mTOR/ HIF-1α/P-gp and MRP1 signaling pathways.Citation97 Furthermore, inhibition of PKM2 by resveratrol induces endoplasmic reticulum stress in tumor cells, promotes mitochondrial division, and leads to apoptosis in tumor cells. Accordingly, overexpression of PKM2 has been shown to relieve the effect of resveratrol.Citation98
Targeting of PDK1 and LDH
Dichloroacetate has been shown to inhibit PDK1 and activate mitochondrial oxidative phosphorylation in GC cells, thereby resulting in an accumulation of mitochondrial ROS and apoptosis.Citation99 Meanwhile, oxamate is an LDH inhibitor, which inhibits mTOR activity, increases levels of ROS, and induces apoptosis in GC cells.Citation100
Targeting of HIF-1α
YC-1 is an inhibitor of HIF-1α, which reduces glycolysis and increases oxidative phosphorylation and intracellular ROS levels in GC cells. Under hypoxic conditions in vitro, treatment of GC cells with YC-1 has led to apoptosis.Citation101 Similarly, rosmarinic acid is another inhibitor of HIF-1α, which reduces glycolysis and induces apoptosis in GC cells.Citation102
While none of these drugs are currently available for clinical use, they have the potential to facilitate in vitro and/or in vivo studies of glycolysis to elucidate mechanistic details.
Conclusion
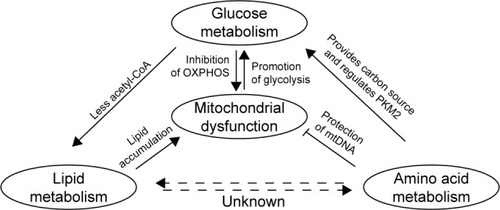
It is clear that a complex relationship exists among the pathways responsible for the metabolism of lipids, amino acids, and glucose, although the components, interactions, and regulatory mechanisms associated with these fundamental pathways remain to be fully characterized. In addition, it has been demonstrated that mitochondria play an important role in metabolic reprogramming in GC, not only by regulating oxidative phosphorylation, but also by regulating the metabolism of lipids and amino acids (). An important observation regarding the studies conducted to date involves differences in the results of in vitro vs in vivo studies. For example, in vitro studies involving low-glucose media have exhibited higher degrees of malignancy and drug resistance than in vivo studies.Citation103 Therefore, it is important for future studies to identify and optimize in vitro experimental conditions so that they are consistent with in vivo conditions. As a result, effects that are cell-specific and/or pathology-specific could be distinguished from those that represent fundamental aspects of glycolysis.
Figure 2 Metabolic reprogramming in GC.
Notes: A model is proposed in which an increase in glycolysis promotes mitochondrial dysfunction. The latter leads to disturbances in the metabolism of lipids and amino acids, and these alterations correlate with changes in the metabolism of glucose.
Abbreviations: ENO1, Enolase; GC, gastric cancer; GLUT, glucose transporter; HIF-1α, hypoxia-inducible factor 1α; HKII, hexokinase II; MCT, monocarboxylic acid transporter; LDH, lactate dehydrogenase; OXPHOS, oxidative phosphorylation; PDH, pyruvate dehydrogenase; PDK, pyruvate dehydrogenase kinase; PKM2, pyruvate kinase M2; TOP1MT, mitochondrial topoisomerase I; VDACs, voltage-dependent anion channels.
We hypothesize that alterations in mitochondrial functions may initiate metabolic reprogramming that can occur in GC, despite this aspect being largely unexplored in studies of GC to date. Thus, we would advocate that future research of GC should include investigations of mitochondria and their functions in order to obtain a better understanding of the genesis and development of GC. In addition, the signifi-cance of changes observed in various metabolites remains to be clarified beyond the identification that these changes are manifestations of metabolic reprogramming. It is anticipated that further studies of these metabolites and their roles could facilitate the development of effective biomarkers for the diagnosis of GC, as well as for prognosis assessments and evaluation of treatment response.
Acknowledgments
We thank Professor Sun Liankun (Department of Pathophysi-ology, School of Basic Medical Sciences, Jilin University) for providing a mitochondria-related study method and Medjaden Bioscience Limited for editing and proofreading. This work was supported by grants from the Jilin Province Science and Technology Development Project (20180101169JC), the Jilin Provincial Health Department Project (2016Q023), the Jilin Provincial Development and Reform Commission Project (2016CO51-2), the National Natural Science Foundation of China (81772794, 81672948), the Jilin Provincial Research Foundation for the Development of Science and Technology Projects (20170623021TC, 20160414005 GH), the Jilin University Bethune Plan B Projects (2015222), and the 13th Five-Year Science and Technology Project of Jilin Provincial Education Department (JJKH20170834KJ, JJKH20170825KJ).
Disclosure
The authors report no conflicts of interest in this work.
References
- BrayFFerlayJSoerjomataramIGLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countriesCA Cancer J Clin201868639442430207593
- LibertiMVLocasaleJWThe Warburg effect: how does it benefit cancer cells?Trends Biochem Sci201641321121826778478
- SitarzRSkieruchaMMielkoJOfferhausGJAMaciejewskiRPolkowskiWPGastric cancer: epidemiology, prevention, classification, and treatmentCancer Manag Res20181023924829445300
- LuoBWangMHouNATP-dependent Lon protease contributes to Helicobacter pylori-induced gastric carcinogenesisNeoplasia201618424225227108387
- ShirokiTYokoyamaMTanumaNEnhanced expression of the M2 isoform of pyruvate kinase is involved in gastric cancer development by regulating cancer-specific metabolismCancer Sci2017108593194028235245
- ZhangBGHuLZangMDHelicobacter pylori CagA induces tumor suppressor gene hypermethylation by upregulating DNMT1 via AKT-NFκB pathway in gastric cancer developmentOncotarget2016799788980026848521
- LeeDYJungDEYuSSLeeYSChoiBKLeeYCRegulation of SIRT3 signal related metabolic reprogramming in gastric cancer by Helicobacter pylori oncoprotein CagAOncotarget2017845783657837829108235
- MoyatMVelinDUse of VacA as a vaccine antigenToxins201686181
- RigoliLCarusoRAMitochondrial DNA alterations in the progression of gastric carcinomas: unexplored issues and future research needsWorld J Gastroenterol20142043161591616625473169
- RassowJMeineckeMHelicobacter pylori VacA: a new perspective on an invasive chloride channelMicrobes Infect201214121026103322796385
- GuaragnellaNGiannattasioSMoroLMitochondrial dysfunction in cancer chemoresistanceBiochem Pharmacol2014921627225107705
- WuJHuLWuFZouLHeTPoor prognosis of hexokinase 2 overexpression in solid tumors of digestive system: a meta-analysisOncotarget2017819323323234428415659
- PedersenPLVoltage dependent anion channels (VDACs): a brief introduction with a focus on the outer mitochondrial compartment’s roles together with hexokinase-2 in the “Warburg effect” in cancerJ Bioenerg Biomembr200840312312618780167
- PastorinoJGShulgaNHoekJBMitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apop-tosisJ Biol Chem200227797610761811751859
- RhoMKimJJeeCDExpression of type 2 hexokinase and mitochondria-related genes in gastric carcinoma tissues and cell linesAnticancer Res2007271A25125817352240
- IsraelsenWJvander HeidenMGPyruvate kinase: function, regulation and role in cancerSemin Cell Dev Biol201543435126277545
- WangCJiangJJiJPKM2 promotes cell migration and inhibits autophagy by mediating PI3K/Akt activation and contributes to the malignant development of gastric cancerSci Rep201771288628588255
- KitayamaKYashiroMMorisakiTPyruvate kinase isozyme M2 and glutaminase might be promising molecular targets for the treatment of gastric cancerCancer Sci2017108122462246929032577
- HamanakaRBChandelNSCell biology. Warburg effect and redox balanceScience201133460601219122022144609
- LeeJKimHKHanYMKimJPyruvate kinase isozyme type M2 (PKM2) interacts and cooperates with OCT-4 in regulating transcriptionInt J Biochem Cell Biol20084051043105418191611
- YangWXiaYJiHCorrigendum: nuclear PKM2 regulates β-catenin transactivation upon EGFR activationNature20174807674142
- LiangJCaoRWangXMitochondrial PKM2 regulates oxi-dative stress-induced apoptosis by stabilizing Bcl2Cell Res201727332935128035139
- WuHYangPHuWOverexpression of PKM2 promotes mitochondrial fusion through attenuated p53 stabilityOncotarget2016747780697808227801666
- StacpoolePWTherapeutic targeting of the pyruvate dehydrogenase Complex/Pyruvate dehydrogenase kinase (PDC/PDK) axis in cancerJ Natl Cancer Inst201710911djx071
- JeoungNHPyruvate dehydrogenase kinases: therapeutic targets for diabetes and cancersDiabetes Metab J201539318819726124988
- QianXXuWXuJEnolase 1 stimulates glycolysis to promote che-moresistance in gastric cancerOncotarget2017829476914770828548950
- LiuYQHuangZGLiGNEffects of α-enolase (ENO1) over-expression on malignant biological behaviors of AGS cellsInt J Clin Exp Med20158123123925784992
- LealMFChungJCalcagnoDQDifferential proteomic analysis of noncardia gastric cancer from individuals of northern BrazilPLoS One201277e4225522860099
- ZhangTBZhaoYTongZXGuanYFInhibition of glucose-transporter 1 (GLUT-1) expression reversed Warburg effect in gastric cancer cell MKN45Int J Clin Exp Med2015822423242825932183
- LiuJWenDFangXWangXLiuTZhuJP38MAPK signaling enhances glycolysis through the up-regulation of the glucose transporter GLUT-4 in gastric cancer cellsCell Physiol Biochem201536115516525925563
- JiangWZhouFLiNLiQWangLFOXM1-LDHA signaling promoted gastric cancer glycolytic phenotype and progressionInt J Clin Exp Pathol2015866756676326261559
- ZhangYZhangXWangXInhibition of LDH-A by lentivirus-mediated small interfering RNA suppresses intestinal-type gastric cancer tumorigenicity through the downregulation of OCT4Cancer Lett20123211455422429998
- JiaDParkJJungKLevineHKaipparettuBElucidating the metabolic plasticity of cancer: mitochondrial reprogramming and hybrid metabolic statesCells20187321
- KamalidehghanBHoushmandMIsmailPPanahiMSAkbariMHDelta mtDNA4977 is more common in non-tumoral cells from gastric cancer sampleArch Med Res200637673073516824932
- LeeHCYinPHLinJCMitochondrial genome instability and mtDNA depletion in human cancersAnn NY Acad Sci20051042110912215965052
- WangLChenXDuZCurcumin suppresses gastric tumor cell growth via ROS-mediated DNA polymerase γ depletion disrupting cellular bioenergeticsJ Exp Clin Cancer Res20173614728359291
- Dalla RosaIZhangHKhiatiSWuXPommierYTranscription profiling suggests that mitochondrial topoisomerase IB acts as a topological barrier and regulator of mitochondrial DNA transcriptionJ Biol Chem201729249201622017229021209
- SobekSBoegeFDNA topoisomerases in mtDNA maintenance and ageingExp Gerontol20145613514124440386
- WangHZhouRSunLTOP1MT deficiency promotes GC invasion and migration via the enhancements of LDHA expression and aerobic glycolysisEndocr Relat Cancer2017241156557828874393
- HirscheyMDShimazuTGoetzmanESIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylationNature2010464728512112520203611
- FinleyLWCarracedoALeeJSIRT3 opposes reprogramming of cancer cell metabolism through HIF1α destabilizationCancer Cell201119341642821397863
- CuiYQinLWuJSIRT3 enhances glycolysis and proliferation in SIRT3-expressing gastric cancer cellsPLoS One2015106e012983426121691
- WangLWangWYCaoLPSIRT3 inhibits cell proliferation in human gastric cancer through down-regulation of Notch-1Int J Clin Exp Med2015845263527126131100
- HuangKHHsuCCFangWLSIRT3 expression as a biomarker for better prognosis in gastric cancerWorld J Surg201438491091724322174
- YuJLiJChenYSnail enhances glycolysis in the epithelial-mesenchymal transition process by targeting FBP1 in gastric cancerCell Physiol Biochem2017431313828848200
- OkuboKUenosonoYArigamiTClinical significance of altering epithelial-mesenchymal transition in metastatic lymph nodes of gastric cancerGastric Cancer201720580281028247164
- ZhuCLHuangQLiuCHLinXSXieFPrognostic value of HIF-1α expression in patients with gastric cancerMol Biol Rep201340116055606224057269
- MaoZJTangQJZhangCAAnti-proliferation and anti-invasion effects of diosgenin on gastric cancer BGC-823 cells with HIF-1α shRNAsInt J Mol Sci20121356521653322754381
- TabataSYamamotoMGotoHThymidine catabolism as a metabolic strategy for cancer survivalCell Rep20171971313132128514652
- PinheiroCLongatto-FilhoAAzevedo-SilvaJCasalMSchmittFCBaltazarFRole of monocarboxylate transporters in human cancers: state of the artJ Bioenerg Biomembr201244112713922407107
- YanPLiYHTangZJShuXLiuXHigh monocarboxylate transporter 4 protein expression in stromal cells predicts adverse survival in gastric cancerAsian Pac J Cancer Prev201415208923892925374230
- HanahanDWeinbergRAHallmarks of cancer: the next generationCell2011144564667421376230
- TilborghsSCorthoutsJVerhoevenYThe role of nuclear factor-kappa B signaling in human cervical cancerCrit Rev Oncol Hematol201712014115029198328
- ZhaoMZhangZGlucose transporter regulation in cancer: a profile and the loopsCrit Rev Eukaryot Gene Expr201626322323827650986
- LiuKLiFHanHParkin regulates the activity of pyruvate kinase M2J Biol Chem201629119103071031726975375
- HongMXiaYZhuYTP53-induced glycolysis and apopto-sis regulator protects from spontaneous apoptosis and predicts poor prognosis in chronic lymphocytic leukemiaLeuk Res201650727727693855
- YueXZhaoYXuYZhengMFengZHuWMutant p53 in cancer: accumulation, gain-of-function, and therapyJ Mol Biol2017429111595160628390900
- ZhangCLiuJLiangYTumour-associated mutant p53 drives the Warburg effectNat Commun201341293524343302
- KimSHChoiSIWonKYLimS-JDistinctive interrelation of p53 with SCO2, COX, and TIGAR in human gastric cancerPathol Res Pract20162121090491027499152
- TaharaTShibataTOkamotoYMutation spectrum of TP53 gene predicts clinicopathological features and survival of gastric cancerOncotarget2016727422524226027323394
- ZhangMDuXNoncoding RNAs in gastric cancer: research progress and prospectsWorld J Gastroenterol201622296610661827547004
- FanCTangYWangJRole of long non-coding RNAs in glucose metabolism in cancerMol Cancer201716113028738810
- ZhangLFJiangSLiuMFMicroRNA regulation and analytical methods in cancer cell metabolismCell Mol Life Sci201774162929294128321489
- LiuLWangYBaiRYangKTianZmiR-186 inhibited aerobic glycolysis in gastric cancer via HIF-1α regulationOncogenesis20165e22427159677
- TangRYangCMaXMiR-let-7a inhibits cell proliferation, migration, and invasion by down-regulating PKM2 in gastric cancerOncotarget2016755972598426745603
- SugiyamaTTaniguchiKMatsuhashiNMiR-133b inhibits growth of human gastric cancer cells by silencing pyruvate kinase muscle-splicer polypyrimidine tract-binding protein 1Cancer Sci2016107121767177527696637
- LiLQYangYChenHZhangLPanDXieWJMicroRNA-181b inhibits glycolysis in gastric cancer cells via targeting hexokinase 2 geneCancer Biomark2016171758127314295
- HongXXuYQiuXMiR-448 promotes glycolytic metabolism of gastric cancer by downregulating KDM2BOncotarget2016716220922210226989077
- DingXLiuJLiuTMaZWenDZhuJmiR-148b inhibits glycolysis in gastric cancer through targeting SLC2A1Cancer Med2017661301131028440026
- XuYDengYYanXZhouTTargeting miR-375 in gastric cancerExpert Opin Ther Targets201115896197221557705
- ZhangLLeiJFangZLXiongJPMiR-128b is down-regulated in gastric cancer and negatively regulates tumour cell viability by targeting PDK1/Akt/NF-κB axisJ Biosci2016411778526949090
- ZhaoYLiuYLinLThe lncRNA MACC1-AS1 promotes gastric cancer cell metabolic plasticity via AMPK/Lin28 mediated mRNA stability of MACC1Mol Cancer20181716929510730
- LiuJPanCGuoLA new mechanism of trastuzumab resistance in gastric cancer: MACC1 promotes the Warburg effect via activation of the PI3K/Akt signaling pathwayJ Hematol Oncol2016917627581375
- LinLHuangHLiaoWMACC1 supports human gastric cancer growth under metabolic stress by enhancing the Warburg effectOncogene201534212700271025043301
- SunKHuPXuFLINC00152/miR-139-5p regulates gastric cancer cell aerobic glycolysis by targeting PRKAA1Biomed Pharmacother2018971296130229156518
- ChenZLiuHYangHGaoYZhangGHuJThe long noncoding RNA, TINCR, functions as a competing endogenous RNA to regulate PDK1 expression by sponging miR-375 in gastric cancerOnco Targets Ther2017103353336228744139
- TugnoliVMucciASchenettiLEx vivo HR-MAS magnetic resonance spectroscopy of human gastric adenocarcinomas: a comparison with healthy gastric mucosaOncol Rep200616354355316865254
- GuJHuXShaoWMetabolomic analysis reveals altered metabolic pathways in a rat model of gastric carcinogenesisOncotarget2016737600536007327527852
- SuganoKSatoKYaoKNew diagnostic approaches for early detection of gastric cancerDig Dis200422432733315812155
- YaoKIwashitaANambuMNature of white opaque substance in gastric epithelial neoplasia as visualized by magnifying endoscopy with narrow-band imagingDig Endosc201224641942523078433
- EnjojiMKohjimaMOhtsuKIntracellular mechanisms underlying lipid accumulation (white opaque substance) in gastric epithelial neoplasms: a pilot study of expression profiles of lipid-metabolism-associated genesJ Gastroenterol Hepatol201631477678126513060
- OttoCKaemmererUIllertBGrowth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglyceridesBMC Cancer20088112218447912
- IchinoeMMikamiTYoshidaTHigh expression of L-type amino-acid transporter 1 (LAT1) in gastric carcinomas: comparison with non-cancerous lesionsPathol Int201161528128921501294
- WangGDaiLLuoLNon-essential amino acids attenuate apoptosis of gastric cancer cells induced by glucose starvationOncol Rep201432133234024858809
- GomesLCDi BenedettoGScorranoLDuring autophagy mitochondria elongate, are spared from degradation and sustain cell viabilityNat Cell Biol201113558959821478857
- YuanMMcNaeIWChenYAn allostatic mechanism for M2 pyruvate kinase as an amino-acid sensorBiochem J2018475101821183729748232
- FeksaLRCornelioADutra-FilhoCSWyseATWajnerMWan-nmacherCMThe effects of the interactions between amino acids on pyruvate kinase activity from the brain cortex of young ratsInt J Dev Neurosci200523650951416023820
- GuoXZhangXWangTXianSLuY3-Bromopyruvate and sodium citrate induce apoptosis in human gastric cancer cell line MGC-803 by inhibiting glycolysis and promoting mitochondria-regulated apoptosis pathwayBiochem Biophys Res Commun20164751374327163639
- WangTAZhangXDGuoXYXianSLLuYF3-Bromopyruvate and sodium citrate target glycolysis, suppress survivin, and induce mitochondrial-mediated apoptosis in gastric cancer cells and inhibit gastric orthotopic transplantation tumor growthOncol Rep20163531287129626708213
- WangTAXianSLGuoXYZhangXDLuYFCombined 18F-FDG PET/CT imaging and a gastric orthotopic xenograft model in nude mice are used to evaluate the efficacy of glycolysis-targeted therapyOncol Rep201839127127929115645
- XuYWangQZhangLZhengM2-Deoxy-D-glucose enhances TRAIL-induced apoptosis in human gastric cancer cells through downregulating JNK-mediated cytoprotective autophagyCancer Chemother Pharmacol201881355556429383484
- ChenFZhuangMZhongCBaicalein reverses hypoxia-induced 5-FU resistance in gastric cancer AGS cells through suppression of glycolysis and the PTEN/Akt/HIF-1α signaling pathwayOncol Rep201533145746325333894
- WuJZhangXWangYLicochalcone A suppresses hexokinase 2-mediated tumor glycolysis in gastric cancer via downregulation of the Akt signaling pathwayOncol Rep20183931181119029286170
- LuJChenMGaoSYuanJZhuZZouXLY294002 inhibits the Warburg effect in gastric cancer cells by downregulating pyruvate kinase M2Oncol Lett20181544358436429541204
- ZhangBYangYShiXProton pump inhibitor pantoprazole abrogates adriamycin-resistant gastric cancer cell invasiveness via suppression of Akt/GSK-β/β-catenin signaling and epithelial-mesenchymal transitionCancer Lett20153562 Pt B70471225449432
- HuangSChenMDingXZhangXZouXProton pump inhibitor selectively suppresses proliferation and restores the chemosensitivity of gastric cancer cells by inhibiting STAT3 signaling pathwayInt Immunopharmacol201317358559223973653
- ChenMHuangSLZhangXQReversal effects of pantoprazole on multidrug resistance in human gastric adenocarcinoma cells by down-regulating the V-ATPases/mTOR/HIF-1α/P-gp and MRP1 signaling pathway in vitro and in vivoJ Cell Biochem201211372474248722396185
- WuHWangYWuCYangPLiHLiZResveratrol induces cancer cell apoptosis through MiR-326/PKM2-mediated ER stress and mitochondrial fissionJ Agric Food Chem201664499356936727960279
- XuanYHurHHamIHDichloroacetate attenuates hypoxia-induced resistance to 5-fluorouracil in gastric cancer through the regulation of glucose metabolismExp Cell Res2014321221923024342832
- ZhaoZHanFYangSWuJZhanWOxamate-mediated inhibition of lactate dehydrogenase induces protective autophagy in gastric cancer cells: involvement of the Akt-mTOR signaling pathwayCancer Lett20153581172625524555
- WakiyamaKKitajimaYTanakaTLow-dose YC-1 combined with glucose and insulin selectively induces apoptosis in hypoxic gastric carcinoma cells by inhibiting anaerobic glycolysisSci Rep2017711265328978999
- HanSYangSCaiZAnti-Warburg effect of rosmarinic acid via miR-155 in gastric cancer cellsDrug Des Devel Ther2015926952703
- BhattacharyaBLowSHSohCIncreased drug resistance is associated with reduced glucose levels and an enhanced glycolysis phenotypeBr J Pharmacol2014171133255326724597478
