Abstract
Background
Cumulatively, evidences revealed that fenofibrate used in the therapy of hyperlipidemia and hypercholesterolemia has anti-cancer effect in multiple cancer types. However, its function and underlying mechanism of chemosensitization in breast cancer remain poorly understood.
Materials and methods
The cytotoxicity of fenofibrate and anti-cancer drugs in breast cancer cells was determined by MTT. Apoptosis and mitochondrial membrane potential were measured using flow cytometry. Caspases and PARP cleavage, the Bcl-2 family members’ protein expression, as well as the activation of AKT and NF-κB signaling pathways were evaluated using Western blot assay. Real-time PCR was used to determine the mRNA expression of Bcl-2 family members.
Results
Our data indicated that fenofibrate suppressed SKBR3 and MDA-MB-231 cell growth in a dose-dependent manner, in the same way as paclitaxel, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), ABT-737, and doxorubicin. Subtoxic levels of fenofibrate significantly augmented paclitaxel, TRAIL, ABT-737, and doxorubicin-induced apoptosis in both these two cell lines. Fenofibrate-promoted chemosensitivity is predominantly mediated by caspase-9 and caspase-3 activation and mitochondrial outer membrane permeabilization. Meanwhile, chemosensitivity promoted by fenofibrate also increased the expression of Bax and Bok and decreased the expression of Mcl-1 and Bcl-xl. Mechanistically, fenofibrate effectively reduced the phosphorylation levels of AKT and NF-κB. In addition, imiquimod, an NF-κB activator, could reverse fenofibrate-induced susceptibility to ABT-737-triggered apoptosis.
Conclusion
The present study provided the evidence of the underlying mechanisms on chemosensitization of fenofibrate by inducing the apoptosis of breast cancer in an AKT/NF-κB-dependent manner and implicated the potential application of fenofibrate in potentiating chemosensitivity in breast cancer therapy.
Introduction
Globally, human breast cancer is the second cause of cancer-correlated mortality in females.Citation1 In China, breast cancer alone accounts for ~15% of all new cancers in women, mostly aged 30–59 years.Citation2 Chemotherapy is effective in decreasing the measure of the primary cancer in neoadjuvant setting; however, it not only causes adverse effects in breast cancer patients, but also inevitably induces apoptosis resistance.Citation3 Therefore, a better understanding of the theory and mechanism of chemoresistance is of great value to exploit novel therapeutic strategy to improve prognosis.
Resistance mechanism by which breast cancer escapes drug-induced cell death has been ascribed to variations in the apoptosis pathway.Citation4 The key apoptotic regulators frequently inhibited by the AKT/NF-κB pathway in breast cancer, which blocks the activity of pro-apoptosis, are Bax, Bok, and Bim.Citation5,Citation6 Thus, it is necessary to investigate the molecular mechanisms of AKT/NF-κB pathway, which is responsible for apoptosis resistance, and to identify potential sensitizers that are competent of inhibiting apoptosis resistance.
Increasingly, evidences showed that fenofibrate, which was utilized in the therapy of hyperlipidemia and hypercholesterolemia, has anti-cancer effects on endometrial cancer, prostate cancer, triple-negative breast cancer, oral cancer, pancreatic cancer, and lung cancer, but only few studies have reported its effect on chemosensitivity of breast cancer.Citation7–Citation13 Researchers found that fenofibrate alone resulted in decreasing the semaphorin 6B gene expression in breast cancer,Citation14 arresting G1 phase in human glioblastoma cells,Citation15 but the detailed mechanisms by which fenofibrate sensitizes breast cancer cells to chemotherapy.
In the present study, we investigated the sensitization effects of fenofibrate on several anti-cancer agents including paclitaxel, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), ABT-737, and doxorubicin in breast cancer cells and provided insight into the molecular mechanisms of the action on fenofibrate-potentiated chemosensitivity.
Materials and methods
Cell culture
The human breast cancer cell lines MDA-MB-231 and SKBR3 were purchased from the American Type Culture Collection (Manassas, VA, USA) and cultured in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (Hyclone; GE Healthcare Life Sciences, Uppsala, Sweden), 100 U/mL penicillin (Thermo Fisher Scientific), and 100 g/mL streptomycin (Thermo Fisher Scientific). All cell lines were cultured in a humidified incubator in an atmosphere of 5% (v/v) CO2 at 37°C.
Cell viability assay
Fenofibrate, paclitaxel, TRAIL, ABT-737, and doxorubicin alone or in combination were tested in vitro for cytotoxicity against breast cancer cell lines in 96-well plates using the MTT assay to determine the cell viability. In short, cells were seeded in 96-well plates and incubated with different concentrations of fenofibrate, paclitaxel, TRAIL, ABT-737, and doxorubicin alone or in combination for indicated times. First, the medium was discarded, and then 50 µL of MTT solution (1 mg/mL in PBS) was added to each well and the plate was incubated at 37°C for additional 4 hours. Then, 150 µL of dimethylsulfoxide was added to dissolve the formazan crystals. Finally, the absorbance of each sample was read at 570 nm using a microplate reader (Molecular Devices LLC, Sunnyvale, CA, USA).
Apoptotic assay by flow cytometry
Flow cytometry was used to assess externalization by fluorescein isothiocyanate (FITC)-labeled Annexin-V and propidium iodide (PI). Briefly, human breast cancer cells were collected after treatment and washed with ice-cold PBS before staining in 500 µL solution containing Annexin-V-FITC in dark at 4°C. After that, PI was added and incubated for 5 minutes at room temperature. Soon afterwards, the fluorescent signal in the cells was detected by flow cytometry (FACS Calibur; BD Biosciences, San Jose, CA, USA). Data analysis was performed by the software WinMDI 2.9 (The Scripps Research Institute, La Jolla, CA, USA).
Mitochondrial membrane potential change assay
5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylc-arbocyanine iodide (JC-1) staining was used to detect the mitochondrial membrane potential change. Human breast cancer cells were seeded in cell culture dish and incubated with various concentrations of fenofibrate, paclitaxel, TRAIL, ABT-737, and doxorubicin alone or in combination for 24 hours. Then, the cells were harvested, washed by PBS, and resuspended in JC-1 staining solution (10 µM) at room temperature for 10 minutes. After that, the cells were detected by a FACSCanto flow cytometer (BD, Franklin Lakes, NJ, USA).
RNA isolation and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from cell lines using the Trizol (Thermo Fisher Scientific) according to the manufacturer’s protocols. Complementary DNA synthesis of mRNA was performed using M-MLV reverse transcriptase (Promega Corporation, Fitchburg, WI, USA). The mRNA expression levels of Mcl-1, Bcl-2, Bim, Bcl-xl, Bok, Bnip3, and Bax were evaluated using PCR with an SYBR green PCR master mix (Thermo Fisher Scientific) and calculated using the 2−ΔΔCq method by normalizing to GAPDH. The thermocycling conditions were as follows: 95°C for 10 minutes, 45 cycles of 95°C for 15 seconds, and 60°C for 1 minute. All the reactions were performed in triplicate and the primer sequences are listed in .
Table 1 The sequences of primers used in real-time PCR
Western blotting
Subsequent to the treatment indicated, the cells were lysed in lysis buffer (2.1 µg/mL aprotinin, 0.5 µg/mL leupeptin, 4.9 mM MgCl2, 1 mM orthovanadate, 1% Triton X 100, and 1 mM phenylmethylsulfonyl fluoride). The protein concentration was determined using a bicinchoninic acid assay. Subsequent to electrophoresis on a 12% or 15% SDS-PAGE gel, proteins were transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5% non-fat milk and incubated with primary antibodies at 4°C overnight. The corresponding horseradish peroxidase (HRP)-conjugated secondary antibody was added and incubated at room temperature for 2 hours. Signals were visualized using an enhanced chemiluminescence reaction with an HRP substrate. The primary antibodies against PARP, caspase-3, caspase-9, Mcl-1, Bcl-2, Bim, Bcl-xl, Bok, Bnip3, Bax, AKT, p-AKT, NF-κB, p-NF-κB, and histone 3 were purchased from Cell Signaling Technologies (Beverly, MA, USA). The antibody against β-actin was purchased from Sigma-Aldrich Co. (St Louis, MO, USA).
Statistical analysis
All data are expressed as mean ± SD from at least three separate experiments. All statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA). Statistical significance was determined using a two-sided Student’s t-test for all data. For statistical analysis, P<0.05 was considered to indicate a statistically significant difference.
Results
Cytotoxicity of fenofibrate, paclitaxel, TRAIL, ABT-737, and doxorubicin on human breast cancer cells
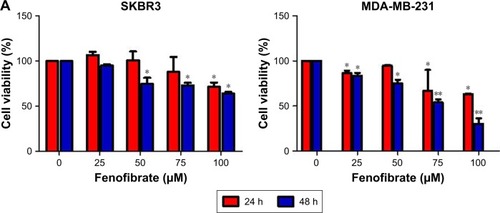
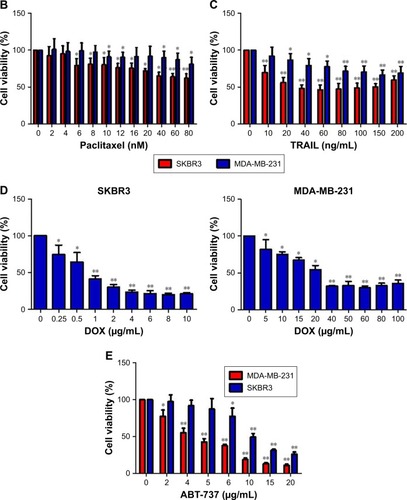
To determine whether fenofibrate could suppress human breast cancer or not, two human breast cancer cell lines and paclitaxel, TRAIL, ABT-737, and doxorubicin were obtained, and the cytotoxicity was evaluated using MTT assay. The results revealed that fenofibrate slightly inhibited SKBR3 cell growth, but significantly suppressed MDA-MB-231 cell growth (). The IC50 of fenofibrate in MDA-MB-231 cells is >100 µM for 24 hours and 79.42±6.25 µM for 48 hours. The IC50 of fenofibrate in SKBR3 cells is >100 µM for both 24 and 48 hours. In addition, cell viability was measured in breast cancer cell lines treated with paclitaxel, TRAIL, ABT-737, and doxorubicin for 24 hours. As presented in , human breast cancer cell lines SKBR3 and MDA-MB-231 involved in the study are highly resistant to paclitaxel and TRAIL, while relatively sensitive to ABT-737 and doxorubicin. The IC50 values of paclitaxel, TRAIL, ABT-737, and doxorubicin in SKBR3 cells are >80 nM, 35±0.69 ng/mL, 11.56±0.93 µg/mL, and 0.71±0.08 µg/mL, respectively. The IC50 values of paclitaxel, TRAIL, ABT-737, and doxorubicin in MDA-MB-231 cells are >80 nM, >200 ng/mL, 4.25±0.21 µg/mL, and 32.41±1.12 µg/mL, respectively.
Figure 1 Cytotoxicity of fenofibrate, paclitaxel, TRAIL, ABT-737, and doxorubicin in human breast cancer cells.
Notes: (A) Fenofibrate inhibited human breast cancer cell growth in vitro. The cancer cells were incubated in the presence of various concentrations of fenofibrate for 24 and 48 hours. Cell viability was determined by the MTT assay. Each point represents the mean of the data of three independent experiments; bars represent SD; *P<0.05 vs control; **P<0.01 vs control. (B–E) Paclitaxel, TRAIL, ABT-737, and doxorubicin suppressed human breast cancer cell growth in vitro. The cancer cells were incubated in the presence of various concentrations of fenofibrate for 24 hours. Cell viability was determined by the MTT assay. Each point represents the mean of the data of three independent experiments; bars represent SD; *P<0.05 vs control; **P<0.01 vs control.
Abbreviations: DOX, doxorubicin; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
Fenofibrate potentiates chemosensitivity of human breast cancer by modulating apoptosis
Next, we examined whether combined treatment of fenofibrate and paclitaxel, TRAIL, ABT-737, and doxorubicin exerts enhanced lethality in human breast cancer cell lines. After co-treatment with indicated concentrations of fenofibrate and paclitaxel, TRAIL, ABT-737, and doxorubicin for 24 hours, MTT assay was performed. Interestingly, combination of fenofibrate and paclitaxel, TRAIL, ABT-737, and doxorubicin dramatically inhibits the cell growth in both human breast cancer cell lines SKBR3 and MDA-MB-231 ().
Figure 2 Fenofibrate potentiates chemosensitivity to human breast cancer by modulating apoptosis.
Notes: (A) SKBR3 cells treated with indicated concentrations of fenofibrate alone and/or combined with indicated concentrations of paclitaxel, TRAIL, ABT-737, and doxorubicin for 24 hours. Cell viability was determined by the MTT assay. Each point represents the mean of the data of three independent experiments; bars represent SD; *P<0.05 vs control; **P<0.01 vs control. (B, C) SKBR3 cells treated as A without doxorubicin treatment. After that, cells were subjected to Annexin-V-FITC and PI staining. Flow cytometry assay was performed to detect the percentage of apoptotic cells. Data are presented as mean ± SD, n=3. **P<0.01 vs control. (D) MDA-MB-231 cells treated with indicated concentrations of fenofibrate alone and/or combined with indicated concentrations of paclitaxel, TRAIL, ABT-737, and doxorubicin for 24 hours. Cell viability was determined by the MTT assay. Each point represents the mean of the data of three independent experiments; bars represent SD; *P<0.05 vs control; **P<0.01 vs control. (E, F) MDA-MB-231 cells treated as D without doxorubicin treatment. **P<0.01 vs control. SKBR3 cells (G) were treated with indicated concentrations of fenofibrate and TRAIL, and MDA-MB-231 cells (H) were treated with indicated concentrations of fenofibrate and ABT-737. MTT assay was performed and the data were analyzed by Chou-Talalay method (combination index >1 indicates antagonism, =1 indicates additivity, and <1 indicates synergy).
Abbreviations: DOX, doxorubicin; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; PI, propidium iodide; FENO, fenofibrate; PAC, paclitaxel; FITC, fluorescein isothiocyanate.
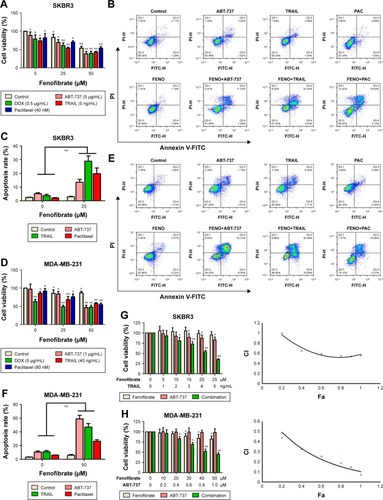
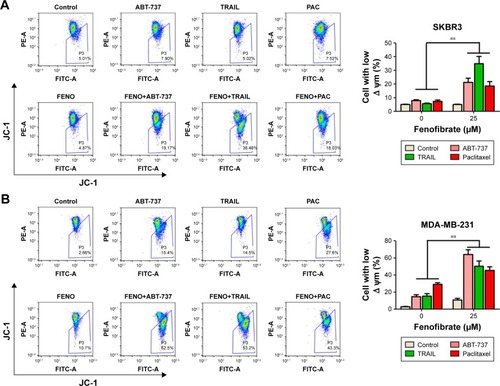
To confirm whether the sensitization effect of fenofibrate involves apoptosis induction, SKBR3 and MDA-MB-231 cells were subjected to flow cytometry analysis after cells were treated with fenofibrate, paclitaxel, TRAIL, and ABT-737, alone or in combination. As shown in , fenofibrate dramatically enhanced paclitaxel-, TRAIL-, and ABT-737-induced apoptosis in SKBR3 and MDA-MB-231 cells (the percentage of Annexin-V-positive cells probably increased from 7.1% and 15.29% to 19.76% and 24.33% when combined with paclitaxel, from 8.71% and 20.31% to 28.87% and 47.07% when combined with TRAIL, from 10.06% and 19.95% to 13.53% and 59.17% when combined with ABT-737 in SKBR3 and MDA-MB-231, respectively). More importantly, combined treatment of fenofibrate and TRAIL (in SKBR3 cells) or ABT-737 (in MDA-MB-231 cells) presented well as synergism (combination index; CI <1, as shown in ). These results suggest that fenofibrate potentiates chemosensitivity to human breast cancer cell via inducing apoptosis.
Initiation of intrinsic apoptotic pathway in the sensitization effect of fenofibrate on ABT-737, TRAIL, and paclitaxel in human breast cancer cells
Here, the underlying mechanisms of the sensitization effect of fenofibrate in breast cancer cells were further elucidated. Our observation indicated that PARP is cleaved in fenofibrate-and-TRAIL- and paclitaxel-treated SKBR3 cells and fenofibrate-and-TRAIL- and ABT-737-treated MDA-MB-231 cells () which supported that the apoptosis is indeed involved in fenofibrate-and-TRAIL-, paclitaxel-, and ABT-737-induced cell death. We further examined the cleavage of caspases, and as shown in , co-treatment with fenofibrate and TRAIL, paclitaxel, and ABT-737 in SKBR3 and MDA-MB-231 cells significantly increased the cleavage of caspase-3 and caspase-9 (caspase-8 was not detected and the data not shown). Therefore, the effect of fenofibrate and ABT-737, TRAIL, and paclitaxel on mitochondrial outer membrane potential (MOMP) was determined by JC-1 staining and flow cytometry experiment. As shown in , the disruption of MOMP induced by fenofibrate and ABT-737, TRAIL, and paclitaxel was significantly increased. After co-treatment with 25 µM fenofibrate and indicated concentration of ABT-737, TRAIL, and paclitaxel, the percentage of cells with depolarized MOMP increased to 19.17%, 36.46%, and 18.03% in SKBR3 cells and 62.5%, 53.2%, and 43.3% in MDA-MB-231 cells, respectively.
Figure 3 Treatment with fenofibrate resulted in downregulation of Mcl-1 and Bcl-xl and upregulation of Bok and Bax.
Notes: (A) SKBR3 and MDA-MB-231 cells treated with indicated concentrations of fenofibrate and combined with indicated concentrations of paclitaxel, TRAIL, and ABT-737 for 24 hours. After that, PARP and caspases proteins were assessed by Western blotting assay. (B) SKBR3 and MDA-MB-231 cells treated with indicated concentrations of fenofibrate for 24 hours. After that, Bcl-2 family proteins were assessed by Western blotting assay. (C) SKBR3 and MDA-MB-231 cells treated with indicated concentrations of fenofibrate for 24 hours. After that, qRT-PCR analyzed expression level of Bcl-2 family proteins. Data were presented as mean ± SD for three independent experiments. *P<0.05 vs control; **P<0.01 vs control.
Abbreviations: TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; PARP, poly (ADP-ribose) polymerase 1; caspase, the cysteine-aspartic acid protease.
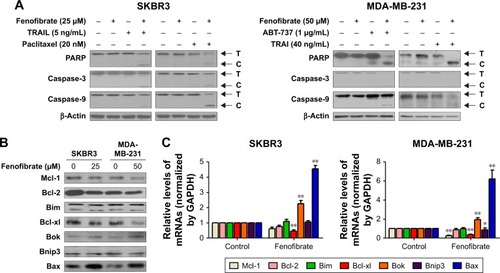
Figure 4 The intrinsic apoptotic pathway is initiated in the sensitization effect of fenofibrate on ABT-737, TRAIL, and paclitaxel in human breast cancer cells.
Notes: SKBR3 (A) and MDA-MB-231 (B) cells were treated as mentioned in . After treatment, the cells were stained with JC-1 and subjected to flow cytometry assay to detect the mitochondrial outer membrane permeabilization. Data are presented as mean ± SD, n=3. **P<0.01 vs control.
Abbreviations: TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; JC-1, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide; FENO, fenofibrate; PAC, paclitaxel.
Treatment with fenofibrate resulted in downregulation of Mcl-1 and Bcl-xl and upregulation of Bok and Bax in human breast cancer cells
Previous studies have shown that fenofibrate inhibits mTOR-p70S6K signaling, regulates autophagy and endoplasmic reticulum stress in human prostate cancer cells, and induces apoptosis of triple-negative breast cancer cells via activation of NF-κB pathway.Citation8–Citation10 However, the role of fenofibrate in human breast cancer is still ambiguous. At first, we evaluated the effect of fenofibrate on Bcl-2 family proteins expression by Western blotting. Surprisingly, as shown in , treatment with fenofibrate dramatically inhibited the protein level of Mcl-1 and Bcl-xl and increased the protein level of Bok and Bax in SKBR3 and MDA-MB-231 cells.
To draw the conclusion whether fenofibrate-mediated decrease of Mcl-1 and Bcl-xl and increase of Bok and Bax in human breast cancer cells was at the transcriptional level, RT-PCR was used to identify the effects of fenofibrate on the mRNA level of Bcl-2 family proteins. It was found that fenofibrate repressed transcription level of Mcl-1 and Bcl-xl and activated transcription level of Bok and Bax in SKBR3 and MDA-MB-231 cells (), suggesting that fenofibrate-mediated Mcl-1 and Bcl-xl decrease and Bok and Bax increase was likely at the transcriptional level.
Fenofibrate potentiated the apoptosis induced by anti-cancer drugs in human breast cancer cells via inhibiting the activation of AKT/NF-κB pathway
AKT/NF-κB pathway is important for the balance between cell survival and apoptosis.Citation16,Citation17 To determine whether AKT/NF-κB signaling pathways are involved in fenofibrate-induced apoptosis, we checked phosphorylated AKT/NF-κB by Western blotting (). Fenofibrate treatment significantly decreased the level of phosphorylated AKT and NF-κB p65, but did not change the total levels of AKT and NF-κB p65 in SKBR3 and MDA-MB-231 cells. As shown in , after treatment of indicated concentration of fenofibrate, nuclear p65 decreased and cytosolic p65 increased both in a dose-dependent manner for 24 hours in MDA-MB-231 cells. These observations indicate that AKT/NF-κB pathway might be a potential target by which fenofibrate can potentiate human breast cancer cells to paclitaxel, TRAIL, ABT-737, and doxorubicin.
Figure 5 Fenofibrate exerted its chemo-sensitization effects in human breast cancer cells apoptosis via activation of AKT/NF-κB pathway.
Notes: (A) SKBR3 and MDA-MB-231 cells treated with indicated concentrations of fenofibrate for 24 hours. After that, AKT and NF-κB proteins were assessed by Western blotting assay. (B) Nuclear translocation of NF-κB in MDA-MB-231 cells was determined by Western blotting after treatment with indicated concentrations of fenofibrate for 24 hours. (C) Activation of NF-κB in MDA-MB-231 cells was determined by Western blotting after treatment with indicated concentrations of imiquimod. (D) MDA-MB-231 cells were pretreated with 50 µM imiquimod for 24 hours followed by co-treatment with 25 µM fenofibrate and 1 µg/mL ABT-737 for another 24 hours. Flow cytometry assay was used to detect the cell apoptosis. Data were presented by mean ± SD for three independent experiments. **P<0.01 vs control.
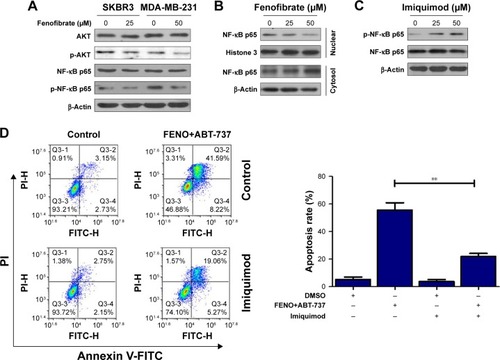
To further confirm whether fenofibrate enhances the apoptosis-induced ability of paclitaxel, TRAIL, and ABT-737 via AKT/NF-κB pathway, MDA-MB-231 cells were pretreated with 50 µM imiquimod, an NF-κB agonist, to activate NF-κB pathway. After pretreatment, MDA-MB-231 cells were co-treated with 25 µM fenofibrate and 1 µg/mL ABT-737 for 24 hours. As shown in , phosphorylation of NF-κB p65 is highly activated in MDA-MB-231 cells treated with imiquimod compared with its control cells. More importantly, high level of phosphorylated NF-κB p65 significantly induced by imiquimod effectively attenuates the apoptosis mediated by the combination of fenofibrate and ABT-737 (). Taken together, fenofibrate sensitizes human breast cancer cells to paclitaxel, TRAIL, ABT-737, and doxorubicin at least partially through inhibiting the activation of AKT/NF-κB signaling pathway.
Discussion
Chemoresistance is one of the most important challenges in cancer treatment and believed to be a main reason for cancer therapy failure, and multiple mechanisms are reported to take part in the development of drug resistance in cancer cells. In addition to Bcl-2 family mentioned above, the abnormal regulation of cell cycle could also mediate the drug resistance in multiple cancer types.Citation18 Furthermore, it was reported that mitochondrial reactive oxygen species play an important role in cancer drug resistance.Citation19 As a key tumor suppressor, P53 exerts a pivotal role in protecting from malignancies. However, overexpression of mutated p53 is frequently associated with resistance to several anti-cancer drugs, including cisplatin, temozolomide, doxorubicin, gemcitabine, tamoxifen, and EGFR-inhibitors (such as cetuximab).Citation20 Although tremendous progress has been made to help us to understand the underlying mechanism of drug resistance of cancer cells, it is still urgent to find novel and more effective sensitizer for chemotherapy.
As an important therapy of hyperlipidemia and hypercholesterolemia, fenofibrate is recently studied in anti-tumor effects in multiple cancer types.Citation10,Citation13,Citation21–Citation23 Its anti-tumor functions such as suppression of cancer cell growth,Citation12 restraining cancer cell metastasisCitation24 and inhibiting carcinogenesis,Citation25 inducing cell-cycle arrest and apoptosis by suppression of Bcl-2, and AKT phosphorylation in prostate cancerCitation26 were discovered during recent years. More recently, fenofibrate was reported to overcome the drug resistance of human prostate cancer cells in a peroxisome proliferator-activated receptor alpha/reactive oxygen species-independent manner.Citation27 However, to date, the function and underlying mechanism of fenofibrate-enhanced chemosensitivity in other cancers have not been well documented, particularly in breast cancer.
The current study attempted to illustrate the role and the underlying mechanism of fenofibrate in the drug susceptibility in human breast cancer. First, the present study verified that fenofibrate inhibited human breast cancer cell growth in a dose- and time-dependent manner. In addition, MTT assay results were also validated by flow cytometry assay with Annexin-V/PI staining. We further illuminated the subcytotoxic level of fenofibrate-sensitized paclitaxel, TRAIL, and ABT-737 on human breast cancer cells by inducing apoptosis. These results suggested that fenofibrate modulates apoptotic pathway which results in an increase in cell sensitivity to paclitaxel-, TRAIL-, and ABT-737-mediated signaling.
Next, since cleavage of caspases and PARP are hallmarks of activation of the apoptosis pathway, we used Western blotting to check the result of cleaved caspase-3, caspase-9, and PARP, which were upregulated in the SKBR3 and MDA-MB-231 cells co-treated with fenofibrate and paclitaxel, TRAIL, as well as ABT-737. Moreover, the augment of pro-apoptosis Bcl-2 family members, such as Bok and Bax, and decrease of pro-survival Bcl-2 family members, such as Mcl-1 and Bcl-xl, resulted in apoptosis by the activation of caspase-9 and caspase-3.Citation28–Citation30 However, in the present study, we detected notable reduction of Mcl-1 and Bcl-xl and activation of Bok and Bax but no significant changes in Bim, Bnip3, and Bcl-2. Thus, we consider downregulation of Mcl-1 and Bcl-xl as well as upregulation of Bok and Bax is an important cause of the augmentation of paclitaxel-, TRAIL-, and ABT-737-induced apoptosis mediated by fenofibrate.
Finally, we investigated the activation of AKT/NF-κB pathway under fenofibrate treatment, since the serine-threonine kinase AKT is best known as a regulator of cell proliferation and survival.Citation31 It is well known that NF-κB has two-way regulation effects on cell apoptosis.Citation32 Our study demonstrated that the level of phosphorylated AKT and NF-κB p65 was reduced by fenofibrate in SKBR3 and MDA-MB-231 cells. Moreover, imiquimod induced phosphorylated NF-κB p65 expression significantly and reversed the apoptosis mediated by the combination of fenofibrate and ABT-737. Thus, these data obviously confirm that fenofibrate potentiates chemosensitivity of human breast cancer cell to paclitaxel, TRAIL, ABT-737, and doxorubicin by suppressing phosphorylated NF-κB p65 at least partially.
Conclusion
Although other downstream target genes of fenofibrate may also take part in modulating apoptosis, the present data demonstrated that fenofibrate could potentiate chemosensitivity to human breast cancer by suppressing the activation of AKT/NF-κB p65 signaling pathway at least partially, which served a prominent function as an activator in apoptosis by downregulating Mcl-1 and Bcl-xl and upregulating Bok and Bax. Therefore, the results of the present study prove that fenofibrate with paclitaxel, TRAIL, ABT-737, and doxorubicin therapies may turn out to be effective new strategies for the treatment of chemo-resistant human breast cancer.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported in part by Zhejiang Medical Association (No 2013ZYC-A134), Technology Division of Taizhou (No 1501KY19), Department of Education of Zhejiang Province (No 201738081), and Department of Science and Technology of Zhejiang Province (No 2016C33230).
Data availability
All data for this study are presented in this published article.
Disclosure
The authors report no conflicts of interest in this work.
References
- BrayFFerlayJSoerjomataramISiegelRLTorreLAJemalAGlobal cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countriesCA Cancer J Clin201868639442430207593
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- PanYZhangFZhaoYBerberine enhances chemosensitivity and induces apoptosis through Dose-orchestrated AMPK signaling in breast cancerJ Cancer2017891679168928775788
- MorinPJDrug resistance and the microenvironment: nature and nurtureDrug Resist Updat20036416917212962682
- Bentires-AljMDejardinEViatourPInhibition of the NF-kappa B transcription factor increases Bax expression in cancer cell linesOncogene200120222805281311420692
- IntaIPaxianSMaegeleIBim and Noxa are candidates to mediate the deleterious effect of the NF-kappa B subunit RelA in cerebral ischemiaJ Neurosci20062650128961290317167080
- SaidiSAHollandCMCharnock-JonesDSSmithSKIn vitro and in vivo effects of the PPAR-alpha agonists fenofibrate and retinoic acid in endometrial cancerMol Cancer200651316569247
- LianXGuJGaoBFenofibrate inhibits mTOR-p70S6K signaling and simultaneously induces cell death in human prostate cancer cellsBiochem Biophys Res Commun20184961707529305864
- TaoTZhaoFXuanQShenZXiaoJShenQFenofibrate inhibits the growth of prostate cancer through regulating autophagy and endoplasmic reticulum stressBiochem Biophys Res Commun201850342685268930098788
- LiTZhangQZhangJFenofibrate induces apoptosis of triple-negative breast cancer cells via activation of NF-κB pathwayBMC Cancer2014149624529079
- TsaiSCTsaiMHChiuCFAMPK-dependent signaling modulates the suppression of invasion and migration by fenofibrate in CAL 27 oral cancer cells through NF-κB pathwayEnviron Toxicol201631786687625545733
- HuDSuCJiangMFenofibrate inhibited pancreatic cancer cells proliferation via activation of p53 mediated by upregulation of LncRNA MEG3Biochem Biophys Res Commun2016471229029526850851
- GoncalvesMDHwangSKPauliCFenofibrate prevents skeletal muscle loss in mice with lung cancerProc Natl Acad Sci U S A20181154E743E75229311302
- MuradHColletPHuin-SchohnCEffects of PPAR and RXR ligands in semaphorin 6B gene expression of human MCF-7 breast cancer cellsInt J Oncol200628497798416525649
- HanDFZhangJXWeiWJFenofibrate induces G0/G1 phase arrest by modulating the PPARα/FoxO1/p27 kip pathway in human glioblastoma cellsTumour Biol20153653823382925566967
- SrinivasKPVijiRDanVMDEPTOR promotes survival of cervical squamous cell carcinoma cells and its silencing induces apoptosis through downregulating PI3K/AKT and by up-regulating p38 MAP kinaseOncotarget2016717241542417126992219
- MiyataSWangLYKitanakaS3EZ, 20Ac-ingenol induces cell-specific apoptosis in cyclin D1 over-expression through the activation of ATR and downregulation of p-AktLeuk Res201864465129179029
- ShahMASchwartzGKCell cycle-mediated drug resistance: an emerging concept in cancer therapyClin Cancer Res2001782168218111489790
- OkonISZouMHMitochondrial ROS and cancer drug resistance: Implications for therapyPharmacol Res201510017017426276086
- HientzKMohrABhakta-GuhaDEfferthTThe role of p53 in cancer drug resistance and targeted chemotherapyOncotarget2017858921894627888811
- KusunokiMSatoDTsutsumiKTsutsuiHNakamuraTOshidaYBlack soybean extract improves lipid profiles in fenofibrate-treated type 2 diabetics with postprandial hyperlipidemiaJ Med Food201518661561825651043
- OikawaSYamashitaSNakayaNSasakiJKonoSfor the Effect of Fenofibrate and Ezetimibe Combination Treatment on Lipid (EFECTL) Study InvestigatorsEfficacy and safety of long-term coadministration of fenofibrate and ezetimibe in patients with combined hyperlipidemia: results of the EFECTL studyJ Atheroscler Thromb2017241779427397061
- Flores-CastilloCZamora-PérezJÁCarreón-TorresEAtorvastatin and fenofibrate combination induces the predominance of the large HDL subclasses and increased apo AI fractional catabolic rates in New Zealand White rabbits with exogenous hypercholesterolemiaFundam Clin Pharmacol201529436237025982284
- PiwowarczykKWybieralskaEBaranJFenofibrate enhances barrier function of endothelial continuum within the metastatic niche of prostate cancer cellsExpert Opin Ther Targets201519216317625389904
- GongYShaoZFuZFenofibrate inhibits cytochrome P450 epoxygenase 2C activity to suppress pathological ocular angiogenesisEBioMedicine20161320121127720395
- LianXWangGZhouHZhengZFuYCaiLAnticancer properties of fenofibrate: a repurposing useJ Cancer2018991527153729760790
- LutyMPiwowarczykKWróbelTPO-235 Fenofibrate overcomes the drug-resistance of human prostate cancer cellsESMO Open20183Suppl 2A112
- HsuSYKaipiaAMcgeeELomeliMHsuehAJBok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective anti-apoptotic Bcl-2 family membersProc Natl Acad Sci U S A1997942312401124069356461
- KeFFSVanyaiHKCowanADEmbryogenesis and adult life in the absence of intrinsic apoptosis effectors Bax, Bak, and BokCell201817351217123029775594
- BudihardjoIOliverHLutterMLuoXWangXBiochemical pathways of caspase activation during apoptosisAnnu Rev Cell Dev Biol19991526929010611963
- BenbrookDMMasamhaCPThe pro-survival function of Akt kinase can be overridden or altered to contribute to induction of apoptosisCurr Cancer Drug Targets201111558659921486222
- YangGXiaoXRosenDGThe biphasic role of NF-kappaB in progression and chemoresistance of ovarian cancerClin Cancer Res20111782181219421339307
