Abstract
Objective
The study aimed to investigate the efficacy of computed tomography (CT)-guided cryoablation debulking of unresectable pelvic recurrent colorectal cancer (CRC).
Patients and methods
From January 2013 to April 2016, 30 patients (18 males and 12 females; aged 57.8±10.5 years) with unresectable pelvic recurrent CRC who had previously received radiotherapy or chemotherapy were included. A total of 35 tumors ranging from 1.2 to 6.3 cm underwent cryoablation. Tumor response was evaluated 1 month after cryoablation according to the Modified Response Evaluation Criteria in Solid Tumors. Logistic regression was used to analyze the risk factors for tumor response. Degree of pain palliation was also determined using the Numerical Rating Scale. Cox proportional hazard models were used to identify predictors of outcomes.
Results
Cryoablation was successfully performed in all patients. Complete response (CR) was achieved for 27 tumors in 23 patients and partial response was achieved for eight tumors in seven patients 1 month after cryoablation. The rate of CR was 77.14%, and tumor size was an independent risk factor for CR. Pain relief was satisfactory in 21 symptomatic patients (P<0.001), and the median duration of pain relief was 6.0 months (95% CI: 2.67–9.33). Serum carcinoembryonic antigen (CEA) was significantly decreased after cryoablation in 15 patients with elevated CEA (P=0.005). The median progression-free survival (PFS) was 10.0 months (95% CI: 4.43–15.67). Multivariate analysis revealed that tumor size (HR =3.089, P<0.001), sex (HR =0.089, P=0.002), and elevated CEA (HR =7.015, P=0.002) were independent predictors of PFS.
Conclusion
CT-guided cryoablation is a safe and effective therapeutic option for pelvic recurrent CRC. Tumor size is an important predictor of poor outcomes.
Introduction
Colorectal cancer (CRC) is the third most frequently diagnosed cancer and the second leading cause of cancer deaths worldwide.Citation1 Despite recent improvements in pretreatment radiological evaluation, total mesorectal excision, radiotherapy, and chemotherapy, 5%–10% of patients develop local recurrence.Citation2 Although radical surgery is a potentially curative option, only 20%–30% of patients with recurrent CRC are eligible to undergo an R0 resection.Citation3 Treatment of local recurrence remains a major challenge, particularly for patients with limited or no response to radiotherapy or chemotherapy.Citation4,Citation5 In addition, these patients always exhibit local compression symptoms and perineal or lower limb pain, which severely impacts their quality of life.Citation6,Citation7
For the past two decades, local ablation therapy for tumors has been an accepted therapeutic strategy.Citation8 Cryoablation is a type of local ablation that can destroy tumors and induce cell apoptosis by mechanisms including ice crystal formation within cells with subsequent cell membrane rupture, dehydration, and tumor ischemia. Because cryoablation can be visualized effectively via computed tomography (CT) imaging, the ablation zone is generally well-controlled. Cryoablation can provide effective pain relief and may be a potential alternative CRC treatment strategy, particularly for patients with unresectable recurrent CRC. Relatively few studies have evaluated cryoablation for treatment of advanced CRC, although some reports have detailed use of radiofrequency ablation (RFA) for treatment of pelvic malignancies.Citation9–Citation11 We retrospectively evaluated the efficacy of CT-guided cryoablation debulking for treatment of patients with unresectable pelvic recurrent CRC who had limited or no response to previous radiotherapy or chemotherapy.
Patients and methods
Patients
This study was approved by the Institutional Review Board of Fudan University Shanghai Cancer Center, and it was conducted in accordance with the ethical standards of the Declaration of Helsinki. Informed consent for retrospective review of our clinical database was waived. No personal data that could reveal a patient entity were used. Patient data were strictly protected for confidentiality when conducting this study. Patients were eligible if they had unresectable pelvic recurrence of CRC with histological confirmation or based on typical radiological features observed by positron emission tomography (PET)/CT, CT, or magnetic resonance imaging (MRI). Exclusion criteria included the following: peritoneal metastases causing intestinal obstruction or bowel obstruction, synchronous liver or lung metastases, a bleeding diathesis or coagulopathy, international normalized ratio ≥1.5 within 2 weeks before treatment, long- term treatment with high-dose aspirin (≥325 mg/day), and uncontrolled active infection.
From January 2013 to April 2016, 30 patients (18 males and 12 females, aged 57.8±10.5 years, range 33–75 years) with unresectable pelvic recurrence of CRC were included in the study. The primary tumors included 29 rectal adenocarcinomas and one sigmoid colon adenocarcinoma. Seven cases (23.33%) were well differentiated adenocarcinomas, while the remaining 23 (76.67%) were moderately to poorly differentiated adenocarcinomas. A total of 23 patients (76.7%) underwent abdominoperineal resection (Miles operation), five (16.7%) underwent low anterior resection (Dixon operation), and two (6.7%) had previously received transsacral local excision (Kraske operation). In addition, nine (30.0%) patients had undergone resection combined with adjuvant chemotherapy. The median recurrence time after resection was 19.5 months (range 3–121 months). All patients were treated with chemotherapy after recurrence and eight (26.7%) patients had previously undergone radiotherapy (). However, the tumors failed to respond to chemoradiotherapy, or exhibited progression. There were no clear boundaries between these tumors and the surrounding tissues, and due to local fiber hyperplasia, local cryoablation was recommended by a multidisciplinary team to control tumor progression and/or provide pain relief.
Table 1 Characteristics of patients at primary diagnosis and previous treatments (n=30)
Prior to cryoablation, 21 patients presented with pain (seven had single lower limb numbness and 14 had perineal pain). A total of 35 tumors, ranging from 1.2 to 6.3 cm (3.35±1.33 cm) in diameter, were treated by cryoablation. Twenty-six patients had solitary tumors and four had multiple tumors. Seventeen tumors across 14 patients were in the presacral region, nine tumors (in nine patients) were in the perineum, and nine tumors (across seven patients) were in the iliac region. Baseline characteristics are summarized in .
CT-guided percutaneous cryoablation
Cryoablation was performed under local anesthesia using a tabletop argon gas-based cryoablation system (Precise Cryoablation System; Galil Medical Ltd., Yokneam, Israel) by two physicians (XHH and LCX) with more than 5 years of experience. Imaging findings on contrast-enhanced CT or MRI less than 2 weeks before cryoablation, particularly tumor size and location with respect to adjacent structures, were used to plan ablation procedures. A Philips 64-slice spiral CT (120 kV, 250 mA, and 3-mm thickness; Philips Healthcare, Andover, MA, USA) was used for imaging guidance, localization, and intraoperative real-time monitoring of the ablation procedures to avoid injuring surrounding critical structures.Citation12
The number of antennas was determined on the basis of preoperative tumor volume. Seventeen-gauge antennas were placed into the tumor within 1.5 cm of the tumor’s edge, with no more than a 2-cm interval between the antennas.Citation13 Once all antennas were placed, a double freeze-thaw session (a total of 15 minutes each time: freeze for 10 minutes followed by thaw for 5 minutes) was performed according to standard protocols. The ablation procedure was ended if a precise ice ball margin exceeded the borders of the target tumor by over 5 mm. One additional session was performed if the ablation zone was insufficient. Repeated ablation was performed if there was new pelvic local recurrence as determined by CT scan or MRI according to routine procedures.
Evaluation of efficacy and follow-up
Follow-up and staging were performed using contrast- enhanced MRI at 1 month after treatment and every 6–8 weeks thereafter. CT or MRI was also performed as required when procedure-related complications were suspected. PET/CT scan was used selectively. Physical examinations and laboratory assessments such as carcino- embryonic antigen (CEA) levels and routine blood tests were performed monthly for all patients. Local tumor response was classified as complete response (CR), partial response (PR), stable disease, or progressive disease, according to the Modified Response Evaluation Criteria in Solid Tumors.Citation14 Degree of pain palliation was determined using the Numerical Rating Scale (NRS).Citation15 Overall survival (OS) was defined as the time from cryoablation to death from any cause. Progression-free survival (PFS) was defined as the time from cryoablation until disease progression or death, whichever occurred first. Complications were assessed according to the Society of Interventional Radiology Clinical Practice Guidelines.Citation12,Citation16
Statistical analysis
Continuous variables are shown as mean ± SD or as medians, and categorical data are represented as frequencies or percentages. Statistical analyses were performed using the statistical software package SPSS 23.0 (IBM Corporation, Armonk, NY, USA). For comparison of NRS scores and serum CEA levels pre- and post-ablation, Wilcoxon signed-rank test was used. Cumulative survival rates were determined by the Kaplan–Meier method. The risk of tumor response was analyzed using logistic regression. Possible prognostic factors for PFS were analyzed using a Cox proportional hazards regression model. P<0.05 was considered statistically significant.
Results
Local tumor response and efficacy
The cryoablation procedure was successfully completed in all patients (technical success rate was 100%). The number of antennas used was 3.5±2.4 (range 1–10; ), freezing time was 20.0±4.1 minutes (range 20–30 minutes), and rewarming time was 11.0±2.0 minutes (range 10–15 minutes). The median follow-up was at 11.0 months (IQR 7.0–18.0 months) for all patients.
Figure 1 Local tumor response and pain palliation efficacy.
Notes: (A) The line chart indicates the correlation between tumor size, number of antennas, tumor response, and location. (B) Kaplan–Meier curves of pain palliation after cryoablation. The median duration of pain relief was 6.0 months (95% CI: 2.67–9.33). (C) Bar graph of NRS score change and corresponding tumor size, response, and location in 21 patients with perineal or lower limb pain.
Abbreviation: NRS, Numerical Rating Scale.
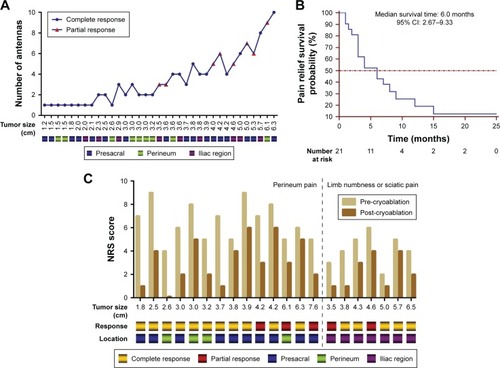
A total of 27 tumors in 23 patients achieved CR, while eight tumors in seven patients showed PR 1 month after cryoablation (). Among these, five tumors were located in the presacral region, two were close to the iliac vessels or nerves, and one was close to the perineum skin. Univariate and multivariate logistic regression analyses showed that tumor size was an independent risk factor for tumor response, with an HR of 2.667 (95% CI: 1.210–5.879, P=0.015).
In 21 patients with symptomatic pain, NRS scores significantly decreased after cryoablation (5.7±1.9 vs 2.9±1.8, P<0.001). The median duration of pain relief was 6.0 months (95% CI: 2.67–9.33; ). Prior to cryoablation, 14 patients (46.67%) had perineal pain with NRS scores of 6.5±1.6 (range 4–9), and NRS scores were 3.1±1.9 (range 0–6) 1 week after treatment (P<0.001). In addition, NRS scores of seven patients (23.33%) with single lower limb numbness or sciatic pain were 4.1±1.3 (range 2–6) and 2.3±1.4 (range 1–4) before and after cryoablation, respectively (). The median duration of pain relief was 3.0 months (95% CI: 0.00–6.67) in 14 patients with perineal pain, and 6.0 months (95% CI: 0.87–11.13) in seven patients with lower limb pain. However, these differences were not statistically significant (P=0.535). Sixteen patients achieved CR and five achieved PR ().
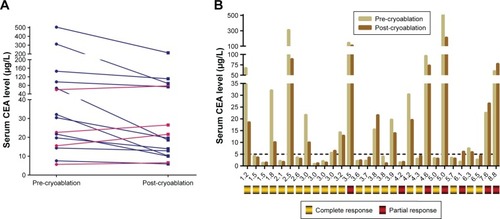
Fifteen patients (50%) had elevated CEA precryoablation (normal value <5 µg/L), and the CEA level post-cryoablation was significantly lower than that at pre-cryoablation (90.5±36.0 µg/L vs 47.4±14.9 µg/L, P=0.002). Ten patients achieved CR and five achieved PR. However, four out of these 15 patients had higher CEA levels post-cryoablation than pre-cryoablation, two of whom achieved PR (). One of the 15 patients with serum CEA (1.89 ng/mL) below the upper limit of normal (ULN) before cryoablation had serum CEA (6.12 ng/mL) higher than the ULN and achieved PR 1 month after cryoablation ().
OS, PFS, and prognostic factors
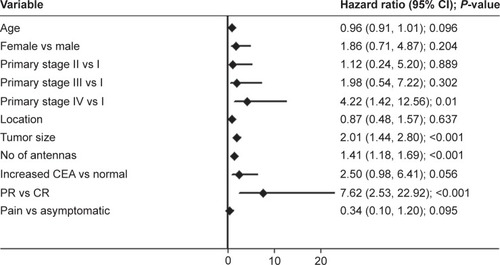
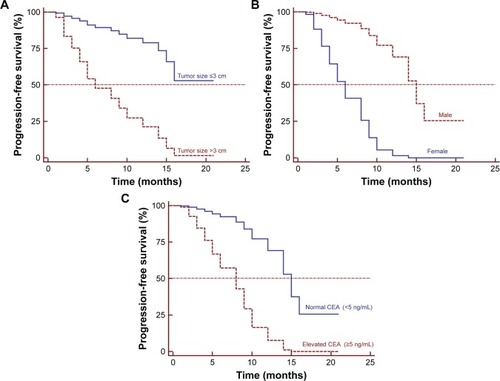
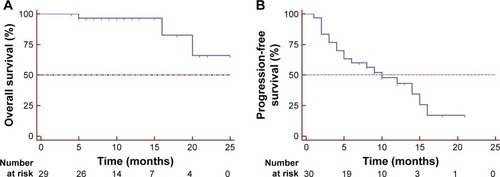
The 1- and 2-year cumulative OS rates were 96.6% and 66.2%, respectively. The median OS was not achieved at the end of follow-up (). Three patients (10.0%) had died, one (3.3%) was lost to follow-up, and 26 (86.7%) were alive. Ten patients (33.3%) who were alive were free of disease. Of the 16 patients who showed local progression, systemic therapy and best supportive care were adopted. Two patients exhibited lung metastasis, and three patients experienced new pelvic local recurrence, and repeated cryoablation was performed. The median PFS was 10.0±2.89 months (95% CI: 4.43–15.67; ). Univariate Cox regression analysis showed that primary tumor stage IV, tumor size, number of antennas, and tumor response were significantly associated with poor survival (; P<0.001). Multiple Cox regression analysis, after adjusting for potential confounders, demonstrated that tumor size >3 cm (HR =3.089, 95% CI: 1.893–5.041, P<0.001), being female (HR =0.089, 95% CI: 0.019–0.422, P=0.002), and elevated CEA levels (HR =7.015, 95% CI: 2.033–24.206, P=0.002) were independent risk factors for PFS ().
Figure 3 Kaplan–Meier estimates of (A) OS and (B) PFS after cryoablation.
Abbreviations: PFS, progression-free survival; OS, overall survival.
Complications
Cryoablation was well tolerated in all patients, without any procedure-related mortality. Seven patients complained of neuralgia or dull pain during the ablation procedure, and 5 mg morphine was administrated to control pain in four patients. Within 3 days after cryoablation, minor complications such as dysuria and urinary retention were observed in three patients (10.00%), and temporary neuralgia and lower limb paralysis were observed in two patients (6.67%). Four patients (13.3%) experienced fever during hospitalization. Short-term complications were managed with antibiotics, analgesia, and supportive treatment. Major complications included abscess formation (one patient who required percutaneous drainage), and perineum skin frostbite and sinus tract formation (one patient). The patients fully recovered after medical treatment. No other major complications were recorded.
Discussion
Despite recent advances, the rate of local recurrence after primary CRC treatment remains high, especially, 2–3 years after resection.Citation11,Citation17 Moreover, many advanced tumors exhibit limited response to radiotherapy or chemotherapy, and supportive care or palliative treatment is required for symptom control. For these patients, image-guided ablation represents a promising method for tumor control and should be considered. Few studies have explored application of RFA in pelvic recurrence of rectal cancer.Citation11,Citation18 Belfiore et al reported that of 14 patients with unresectable recurrent rectal cancer who received RFA treatment, eleven achieved satisfactory pain reduction, and ten showed locoregional control of tumor progression.Citation10 RFA combined with surgical debulking was also feasible in locally advanced abdominopelvic malignancies.Citation19,Citation20 However, heat-based ablation could cause rapid coagulation necrosis, resulting in irreversible nerve damage. As such, cryoablation has particular advantages over RFA in terms of tolerability and safety.Citation18 Because the freeze zone shows slow-density changes on CT imaging, physicians can accurately assess the ablated region during the procedure, and thus avoid damaging adjacent vulnerable structures. Recent evidence suggested that cryoablation can provide a survival advantage in comparison to other interventional procedures.Citation15
Cryoablation is now offered as primary treatment in many advanced pelvic tumors, such as prostate cancer, also recommend as salvage treatment for bone and soft-tissue tumors, pelvic local recurrence, or metastasis.Citation21,Citation22 However, there are few reports on CT-guided cryoablation in the treatment of recurrent CRC. At our institution, cryoablation has become a routine treatment for recurrent tumors at various sites. In this study, CR was achieved in 23 patients (76.7%), PR was achieved in seven patients (23.3%), and the tumor response was superior to other studies with regards to palliative treatment.Citation11,Citation18,Citation23 Tumor size was an independent risk factor response, and the ablation margin was also an important prognostic factor which depends on tumor location and surrounding tissues. Firstly, there are limited ablation volume avoiding tumor remnant in these locations close to major structures, and secondly the risk of complications could be higher. In the seven patients who achieved PR, five tumors were located at the presacral region, two were close to the iliac vessels or nerves, and one was close to the perineum skin. Although ablation margin >5 mm was achieved, there were no more than 10 mm safe ablation zones to obtain more desirable outcomes and avoid serious complications. PFS was not significantly associated with tumor location as determined by univariate/multiple Cox regression analyses. Due to the small sample size in this study, it was not possible to define an association between tumor response and location.
Patients with pelvic recurrence of CRC experience poor quality of life due to systemic or local complications such as pain, lower extremity edema, or dysuria.Citation15,Citation24 Cryoablation was effective for pain control, improvement of related disability, and quality of life.Citation25 Twenty-one patients (70.0%) in our study suffered pain before treatment, and the primary objective of treatment for these patients was pain control. The target tumors were mostly fixed to the pelvic wall or urinary system. Tumors associated with perineal pain were located at the anterior presacral space or perineum, while lower limb pain was attributed to iliac neurological invasion. Pain was alleviated 1 week after cryoablation, as measured by NRS. The median duration of pain relief was 6.0 months, which suggests efficient pain control. Although most patients in these studies exhibited efficient pain palliation and tumor necrosis, analgesia management was frequently needed during the procedure in patients who experienced intolerable pain. Overall, cryoablation with a >5 mm margin was tolerable and safe.
Serum CEA determination had definite value in terms of CRC screening and diagnosis, curative effect evaluation, and follow-up after treatment. Fifteen patients in the study showed elevated CEA pre-cryoablation, and serum CEA was significantly reduced post-cryoablation. Elevated CEA pre-cryoablation was an independent predictive factor for PFS as determined by multiple Cox regression analysis. However, PRs were observed in five patients 1 month after cryoablation.
At a median follow-up time of 11.0 months, the median PFS was 10.0 months, but median OS was not achieved. The nodal status of primary CRC, number of tumors, largest tumor size, and pre-ablation CEA level have been traditionally associated with oncologic outcome of CRC recurrence/metastases. Sofocleous et alCitation26 modified a surgical clinical risk score (CRS) and assessed use of this modified scale to predict outcomes for ablation of CRC liver metastasis (CLM). Another study found that the modified CRS (including CEA level) was a predictor of OS and local tumor PFS (LTPFS).Citation27 These studies showed that a modified CRS for ablation can be used as a prognostic stratification tool. In this cohort, few patients had CEA levels >200 ng/mL at the time of RFA. To compensate, the CEA threshold level was modified, and CEA level >30 ng/mL was associated with shorter OS (P=0.003). However, other studiesCitation28,Citation29 set CEA level >5 ng/mL as the stratification level, and no significant differences were found. These results also showed that tumors ≤3 cm and ablation margins >5 mm can significantly lower local tumor progression rates after RFA. A series of studiesCitation27,Citation30 demonstrated that ablation margins larger than 5 mm (ideally >10 mm) are critical for local tumor control. Odisio et alCitation28,Citation29 reported that minimal ablation margins and RAS status interacted as independent predictors of LTPFS following CLM ablation. These results showed that achieving minimum ablation margins >10 mm provided significantly improved LTPFS among RAS mutant CLM. In addition, Shady et alCitation31 also reported that KRAS mutation was a significant predictor of local tumor progression (LTP) after RFA of CLM with margins of 1–5 mm. Therefore, a minimal radiographic ablation margin of at least 5 mm is essential for local tumor control, especially, for mutant CLM. Yamashita et alCitation32 reported that midgut origin, multiple CLM, and RAS mutation were associated with poor survival rates after ablation of CLM. However, the sample size was small and did not include patients with transverse colon cancer or rectal cancer. The embryonic origin of CRC alone cannot be recommended as grounds for excluding patients from ablation. In our study, the cryoablation zone included >5 mm margin around all target tumors in which there was intent to debulk. Multivariate analysis suggested that tumor size >3 cm, being female, and elevated CEA levels were independent risk factors for PFS. However, these comparisons and conclusions should be interpreted with caution due to small sample size and limited statistical power.
In our study, one patient had skin frostbite due to the short distance between the tumor and skin. Notably, neural functional injury related to the cryoablation zone was transient and recovered over time. Neurologic symptoms were only recorded in two patients. Despite the occurrence of some side effects, cryoablation may be a feasible palliative treatment for pain control and tumor debulking.Citation15,Citation19,Citation33
There were several limitations in this study. It was a retrospective study with a small sample size and short follow-up period, and no control group was included. The clinical stage of CRC in the study was not homogeneous and the locations of the target tumors varied. Only patients with obvious invasion into adjacent structures were included in the study, which limited sample size and may have resulted in underestimation of the incidence of complications. Although high pre-cryoablation CEA level was an independent risk factor for PFS in this study, the stratification of CEA level varied in other studies, and its prognostic value also varied. The potentially curative effect of cryoablation in management of patients with pelvic malignancy should be further studied, and randomized controlled trials are needed.
Conclusion
CT-guided cryoablation is an attractive alternative for treatment of pelvic recurrence of CRC that provides effective pain relief and tumor control.
Acknowledgments
This study was supported in part by grants from the National Natural Science Foundation of China (No 81501562) and the National Key Research and Development Program of China (No 2016YFC0106203).
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2015CA Cancer J Clin201565152925559415
- van GijnWMarijnenCANagtegaalIDPreoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trialLancet Oncol201112657558221596621
- NielsenMBLaurbergSHolmTCurrent management of locally recurrent rectal cancerColorectal Dis201113773274220041928
- JanjanNACraneCFeigBWImproved overall survival among responders to preoperative chemoradiation for locally advanced rectal cancerAm J Clin Oncol2001242107p112p11319280
- KhrizmanPNilandJCTer VeerAPostoperative adjuvant chemotherapy use in patients with stage II/III rectal cancer treated with neoadjuvant therapy: a national comprehensive cancer network analysisJ Clin Oncol2013311303823169502
- GuyotFFaivreJManfrediSTime trends in the treatment and survival of recurrences from colorectal cancerAnn Oncol200516575676115790673
- PfisterDGBensonABSomerfieldMRClinical practice. surveillance strategies after curative treatment of colorectal cancerN Engl J Med2004350232375238215175439
- HinshawJLLubnerMGZiemlewiczTJLeeFTBraceCLPercutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation – what should you use and why?Radiographics20143451344136225208284
- GreenSHKhatriVPMcgahanJPRadiofrequency ablation as salvage therapy for unresectable locally recurrent rectal cancerJ Vasc Interv Radiol200819345445818295709
- BelfioreGTedeschiERonzaFMCT-guided radiofrequency ablation in the treatment of recurrent rectal cancerAJR Am J Roentgenol2009192113714119098192
- LefevreJHParcYLewinMRadiofrequency ablation for recurrent pelvic cancerColorectal Dis200810878178418028468
- AhmedMSolbiatiLBraceCLImage-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year updateJ Vasc Interv Radiol201425111691170525442132
- WangHLittrupPJDuanYThoracic masses treated with percutaneous cryotherapy: initial experience with more than 200 proceduresRadiology2005235128929815798173
- SatoYWatanabeHSoneMTumor response evaluation criteria for HCC (hepatocellular carcinoma) treated using TACE (transcatheter arterial chemoembolization): RECIST (response evaluation criteria in solid tumors) version 1.1 and mRECIST (modified RECIST): JIVROSG-0602Ups J Med Sci20131181162223167460
- MylonaSKaragiannisGPatsouraSPalliative treatment of rectal carcinoma recurrence using radiofrequency ablationCardiovasc Intervent Radiol201235487588222167304
- CardellaJFKunduSMillerDLSociety of interventional radiology clinical practice guidelinesJ Vasc Interv Radiol2009207 SupplS189S19119559998
- YunHRLeeLJParkJHLocal recurrence after curative resection in patients with colon and rectal cancersInt J Colorectal Dis200823111081108718688621
- OhhigashiSWatanabeFRadiofrequency ablation is useful for selected cases of pelvic recurrence of rectal carcinomaTech Coloproctol20037318619114628164
- GajdosCMacdermottTMccarterMDPearlmanNWCombined thermal-surgical ablation of locally advanced abdominopelvic malignanciesAnn Surg Oncol20111851267127321174157
- SpiliotisJHadjicostasPRogdakisAManagement of advanced abdominopelvic tumors with combined radiofrequency ablation and surgical debulkingDig Surg200825318819018577862
- GuoZSiTYangXXuYOncological outcomes of cryosurgery as primary treatment in T3 prostate cancer: experience of a single centreBJU Int20151161798425168692
- BingFGarnonJTsoumakidouGImaging-guided percutaneous cryotherapy of bone and soft-tissue tumors: what is the impact on the muscles around the ablation site?AJR Am J Roentgenol201420261361136524848836
- WangZLuJLiuLClinical application of CT-guided (125)I seed interstitial implantation for local recurrent rectal carcinomaRadiat Oncol2011613822004599
- ColibaseanuDTDozoisEJMathisKLExtended sacropelvic resection for locally recurrent rectal cancer: can it be done safely and with good oncologic outcomes?Dis Colon Rectum2014571475524316945
- SimonCJDupuyDEImage-guided ablative techniques in pelvic malignancies: radiofrequency ablation, cryoablation, microwave ablationSurg Oncol Clin N Am200514241943115817247
- SofocleousCTPetreENGonenMCT-guided radiofrequency ablation as a salvage treatment of colorectal cancer hepatic metastases developing after hepatectomyJ Vasc Interv Radiol201122675576121514841
- ShadyWPetreENGonenMPercutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes – a 10-year experience at a single centerRadiology2016278260161126267832
- CalandriMYamashitaSGazzeraCAblation of colorectal liver metastasis: interaction of ablation margins and ras mutation profiling on local tumour progression-free survivalEur Radiol20182872727273429417253
- OdisioBCYamashitaSHuangSYLocal tumour progression after percutaneous ablation of colorectal liver metastases according to ras mutation statusBr J Surg2017104676076828240361
- ShadyWPetreENDoKGPercutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor controlJ Vasc Interv Radiol201829226827529203394
- ShadyWPetreENVakianiEKRAS mutation is a marker of worse oncologic outcomes after percutaneous radiofrequency ablation of colorectal liver metastasesOncotarget2017839661176612729029497
- YamashitaSOdisioBCHuangSYEmbryonic origin of primary colon cancer predicts survival in patients undergoing ablation for colorectal liver metastasesEur J Surg Oncol20174361040104928187878
- PuscedduCSotgiaBMelisLFeleRMMeloniGBPainful pelvic recurrence of rectal cancer: percutaneous radiofrequency ablation treatmentAbdom Imaging20133861225123323736888