Abstract
Since the blood vessel epicardial substance or Popeye domain-containing protein 1 (BVES/POPDC1) was first identified in the developing heart by two independent laboratories in 1999, an increasing number of studies have investigated the structure, function, and related diseases of BVES/POPDC1. During the first 10 years following the discovery of BVES/POPDC1, studies focused mainly on its structure, expression patterns, and functions. Based on these studies, further investigations conducted over the previous decade examined the role of BVES/POPDC1 in human diseases, such as colitis, heart diseases, and human cancers. This review provides an overview of the structure and expression of BVES/POPDC1, mainly focusing on its potential role and mechanism through which it is involved in human cancers.
Introduction
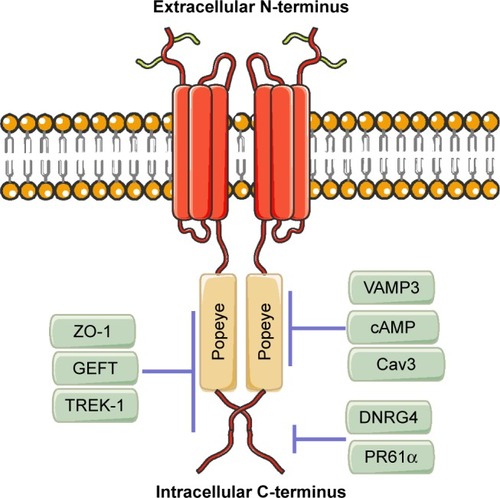
Blood vessel epicardial substance (BVES), also known as Popeye domain-containing protein 1 (POPDC1), belongs to the POPDC family, which shares the same Popeye structure within the intracellular C-terminus.Citation1,Citation2 The POPDC family consists of BVES/ POPDC1, POPDC2, and POPDC3. These genes produce evolutionarily conserved transmembrane proteins and do not share significant structural homology with any other identified proteins.Citation2 BVES/POPDC1 is 359 amino acids long, is principally localized to the plasma membrane, and contains an extracellular amino terminus, three transmembrane domains, and a cytoplasmic Popeye domain.Citation3 The extracellular N-terminus of BVES/POPDC1 contains two invariant N-glycosylation sites, which are thought to be dispensable. However, they may be involved in the protection of BVES/ POPDC1 from proteolysis and localization of BVES/POPDC1 to the membrane.Citation4,Citation5 The intracellular C-terminus of BVES/POPDC1 includes the novel but highly conserved Popeye domain. BVES/POPDC1 protein forms a dimer or multimer in the cell within the Popeye domain (). These BVES–BVES interactions are necessary to maintain epithelial integrity and junctional stability.Citation6
Figure 1 BVES/POPDC1 structure.
Notes: BVES/POPDC1 protein consists of an extracellular N-terminus, a three- pass transmembrane domain, and an intracellular C-terminus containing the highly conserved Popeye domain. BVES/POPDC1 directly binds to cAMP, VAMP3, and Cav3 in the Popeye domain, directly interacts with DNRG4 and PR61α in the intracellular C-terminus outside of Popeye domain, and directly interacts with ZO-1, GEFT, and TREK-1 in the intracellular C-terminus both inside and outside of Popeye domain.
BVES/POPDC1 transcripts have been identified in an array of eukaryotes ranging from honey bees to human beings.Citation7 Human BVES/POPDC1 has been identified in the heart, smooth and skeletal muscle, brain, liver, gastrointestinal tract, and various epithelia.Citation2,Citation8–Citation13 BVES/POPDC1 is a newly discovered cyclic 3′,5′-adenosine monophosphate (cAMP) effectorCitation14 and a caveolae-associated protein important for the tolerance of environmental stress (eg, oxygen deficiency and nutrient deprivation). Moreover, it is involved in the regulation of cell calcium and survival pathway signaling.Citation15,Citation16 Interestingly, BVES/POPDC1-expressing cells have a common phenotype or function: they are adherent or are at least highly interactive in nature.Citation7 Subcellular localization studies revealed that BVES/POPDC1 is mainly localized in the cytomembrane, especially in the lateral cell membrane, cell junction, and tight junction.Citation17 Its movement from the cytoplasm to membrane is an early event occurring concurrently with cell–cell contact.Citation17,Citation18 BVES/POPDC1 colocalizes in epithelial cells with Occludin and ZO-1 in an apical–lateral position within the z-axis.Citation17 This expression pattern indicates a role of BVES/POPDC1 in cell communication or cell–cell adhesion.
The expression of BVES/POPDC1 during embryogenesis has been studied in several organisms, including chick,Citation13 mouse,Citation12 Xenopus laevis,Citation19 and Drosophila.Citation20 In these studies, BVES/POPDC1 was found in a multitude of tissue types derived from all three germ layers both in the embryo and adults. In 2008, Feng et alCitation21 were the first to link the expression of BVES/POPDC1 to non-small-cell lung cancer (NSCLC) in human beings. In the following 10 years, accumulating evidence has revealed the role and mechanism of BVES/POPDC1 in human diseases, especially in cancers.
BVES/POPDC1 in cancers
We have summarized the articles discussing the role of BVES/POPDC1 in human cancers in .
Table 1 The expression and function of BVES in human cancers
BVES/POPDC1 in lung cancer
Feng et alCitation21 performed MethyLight assays to detect the DNA methylation status of 27 genes in 49 paired cancerous and noncancerous tissues obtained from patients with NSCLC who underwent surgical resection. They found that seven genes significantly more frequently methylated at high levels (percentage of methylated reference ≥4%) in cancerous vs noncancerous tissue, and among which, BVES/POPDC1 had high levels of methylation in cancerous but never noncancerous tissue.Citation21 Feng et al’s study was the first to incorporate BVES/POPDC1 into the genes frequently methylated in NSCLC.Citation21 In 2010, this research group studied the early events of lung cancer by analyzing lung tissue samples obtained from 151 cancer-free subjects (121 smokers and 30 nonsmokers) for hypermethylation of genes previously observed to be hypermethylated in NSCLC. They found that BVES/POPDC1 was rarely hypermethylated (<2%) in these subjects, suggesting that BVES/POPDC1 methylation is not the preneoplastic event leading to NSCLC.Citation22 However, they speculated that the detection of BVES/POPDC1 methylation may be conducive to monitor and detect tumor recurrence in early-stage NSCLC after curative surgical resection.Citation22
BVES/POPDC1 in gastric cancer
Kim et alCitation23 examined the hypermethylation of POPDCs in gastric cancer and found that the expression of BVES/ POPDC1 and POPDC3 was silenced in 8/11 (73%) gastric cancer cell lines investigated. Further analysis suggested that the hypermethylation of the BVES/POPDC1 and POPDC3 promoters correlated with their decreased expression in gastric cancer cell lines. BVES/POPDC1 and POPDC3 were hypermethylated in 69% and 64% of gastric cancer tissues, respectively. Treatment with the DNA methylation inhibitor 5-aza-dC and the histone deacetylase inhibitor trichostatin A (TSA) restored the expression of BVES/POPDC1 in gastric cancer cell lines SNU-601, SNU-620, and SNU-638.Citation23 In addition, they found that BVES/POPDC1 and POPDC3 were downregulated in EGF-induced epithelial–mesenchymal transition (EMT). Moreover, silencing of POPDC3 promoted the migration and invasion of gastric cancer cells. However, these results did not reveal a direct role of BVES/POPDC1 in gastric cancer.Citation23 There was no significant correlation between the expression of BVES/POPDC1 or POPDC3 and clinical characteristics. The investigators speculated that the inactivation of BVES/POPDC1 and POPDC3 is an early-stage process in gastric cancer prior to the occurrence of metastasis. However, Luo et alCitation24 examined 306 human gastric cancer and 78 noncancerous gastric tissues and found that the expression of BVES/POPDC1 was decreased in gastric cancer tissue. The low expression of BVES/POPDC1 correlated with histological differentiation, depth of invasion, regional lymph node and distant metastasis, and TNM stage. They also suggested that the reduced expression of BVES/POPDC1 is associated with the progression of gastric cancer and poor survival.Citation24 The contradictory results obtained from these two studies may be attributed to the following: 1) Kim et al analyzed the level of BVES/POPDC1 methylation and clinicopathological characteristics, whereas Luo et al compared the protein level of BVES/POPDC1 with clinicopathological characteristics through immunohistochemistry; 2) Kim et al only analyzed a limited range of clinicopathological characteristics (ie, age, gender, histology, and TNM stage), whereas the research conducted by Luo et al included a more comprehensive clinicopathological profile (ie, age, gender, location, size, histology, histological differentiation, invasion depth, regional lymph node metastasis, distant metastasis, and TNM stage); 3) The number of experimental samples and experimental groups were different: Kim et al studied the expression of BVES/POPDC1 in 96 pairs of gastric cancer tissues and adjacent healthy tissues, whereas Luo et al performed their investigation in 306 gastric cancer tissues and 78 noncancerous gastric tissues. Further research is warranted to clarify the role of BVES/POPDC1 in gastric cancer metastasis and the molecular mechanism involved in this process.
BVES/POPDC1 in colorectal cancer
The expression of BVES/POPDC1 was found to be decreased in all stages of human colorectal carcinoma and in adenomatous polyps.Citation25 Similar to lung cancer and gastric cancer, the low expression of BVES/POPDC1 in colorectal carcinoma and adenoma were mainly due to hypermethylation of the gene promoter on the cytosine–phosphate–guanine island.Citation25 Overexpression of BVES/POPDC1 promoted the epithelial phenotype of colorectal cancer cells, including decreased proliferation, migration, invasion, and anchorage-independent growth as well as impaired growth and metastasis in an orthotopic xenograft.Citation25 More recently, BVES/POPDC1 was shown to be reduced in human ulcerative colitis and colitis-associated cancer biopsy specimens.Citation26 Furthermore, BVES/POPDC1-knockout mice (BVES/POPDC1−/−) presented with increased crypt height, elevated proliferation, decreased apoptosis, altered intestinal lineage allocation, and dysregulation of permeability and intestinal immunity.Citation26 Furthermore, BVES/POPDC1−/− mice exhibited increased tumor multiplicity and degree of dysplasia after treatment with azoxymethane and dextran sodium sulfate.Citation26 Molecular analysis revealed that the knockdown of BVES/POPDC1 increased c-Myc stability – an oncogene that is dysregulated in numerous malignancies – and subsequently increased the expression of downstream key target genes, such as ornithine decarboxylase and carbamoyl phosphate synthetase 2 aspartate transcarbamylase and dihydroorotase. However, the overexpression of BVES/POPDC1 reduced c-Myc stability and increased c-Myc ubiquitylation.Citation26 In addition, BVES/POPDC1 was shown to regulate tumor proliferation in colorectal cancer via the WNT signaling pathway.Citation25 Interestingly, Xing et alCitation27 performed a bioinformatics analysis of colon adenocarcinoma (COAD) RNA-seq V2 exon data obtained from The Cancer Genome Atlas data portal, which included 285 tumor samples and 41 pericarcinomatous tissue samples. The results showed that BVES/POPDC1-AS1 and other three lncRNAs (MYLK-AS1, ADAMTS9-AS1, and FENDRR) were shown to have significant co-regulatory relationships or functional synergistic effects. All these lncRNAs were downregulated in COAD, suggesting their critical roles in the development and progression of COAD.Citation27 The research also revealed another posttranscriptional modification except for hypermethylation. However, further studies are warranted to illustrate the role of BVES/POPDC1-AS1 in colorectal cancer.
BVES/POPDC1 in hepatocellular carcinoma (HCC)
The tight junction is one of the most important intercellular structures of the liver.Citation28 The tight junction and its associated proteins are usually found to be decreased in HCC, such as ZO-1, Claudin-1, and twist.Citation29–Citation31 Our experimental group investigated the expression of BVES/POPDC1 in human HCC tissues and found that it was downregulated compared with that observed in corresponding paracancerous tissues.Citation32,Citation33 The expression of BVES/POPDC1 was also decreased in HCC cell lines. Interestingly, the decreased expression of BVES/POPDC1 was related to the invasion potential of HCC cell lines, ie, BVES/POPDC1 expression was inversely correlated with the metastatic potential of HCC cell lines.Citation32 Knockdown of BVES/POPDC1 triggered EMT, including morphological changes in cytoskeleton rearrangement and junctional disruption, elevated expression of matrix metalloproteinase (MMP)2, MMP9, and IL-6 and decreased E-cadherin, and importantly, increased cell migration and invasion.Citation32 Furthermore, we found that the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway may be another important upstream regulating signal pathway for BVES/POPDC1. Of note, the PI3K/Akt pathway inhibitor LY-294002 restored the decreased expression of BVES/POPDC1 inhibited by rhnetrin-1.Citation33 DNA methylation remains an important modification leading to the decreased expression of BVES/POPDC1 in HCC.Citation34 Dong et alCitation35 detected the methylation status of multiple gene promoters in the serum of patients with hepatitis B virus (HBV)-related HCC, liver cirrhosis, chronic hepatitis B, and healthy individuals. The rate of hypermethylation of the BVES/POPDC1 promoter in HCC patients was 29.59%, higher than that observed in those with liver cirrhosis (4%), chronic hepatitis B (1.11%), and healthy individuals (0). The combined detection of BVES/POPDC1 methylation with that of RASSF1A, HOXA9, and AFP in HCC patients yielded a sensitivity and specificity of 83.7% and 78.9%, respectively.Citation35 Therefore, the use of a combined methylation kit for the promoters of the BVES/POPDC1, RASSF1A, and HOXA9 genes in the serum and AFP may improve the diagnosis of HBV-related HCC in clinical practice. However, additional research is necessary to confirm the effectiveness of this approach.
BVES/POPDC1 in breast cancer
Contrary to the high expression of POPDC2 and POPDC3 in breast cancer, the expression of BVES/POPDC1 is decreased at all stages of ductal breast carcinoma tissues and in all molecular subtypes of breast cancer (ie, luminal A, luminal B, human EGFR2 positive, and triple negative). Moreover, it is suppressed in the more aggressive breast cancer cell lines compared with that observed in nonmalignant breast cancer cells.Citation36 Consistently with previous studies in HCC and gastric cancer, BVES/POPDC1 is not correlated with the clinical progression of breast cancer.Citation36 This phenomenon suggests that the inhibition of BVES/POPDC1 is a feature of all clinical stages of these cancers, and the early molecular alteration of BVES/POPDC1 may represent an initiation step in the malignant process. Indeed, the suppression of BVES/POPDC1 promotes the migration and proliferation of breast cancer cells, whereas the overexpression of BVES/ POPDC1 inhibits this malignant phenotype.Citation37 There is a significant inverse correlation between BVES/POPDC1 and EGFR expression in both stage 2 and stage 3 breast cancer tissues. Notably, EGF protein significantly suppressed BVES/POPDC1 expression in MCF7, MDA231, and SKBR3 breast cancer cells, whereas the use of the EGFR inhibitor AG1478 (1 mM concentration) increased the level of BVES/ POPDC1.Citation36 Further study found that the overexpression of BVES/POPDC1 attenuated EGF-mediated cell migration and proliferation in breast cancer cells.Citation36 Previous studies also proved that the EGFR signaling pathway regulates BVES/ POPDC1 expression in certain follicle cells of Drosophila during oogenesis and in gastric cancer cells.Citation20 The increased expression of EGF and EGF receptors has been reported to be a potent stimulator of cancer cell migration and invasion.Citation38–Citation40 Furthermore, EGFR-targeted therapies, including monoclonal antibodies,Citation41,Citation42 tyrosine kinase inhibitors,Citation43–Citation45 PI3K inhibitors,Citation46,Citation47 and antisense gene therapy,Citation48 have been shown to be effective in cancer cells, especially those of breast cancer. Therefore, in the following 10 years, molecular drugs targeting the EGF/BVES/POPDC1 pathway may provide new strategies for cancer therapy.
BVES/POPDC1 in eye neoplasms
BVES/POPDC1 is localized to an apical–lateral position in the initial epithelial primordia of the eye.Citation9 Later, during morphogenesis and in the adult, BVES/POPDC1 is redistributed in a cell type-specific manner in the cornea, lens, and retina.Citation9 In an in vitro model of corneal wound healing, BVES/POPDC1 was found to be lost at the epithelial surface during cellular migration across the wound. However, it was restored at the contact area during the reinitiation of epithelial continuity.Citation9 Morpholino knockdown of BVES/ POPDC1 expression disrupted human corneal epithelial integrity. Following injury, this treatment accelerated cell movement at the wound surface but impacted the regeneration of an intact epithelium.Citation9 These results confirmed that BVES/POPDC1 regulates epithelial adhesion and movement during organogenesis of the eye.
Russ et alCitation49 verified that the upregulation of BVES/ POPDC1 expression in trabecular meshwork cells leads to increased tight junction (TJ) formation with decreased activation of RhoA. Manipulation of BVES/POPDC1 expression in human corneal epithelial cell line resulted in reciprocal changes in epithelial–mesenchymal phenotypes.Citation25 These observations indirectly identify BVES/POPDC1 as a regulator of EMT. EMT in tumor cells is similar to that observed in wound healing and organogenesis,Citation50 suggesting that BVES/ POPDC1 may play an important role in regulating EMT processes in ocular tumor cells.
BVES/POPDC1 in other diseases
BVES/POPDC1 in heart disease
BVES/POPDC1 is strongly expressed in the heart and skeletal muscle, and this expression pattern is observed in all animal models that have been studied thus far, eg, Drosophila,Citation20 chick,Citation1 zebrafish,Citation51 Xenopus,Citation19 and mice.Citation12 In the adult heart, BVES/POPDC1 is highly expressed in the atria vs the ventricles.Citation14 In addition, it is elevated in the cardiac conduction which includes the sinoatrial node, the atrioventricular node, the His bundle, the bundle branches, and the Purkinje fibers.Citation14 Functional analysis of BVES/POPDC1 suggested an overlapping role for proper electrical conduction in the heart and maintenance of structural integrity in skeletal muscle. In BVES/POPDC1−/− mice, the presence of stress-induced sinus bradycardia has been reported, following the exposure of BVES/POPDC1−/− mice to physical exercise, mental stress, or injection of isoproterenol.Citation14 The mean heart rate of the null mouse mutants was significantly lower, and the sinoatrial node (SAN) pacemaker was pausing for different lengths of time.Citation14 Interestingly, this pathological phenotype was not present in young mice; however, at 5–8 months of age, these mutants displayed severe stress-induced bradycardia with episodes of sinus node dysfunction.Citation14 The age-dependent phenotype in the mutants is reminiscent of the sick sinus syndrome (SSS), which is the most frequent reason for the implantation of a pacemaker and the most prevalent condition in elderly individuals without other heart diseases. Therefore, it has been speculated that, in SSS patients, the disease may be caused by abnormal expression or function of BVES/POPDC1.
During myocardial ischemia/reperfusion (I/R), a series of damaging changes that occur in myocardial ultrastructure, energy metabolism, cardiac function, and electrophysiology are more prominent after vascular recanalization.Citation52 This pathological phenotype is termed myocardial I/R injury. Alcalay et alCitation16 found that both the protein and mRNA levels of BVES/POPDC1 were decreased during I/R. Induction of myocardial I/R caused a marked lower functional recovery in BVES/POPDC1 null mutants compared with the wild-type (WT) as well as a larger infarct size.Citation16 Cardiac myocytes isolated from BVES/POPDC1 null mutants appeared impaired Ca+2 transients, increased vulnerability to oxidative stress and no pharmacologic preconditioning.Citation16 Further research revealed the colocalization of BVES/POPDC1 with Caveolin-3 (Cav3), and BVES/POPDC1 is a caveolae-associated protein and is important for the preservation of caveolae structural and functional integrity.Citation16 A recent study performed by this research group using myocytes treated with BVES/ POPDC1 siRNA revealed that BVES/POPDC1 is required for the survival of cardiac myocytes under serum deficiency.Citation15 Moreover, silencing of BVES/POPDC1 in rat neonatal cardiomyocytes increases the expression of cell death regulator Bcl-2/adenovirus E1B 19-kDa interacting protein 3 (Bnip3), while attenuating Rac1 activity and modifying the interaction of FoxO3 and NF-κB transcription factors with the Bnip3 promoter.Citation15 These results suggest that BVES/ POPDC1 may serve as a potential target to enhance heart protection. However, at present, the evidence regarding the role of BVES/POPDC1 in human heart disease is limited.
Zhang et alCitation53 found that BVES/POPDC1 is one of the differentially expressed genes identified in ventricular septal defect and normal human ventricular septum myocardium using suppression subtractive hybridization.Citation53 These genes are mainly involved in energy metabolism, cell cycle and growth, cytoskeleton and cell adhesion, LIM protein, zinc finger protein, and development. Gingold-Belfer et alCitation54 detected the expression of POPDC proteins in biopsies from non-failing and failing human hearts and found that the levels of BVES/POPDC1 and POPDC3 were decreased in failing hearts. However, inconsistent with the expression pattern observed in mice, BVES/POPDC1 was expressed in the four human heart chambers and its expression levels were higher in the ventricles than in the atria.Citation54 These differences may be due to compartment-dependent differences. Schindler et al identified a BVES/POPDC1 missense variant (S201F) via whole-exome sequencing in a family of four individuals with cardiac arrhythmias and limb-girdle muscular dystrophy.Citation55 Interestingly, forced expression of BVES/POPDC1S201F in murine cardiac muscle cells increased the hyperpolarization and upstroke velocity of the action potential.Citation55 Furthermore, expressing the homologous mutation of BVES/POPDC1S191F in zebrafish resulted in heart and skeletal muscle phenotypes, as observed in patients.Citation55 This was the first and only study to identify BVES/POPDC1 as a disease-causing gene in human heart diseases.
BVES/POPDC1 and stem cells
Stem cells are an important resource for tissue repair, regeneration, and tumorigenesis.Citation56 A recent study investigated the role of BVES/POPDC1 in mice intestine after ionizing radiation exposure.Citation57 In the study, BVES/POPDC1−/− mice presented with increased crypt size, and the elevated proliferation and expression of stem cell markers (eg, Lgr5, Ascl2, Olfm4, Nanog, Sox9, Lrig1, and Bmi1) and the ex vivo BVES/POPDC1−/− enteroid model exhibited increased stemness with increased plating efficiency, proportion of stem spheroids, retention of cystic structures, amplified WNT signaling, and responsiveness to WNT activation.Citation57 BVES/POPDC1 expression was decreased in WT mice that underwent radiation.Citation57 Moreover, after radiation, BVES/ POPDC1−/− mice showed significantly greater crypt viability compared with WT mice.Citation57 Therefore, these data suggested that BVES/POPDC1 – apart from an intestinal epithelial adhesion molecule – is a key regulator of intestinal stem cell programming and intestinal homeostasis. However, the role of BVES/POPDC1 in cancer stem cells remains unclear.
Potential downstream signaling pathway of BVES/POPDC1
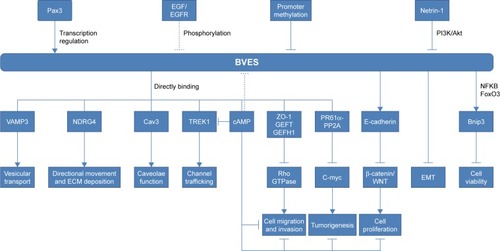
Previous studies have shown that BVES/POPDC1 is a cell adhesion molecule, inhibited in multiple types of solid tumors. However, an increasing number of recent studies have found that BVES/POPDC1 regulates EMTCitation25,Citation32 and modifies intestinal permeabilityCitation58 and stem cell programs,Citation57 etc. Determining the downstream signaling pathways of BVES/ POPDC1 may provide new insights into tumor research and intervention targeting BVES/POPDC1. We have shown the possible upstream and downstream mechanisms of BVES/ POPDC1 in .
Figure 2 The upstream effectors and downstream targets of POPDC1/BVES.
Notes: → represents promotion or elevation; ⊣ represents inhibition or decrease.
Abbreviations: ECM, extracellular matrix; EMT, epithelial–mesenchymal transition.
GEFT/Rho signaling
GEFT, also known as p63RhoGEF or ARHGEF25, belongs to the Rho guanine nucleotide exchange factor (GEF) family.Citation59,Citation60 GEFT modulates the active state of Rho GTPases by stimulating the exchange of guanosine diphosphate for GTPCitation52,Citation61,Citation62 and further regulates cell proliferation, migration, cell–cell adhesion, and cell cycle regulation.Citation63–Citation66 Rac1, Cdc42, and RhoA are the main members of the Rho family of GTPases. These proteins are found to be upregulated in human tumors, such as breast, testicular, ovarian, liver, and colorectal cancers, and promote the proliferation, migration, and invasiveness of cancer cells.Citation67–Citation70 GEFT is the first identified protein directly interacting with BVES/POPDC1. BVES/POPDC1 is mainly localized in the plasma membrane; thus, the BVES/POPDC1–GEFT interaction results in GEFT being “detained” in the plasma membrane, leading to a decreased number and activation of GEFT in the cytoplasm.Citation71 Exogenous expression of BVES/POPDC1 reduced Rac1 and Cdc42 activity; however, it does not affect the levels of active RhoA in NIH 3T3 cells.Citation71 Another research group found that the increased expression of BVES/POPDC1 in trabecular meshwork cells and human corneal epithelial cells led to increased formation of the tight junction with decreased activation of RhoA.Citation49,Citation72 GEF-H1, also known as ARHGEF2, is another Rho GTPase.Citation73,Citation74 Consistent with the aforementioned study, in the research, the investigators speculated that BVES/POPDC1 is combined with ZO-1 and cingulin to form a tight junction complex, sequestering GEF-H1 in the plasma membrane and modulating RhoA signaling.Citation72 Recently, upregulation of BVES/POPDC1 and inactivation of the RhoA/Rock pathway were observed in the mesenchymal–epithelial transition (MET) of pig fibroblastsCitation75 and human HCC cells.Citation76 Using an RhoA-GTPase activation assay, our group demonstrated that the suppression of BVES/ POPDC1 increased RhoA activity, thereby promoting cell migration and invasion. In contrast, the overexpression of BVES/POPDC1 decreased RhoA activity in HCC cells.Citation33 More recently, we observed the colocalization of BVES/ POPDC1, ZO-1, and GEFT in liver tissues and cells and disappearance in HCC tissues and cells (unpublished data). Based on these findings, this colocalization may be the mechanism through which BVES/POPDC1 directly inhibits Rho activity.
WNT signaling
WNT signaling plays central roles in embryogenesis, tissue homeostasis, wound repair, and malignancy.Citation77,Citation78 Cellular adhesion complexes, including tight and adherens junctions, have been confirmed to be closely related to WNT signaling.Citation79–Citation81 Adherens junctions are modulators of canonical WNT signaling through sequestration of β-catenin at the cell membrane.Citation82,Citation83 Similarly, tight junctions play a fundamental role in outside-in signaling cascades for WNT signaling.Citation84 BVES/POPDC1 is an important regulator of intercellular connection, regulating the expression of E-cadherin in multiple cell lines and tumors.Citation25,Citation32 In thymosin β4-treated mice, BVES/POPDC1 was increased and accompanied by elevated expression of β-catenin.Citation85 Furthermore, BVES/POPDC1 modulates the localization of β-catenin and WNT transcriptional activity in human colorectal carcinoma cells. In addition, the overexpression of BVES/POPDC1 increased the membrane-bound localization of β-catenin, whereas it decreased cytoplasmic expression.Citation25 These in vitro results are consistent with those observed in vivo. The cytoplasmic levels of β-catenin were excessive, and its nuclear localization in BVES/POPDC1−/− mice tumors was higher compared with that observed in WT micetumors.Citation26 Meanwhile, WNT targets (ie, Mmp7, Wisp2, and Rspo4), were upregulated in BVES/POPDC1−/− mice tumors, further confirming that BVES/POPDC1 regulates the WNT signaling pathway.Citation26,Citation57
Protein phosphatase 2A (PP2A)
PP2A is one of the main serine–threonine phosphatases in mammalian cells. It is composed of a 65 kDa structural A subunit (PP2AA or PR65; α and β isoforms) and a 36-kDa catalytic C subunit (PP2AC; α and β isoforms).Citation86 PP2A is a well-established regulator of the cell cycle and apoptosis by counteracting most of the kinase-driven intracellular signaling pathways.Citation87 PP2A complexes inhibit mitogenic and anti-apoptotic signals by dephosphorylating and inactivating MEK1 and ERK kinases, decreasing stability, and inhibiting the function of c-MYC and STAT5.Citation88,Citation89 In addition, PP2A suppresses the translation of oncogenes such as MCL1 and c-MYC through direct and indirect dephosphorylation of EIF4E.Citation90 Similarly, PP2A exhibits pro-apoptotic activity by negatively regulating the PI3K/Akt pathway through direct Akt dephosphorylation, inactivation of anti-apoptotic BCL2, and activation of pro-apoptotic factors BAD and BIM.Citation91 PP2A has been suggested to be genetically altered or functionally inactivated in many solid cancersCitation92–Citation94 and leukaemiasCitation95–Citation97 and therefore acts as a tumor suppressor. Recently, Parang et al found that BVES/POPDC1 interacted with PR61α to promote c-Myc degradation through yeast- two-hybrid, co-immunoprecipitation, and a proximity ligation assay.Citation26 They identified a fragment (15 amino acids) of human BVES/POPDC1 required for the BVES/ POPDC1-PR61α interaction and c-Myc degradation. They also demonstrated that the regulation of WNT signaling and the oncogene c-Myc by BVES/POPDC1 was a key event in colitis-induced tumourigenesis.Citation26 PP2A dephosphorylates a number of target proteins, many of which are implicated in tumorigenesis. Therefore, the interaction of BVES/POPDC1 and PP2A warrants further study.
Other possible targets and signaling pathways
TWIK-related K+ channel 1 (TREK-1)
TREK-1 belongs to the two-pore domain potassium channel family, which is regulated by a large number of stimulating factors, such as pH, stretch, temperature, phosphorylation, and interacting proteins.Citation98 TREK-1 has recently been discovered to play an important role in human prostate cancer. TREK-1 is highly expressed in prostate cancer, unlike in healthy prostate or benign prostatic hyperplasia.Citation99,Citation100 In addition, its expression is strongly correlated with the grade and stage of prostate cancer.Citation100 Overexpression of TREK-1 in healthy prostate epithelial cells increased their proliferation ability.Citation100 In contrast, the knockdown of TREK-1 significantly inhibited the proliferation of prostate cancer cells.Citation99 Recently, an investigation of ion channels and electrogenic proteins in Xenopus oocytes demonstrated that TREK-1 functionally interacts with BVES/POPDC1.Citation101 Co-expression of BVES/ POPDC1 and TREK-1 stimulates a twofold higher current than that measured in the absence of BVES/POPDC1.Citation101 However, the role of BVES/POPDC1–TREK-1 interaction in human cancers has not been examined.
Caveolin-3
Caveolins are the major constructive component of the caveolae. Currently, there are three identified caveolin isoforms (Cav1, Cav2, and Cav3).Citation102 Caveolins play an important role in the transcytosis of molecules into cells and regulation of signal transductions.Citation103,Citation104 Cav3 is the muscle- specific isoform, which is localized to the sarcolemma in skeletal muscle fibers and in sarcolemma and transverse tubules in cardiac myocytes.Citation105 However, its presence has been observed in certain solid tumors. Cav3 is frequently expressed in seminoma and anaplastic carcinoma.Citation106,Citation107 Genetic ablation of Cav3 expression induces a lactogenic microenvironment, which is protective against mammary tumor formation.Citation108 Cav3 has recently been identified as an interaction partner of BVES/POPDC1.Citation16,Citation101 This interaction is important for the preservation of caveolae structural and functional integrity.Citation101 However, its role in the development and progression of cancer requires further investigation.
Vesicle-associated membrane protein 3 (VAMP3)
VAMP3 is a vesicular transport protein regulating the recycling of transferrins, the transferrin receptor, and integrins.Citation109,Citation110 It is also involved in the secretion of MMPs, degradation of the extracellular matrix (ECM), and invasiveness of human fibrosarcoma cells.Citation111 Loss of VAMP3 function promotes cell migration and adhesion in human pancreatic cancer cells.Citation112 BVES/POPDC1 has recently been shown to directly interact with VAMP3, and loss of BVES/POPDC1 function impairs VAMP3-mediated vesicular transport by disrupting the recycling of transferrin and β-1-integrin in MDCK epithelial cells.Citation113 Furthermore, the expression of mutated BVES/POPDC1 disrupted integrin functions, resulting in impaired uptake during cell movement and disrupted cell spreading and adhesion.Citation113 Based on these findings, the roles of BVES/POPDC1–VAMP3 in cancer may involve a novel BVES/POPDC1 signaling mechanism.
N-myc downstream regulated gene 4 (NDRG4)
Loss of BVES/POPDC1 or NDRG4 functions have been demonstrated to result in disrupted cell movement.Citation37,Citation114,Citation115 Using SPOTs and pull-down analysis, Benesh et alCitation116 found that NDRG4 directly binds to BVES/POPDC1 C-terminus which is outside of the conserved Popeye domain. The BVES/POPDC1/NDRG4 interaction is important for the regulation of epicardial cell directional migration, and disruption of this interaction randomizes migratory patterns.Citation116 Furthermore, BVES/POPDC1 and NDRG4 specifically mediate the trafficking of internalized fibronectin via “autocrine ECM deposition” fibronectin recycling pathway.Citation116 BVES/POPDC1/NDRG4 interaction also regulates fusion of cell surface-bound vesicles.Citation116 These data suggest that BVES/POPDC1 may have multiple roles on cellular behaviors affecting development, repair, and cancer invasiveness.
Bnip3
Bnip3 is one of the Bcl-2 families of cell death regulatory factors that functions via both the activation of Bax/Bak and the opening of the mitochondria permeability transition pores.Citation117 The expression of Bnip3 increases during stress such as hypoxia through hypoxia-inducing factor-1 dependent or independent mechanisms, such as transcriptionally regulated by RB1-E2F1, TP53, FOXO3, NF-κB, and other tumor-relevant transcription factors.Citation117 Bnip3 is well studied in cell death, autophagy, and mitophagy.Citation118 Recently, Kliminski et alCitation15 found that the knockdown of BVES/POPDC1 in rat neonatal cardiomyocytes grown in serum-free medium reduced cell viability, facilitated mitochondrial injury, and upregulated Bnip3. Further, they found that BVES/ POPDC1 regulated the Bnip3 expression by modifying the competitive binding of FoxO3 and NFκB transcription factors with the Bnip3 promoter.Citation15 In consideration of the multifaceted role of autophagy and mitophagy in cancer, the role of BVES/POPDC1-regulated Bnip3 in cancer warrants further study.Citation119
cAMP is a second messenger molecule which is involved in many human normal physiological functions and pathological state.Citation120 There are four effectors that directly bind to cAMP in mammalian cells as known previously, namely protein kinase A (PKA), exchange protein directly activated by cAMP (Epac), cyclic nucleotide receptor involved in sperm function (CRIS), and cyclic nucleotide-gated ion (CNG) channels.Citation120 POPDC proteins have recently demonstrated as a novel class of cAMP effector proteins in striated muscle, the Popeye domain functioned as a high-affinity cAMP-binding site, and the binding affinity is ~10-fold higher than Epac and in the same level as that of PKA.Citation14 Amunjela et alCitation37 have recently demonstrated that BVES/ POPDC1 co-immunoprecipitates with cAMP in breast cancer lines. Interestingly, cAMP increases BVES/POPDC1 protein levels in these cells. In addition, cAMP was supposed to negatively modulate the interaction of TREK-1 with POPDC proteins.Citation37 Additional studies defining the BVES/POPDC1- cAMP and its roles on cancer will be required.
Future studies
Over the previous 10 years, the expression and function of BVES/POPDC1 in cancer have been gradually recognized. All studies suggest that BVES/POPDC1 is a tumor suppressor gene.Citation84 However, the correlation between BVES/POPDC1 and tumor prognosis requires further study to provide the possibility of BVES/POPDC1-targeted tumor intervention and follow-up. Decreased BVES/POPDC1 expression occurs in the early stage of cancer, and promoter methylation is the main mechanism of BVES/POPDC1 inhibition. Therefore, detection of the level of BVES/POPDC1 methylation may be important for the early detection of tumors and discovery of precancerous lesions.
BVES/POPDC1 is highly expressed in the developing heart and markedly downregulated during end-stage heart failure.Citation54 Deficiency in BVES/POPDC1 leads to sinus node dysfunction in aged miceCitation14,Citation121 and humans born with Fallot’s tetralogy,Citation122 suggesting its involvement in heart pathology and morphogenesis.Citation101 Considering the differential expression of BVES/POPDC1 in the heart and tumors, we speculate that BVES/POPDC1 may play a role in tumor-related heart disease and cardiac disease caused by antineoplastic drugs. However, this potential role of BVES/POPDC1 requires further study by oncologists and cardiologists.
We recently found that BVES/POPDC1 participates in “cancer cell extrusion”, by which cancer cells leave the primary tumor and initiate local or distant metastasis.Citation123 Mediation of this process by BVES/POPDC1 may be another important mechanism by which it inhibits tumor metastasis, especially in gastrointestinal tumors.Citation124–Citation126 More recently, BVES/POPDC1 was found to regulate intestinal stem cell programming after exposure to radiation,Citation57 suggesting a potential role in the treatment of cancer using stem cells.
Overall, in the previous decade, the research on BVES/ POPDC1 has shown great promise. Further research, based on the currently available evidence, is warranted to evaluate the role of BVES/POPDC1 in human disease.
Acknowledgments
This review is supported by the National Natural Science Foundation of China (No 81472311, No 81572419, and No 81702396).
Disclosure
The authors report no conflicts of interest in this work.
References
- deRZavaljevskiMNlSBaderDReeseDEStreiffNLBves: a novel gene expressed during coronary blood vessel developmentDev Biol1999209115917110208750
- AndreeBHillemannTKessler-IceksonGIsolation and characterization of the novel Popeye gene family expressed in skeletal muscle and heartDev Biol2000223237138210882522
- deRBaderDMCloning and expression of hbves, a novel and highly conserved mRNA expressed in the developing and adult heart and skeletal muscle in the humanMamm Genome199910991391510441744
- KnightRFBaderDMBackstromJRMembrane topology of Bves/ Pop1A, a cell adhesion molecule that displays dynamic changes in cellular distribution during developmentJ Biol Chem200327835328723287912815060
- KukuruzinskaMALennonKProtein N-glycosylation: molecular genetics and functional significanceCrit Rev Oral Biol Med1998944154489825220
- KawaguchiMHagerHAWadaAKoyamaTChangMSBaderDMIdentification of a novel intracellular interaction domain essential for Bves functionPLoS One200835e226118493308
- HagerHABaderDMBves: ten years afterHistol Histopathol200924677778719337975
- OslerMEBaderDMBves expression during avian embryogenesisDev Dyn2004229365866714991721
- RipleyANChangMSBaderDMBves is expressed in the epithelial components of the retina, lens, and corneaInvest Ophthalmol Vis Sci20044582475248315277466
- VasavadaTKDiAngeloJRDuncanMKDevelopmental expression of Pop1/BvesJ Histochem Cytochem200452337137714966204
- McCarthyMAllen brain atlas maps 21,000 genes of the mouse brainLancet Neurol200651190790817086647
- SmithTKBaderDMCharacterization of Bves expression during mouse development using newly generated immunoreagentsDev Dyn200623561701170816538658
- TorloppABreherSSSchluterJBrandTComparative analysis of mRNA and protein expression of Popdc1 (Bves) during early development in the chick embryoDev Dyn2006235369170016444735
- FroeseABreherSSWaldeyerCPopeye domain containing proteins are essential for stress-mediated modulation of cardiac pace- making in miceJ Clin Invest201212231119113022354168
- KliminskiVUzielOKessler-IceksonGPopdc1/Bves functions in the preservation of cardiomyocyte viability while affecting Rac1 activity and BNIP3 expressionJ Cell Biochem201711861505151727886395
- AlcalayYHochhauserEKliminskiVPopeye domain containing 1 (Popdc1/Bves) is a caveolae-associated protein involved in ischemia tolerancePLoS One201389e7110024066022
- OslerMEChangMSBaderDMBves modulates epithelial integrity through an interaction at the tight junctionJ Cell Sci2005118Pt 204667467816188940
- WadaAMReeseDEBaderDMBves: prototype of a new class of cell adhesion molecules expressed during coronary artery developmentDevelopment2001128112085209311493530
- RipleyANOslerMEWrightCVBaderDXbves is a regulator of epithelial movement during early Xenopus laevis developmentProc Natl Acad Sci U S A2006103361461916407138
- LinSZhaoDBownesMBlood vessel/epicardial substance (Bves) expression, essential for embryonic development, is down regulated by Grk/EFGR signallingInt J Dev Biol2007511374417183463
- FengQSeHSternJEDNA methylation in tumor and matched normal tissues from non-small cell lung cancer patientsCancer Epidemiol Biomarkers Prev200817364565418349282
- SalskovAHawesSESternJEHypermethylation of CCND2 may reflect a smoking-induced precancerous change in the lungJ Oncol2011201195014021577262
- KimMJangHRHaamKFrequent silencing of Popeye domain- containing genes, Bves and POPDC3, is associated with promoter hyper- methylation in gastric cancerCarcinogenesis20103191685169320627872
- LuoDHuangHLuMLAbnormal expression of adhesion protein Bves is associated with gastric cancer progression and poor survivalPathol Oncol Res201218249149722109561
- CsWZhangBSmithJJBves regulates EMT in human corneal and colon cancer cells and is silenced via promoter methylation in human colorectal carcinomaJ Clin Invest2011121104056406921911938
- ParangBKazAMBarrettCWBVES regulates c-Myc stability via PP2A and suppresses colitis-induced tumourigenesisGut201766585286228389570
- XingYZhaoZZhuYZhaoLZhuAPiaoDComprehensive analysis of differential expression profiles of mRNAs and lncRNAs and identification of a 14-lncRNA prognostic signature for patients with colon adenocarcinomaOncol Rep20183952365237529565464
- ZeiselMBDhawanPBaumertTFTight junction proteins in gastro- intestinal and liver diseaseGut2019653547561
- NagaiTAraoTNishioKImpact of tight junction protein ZO-1 and TWIST expression on postoperative survival of patients with hepatocellular carcinomaDig Dis201634670270727750241
- HigashiYSuzukiSSakaguchiTLoss of Claudin-1 expression correlates with malignancy of hepatocellular carcinomaJ Surg Res20071391687617270214
- SchmittMHorbachAKubitzRFrillingAHaussingerDDisruption of hepatocellular tight junctions by vascular endothelial growth factor (VEGF): a novel mechanism for tumor invasionJ Hepatol200441227428315288477
- HanPFuYLuoMBves inhibition triggers epithelial- mesenchymal transition in human hepatocellular carcinomaDig Dis Sci2014595992100024442236
- HanPFuYLiuJNetrin-1 promotes cell migration and invasion by down-regulation of Bves expression in human hepatocellular carcinomaAm J Cancer Res2015541396140926101705
- LeiYHanPLinZYYanWTianDHypoxia regulates epithelial- mesenchymal transition (EMT) in hepatocellular carcinoma cells via promoting Bves methylationHepatology2018681264A1264A
- DongXHouQChenYWangXDiagnostic value of the methylation of multiple gene promoters in serum in hepatitis B virus-related hepatocellular carcinomaDis Markers20172017292938128951629
- AmunjelaJNTuckerSJPOPDC1 is suppressed in human breast cancer tissues and is negatively regulated by EGFR in breast cancer cell linesCancer Lett2017406819228807821
- AmunjelaJNTuckerSJDysregulation of POPDC1 promotes breast cancer cell migration and proliferationBiosci Rep2017376BSR2017103928954821
- DengWGuLLiXCD24 associates with EGFR and supports EGF/EGFR signaling via RhoA in gastric cancer cellsJ Transl Med2016143226830684
- ClaperonAMergeyMNguyen Ho-BouldoiresTHEGF/EGFR axis contributes to the progression of cholangiocarcinoma through the induction of an epithelial-mesenchymal transitionJ Hepatol201461232533224704591
- GanYShiCIngeLHibnerMBalducciJHuangYDifferential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cellsOncogene201029354947495820562913
- PatelDBassiRHooperAPrewettMHicklinDJKangXAnti- epidermal growth factor receptor monoclonal antibody cetuximab inhibits EGFR/HER-2 heterodimerization and activationInt J Oncol2009341253219082474
- KimuraHSakaiKAraoTShimoyamaTTamuraTNishioKAntibody-dependent cellular cytotoxicity of cetuximab against tumor cells with wild-type or mutant epidermal growth factor receptorCancer Sci20079881275128017498200
- RusnakDWLackeyKAffleckKThe effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivoMol Cancer Ther200112859412467226
- FeldingerKKongAProfile of neratinib and its potential in the treatment of breast cancerBreast Cancer (Dove Med Press)2015714716226089701
- SchulerMAwadaAHarterPA phase II trial to assess efficacy and safety of afatinib in extensively pretreated patients with HER2- negative metastatic breast cancerBreast Cancer Res Treat201213431149115922763464
- LiuTYacoubRTaliaferro-SmithLDCombinatorial effects of lapatinib and rapamycin in triple-negative breast cancer cellsMol Cancer Ther20111081460146921690228
- SoulieresDFaivreSMesiaRBuparlisib and paclitaxel in patients with platinum-pretreated recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL-1): a randomised, double-blind, placebo-controlled phase 2 trialLancet Oncol201718332333528131786
- LaiSYKoppikarPSmTIntratumoral epidermal growth factor receptor antisense DNA therapy in head and neck cancer: first human application and potential antitumor mechanismsJ Clin Oncol20092781235124219204206
- RussPKKuppermanAIPresleySHHaseltonFRChangMSInhibition of RhoA signaling with increased Bves in trabecular meshwork cellsInvest Ophthalmol Vis Sci201051122323019628742
- CampbellKContribution of epithelial-mesenchymal transitions to organogenesis and cancer metastasisCurr Opin Cell Biol201855303530006053
- WuYCLiuCYChenYHChenRFHuangCJWangIJBlood vessel epicardial substance (Bves) regulates epidermal tight junction integrity through atypical protein kinase CJ Biol Chem201228747398873989723019331
- FerdinandyPSchulzRBaxterGFInteraction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioningPharmacol Rev200759441845818048761
- ZhangHZhouLYangRIdentification of differentially expressed genes in human heart with ventricular septal defect using suppression subtractive hybridizationBiochem Biophys Res Commun2006342113514416472770
- Gingold-BelferRBergmanMAlcalayYPopeye domain- containing 1 is down-regulated in failing human heartsInt J Mol Med2011271253121069264
- SchindlerRFScottonCZhangJPOPDC1(S201F) causes muscular dystrophy and arrhythmia by affecting protein traffickingJ Clin Invest2016126123925326642364
- LiuMTuJGingoldJAKongCSLLeeDFCancer in a dish: progress using stem cells as a platform for cancer researchAm J Cancer Res20188694495430034933
- ReddyVKShortSPBarrettCWBVES regulates intestinal stem cell programs and intestinal crypt viability after radiationStem Cells20163461626163626891025
- YaCReddyVKSinghKBVES is required for maintenance of colonic epithelial integrity in experimental colitis by modifying intestinal permeabilityMucosal Immunol20181151363137429907869
- SouchetMPortales-CasamarEMazuraisDHuman p63RhoGEF, a novel RhoA-specific guanine nucleotide exchange factor, is localized in cardiac sarcomereJ Cell Sci2002115Pt 362964011861769
- LutzSFreichel-BlomquistARumenappUSchmidtMJakobsKHWielandTp63RhoGEF and GEFT are Rho-specific guanine nucleotide exchange factors encoded by the same geneNaunyn Schmiedebergs Arch Pharmacol2004369554054615069594
- LutzSShankaranarayananACocoCStructure of Galphaq- p63RhoGEF-RhoA complex reveals a pathway for the activation of RhoA by GPCRsScience200731858581923192718096806
- KiS-FSandquistJCRossol-AllisonJMLK3 limits activated Galphaq signaling to Rho by binding to p63RhoGEFMol Cell2008321435618851832
- HayashiAHiatariRTsujiTOhashiKMizunoKp63RhoGEF-mediated formation of a single polarized lamellipodium is required for chemotactic migration in breast carcinoma cellsFEBS Lett2013587669870523380069
- TangXJinRQuGGPR116, an adhesion G-protein-coupled receptor, promotes breast cancer metastasis via the Galphaq-p63Rho- GEF-Rho GTPase pathwayCancer Res201373206206621824008316
- AbrahamCGLudwigMPAndrysikZDeltaNp63alpha suppresses TGFB2 expression and RhoA activity to drive cell proliferation in squamous cell carcinomasCell Rep201824123224323630232004
- XuLi CXiaoHLCRMP4a suppresses cell motility by sequestering RhoA activity in prostate cancer cellsCancer Biol Ther2018111 Epub201886
- BusteloXRRHO GTPases in cancer: known facts, open questions, and therapeutic challengesBiochem Soc Trans201846374176029871878
- NarumiyaSThumkeoDRho signaling research: history, current status and future directionsFEBS Lett2018592111763177629749605
- LawsonCDRidleyAJRho GTPase signaling complexes in cell migration and invasionJ Cell Biol2018217244745729233866
- JansenSGosensRWielandTSchmidtMPaving the Rho in cancer metastasis: Rho GTPases and beyondPharmacol Ther201818312128911825
- SmithTKHagerHAFrancisRKilkennyDMLoCWBaderDMBves directly interacts with GEFT, and controls cell shape and movement through regulation of Rac1/Cdc42 activityProc Natl Acad Sci U S A2008105248298830318541910
- RussPKPinoCJWilliamsCSBaderDMHaseltonFRChangMSBves modulates tight junction associated signalingPLoS One201161e1456321283798
- RenYLiRZhengYBuschHCloning and characterization of GEF-H1, a microtubule-associated guanine nucleotide exchange factor for Rac and Rho GTPasesJ Biol Chem19982735234954349609857026
- Benais-PontGPunnAFlores-MaldonadoCIdentification of a tight junction-associated guanine nucleotide exchange factor that activates Rho and regulates paracellular permeabilityJ Cell Biol2003160572974012604587
- ShiJWLiuWZhangTTThe enforced expression of c-myc in pig fibroblasts triggers mesenchymal-epithelial transition (MET) via F-actin reorganization and RhoA/ROCK pathway inactivationCell Cycle20131271119112723466707
- WangSCLinXLLiJMicroRNA-122 triggers mesenchymal- epithelial transition and suppresses hepatocellular carcinoma cell motility and invasion by targeting RhoAPLoS One201497e10133024992599
- NusseRCleversHWnt/beta-catenin signaling, disease, and emerging therapeutic modalitiesCell2017169698599928575679
- Murillo-GarzonVKyptaRWNT signalling in prostate cancerNat Rev Urol2017141168369628895566
- ArtusCGlacialFGaneshamoorthyKThe Wnt/planar cell polarity signaling pathway contributes to the integrity of tight junctions in brain endothelial cellsJ Cereb Blood Flow Metab201434343344024346691
- ShahGVMuralidharanAGokulgandhiMSoanKThomasSCadherin switching and activation of beta-catenin signaling underlie proinvasive actions of calcitonin-calcitonin receptor axis in prostate cancerJ Biol Chem200928421018103019001380
- PredaVLarkinSJKaravitakiNAnsorgeOGrossmanABThe Wnt signalling cascade and the adherens junction complex in craniopharyn- gioma tumorigenesisEndocr Pathol20152611825355426
- KlinkeDJ2ndHorvathNCuppettVWuYDengWKanjRInterlocked positive and negative feedback network motifs regulate beta-catenin activity in the adherens junction pathwayMol Biol Cell201526224135414826224311
- FrancisHKennedyLAlpiniGDual ablation of beta- and gamma- catenin: critical regulators of junctions and their functionsHepatology20186762079208129272046
- ParangBThompsonJJCsWWilliamsCSBlood vessel epicardial substance (Bves) in junctional signaling and cancerTissue Barriers2018112 Epub20181011
- Bock-MarquetteIShrivastavaSPipesGCThymosin beta4 mediated PKC activation is essential to initiate the embryonic coronary developmental program and epicardial progenitor cell activation in adult mice in vivoJ Mol Cell Cardiol200946572873819358334
- PerrottiDNevianiPProtein phosphatase 2A: a target for anticancer therapyLancet Oncol2013146e229e23823639323
- EichhornPJCreyghtonMPBernardsRProtein phosphatase 2A regulatory subunits and cancerBiochim Biophys Acta20091795111518588945
- JunttilaMRPuustinenPNiemelaMCIP2A inhibits PP2A in human malignanciesCell20071301516217632056
- RossJAChengHZsNFrostJAKirkenRAProtein phosphatase 2A regulates interleukin-2 receptor complex formation and JAK3/STAT5 activationJ Biol Chem201028563582359119923221
- LiYYuePDengXProtein phosphatase 2A negatively regulates eukaryotic initiation factor 4E phosphorylation and eIF4F assembly through direct dephosphorylation of Mnk and eIF4ENeoplasia2010121084885520927323
- JanssensVRebolloAThe role and therapeutic potential of Ser/Thr phosphatase PP2A in apoptotic signalling networks in human cancer cellsCurr Mol Med201212326828722300139
- BockelmanCLassusHHemmesAPrognostic role of CIP2A expression in serous ovarian cancerBr J Cancer2011105798999521897396
- TsengLMLiuCYChangKCChuPYShiauCWChenKFCIP2A is a target of bortezomib in human triple negative breast cancer cellsBreast Cancer Res2012142R6822537901
- LinYCChenKCChenCCChengALChenKFCIP2A-mediated Akt activation plays a role in bortezomib-induced apoptosis in head and neck squamous cell carcinoma cellsOral Oncol201248758559322342571
- CristobalIGarcia-OrtiLCirauquiCOverexpression of SET is a recurrent event associated with poor outcome and contributes to protein phosphatase 2A inhibition in acute myeloid leukemiaHaematologica201297454355022133779
- LucasCMHarrisRJGiannoudisACoplandMSlupskyJRClarkRECancerous inhibitor of PP2A (CIP2A) at diagnosis of chronic myeloid leukemia is a critical determinant of disease progressionBlood2011117246660666821490338
- RuvoloPPQuiYHCoombesKRLow expression of PP2A regulatory subunit B55alpha is associated with T308 phosphorylation of AKT and shorter complete remission duration in acute myeloid leukemia patientsLeukemia201125111711171721660042
- CheminJPatelAJDupratFLauritzenILazdunskiMHonoreEA phospholipid sensor controls mechanogating of the K+ channel TREK-1EMBO J2005241445315577940
- ZhangGMWanFNQinXJPrognostic significance of the TREK-1 K2P potassium channels in prostate cancerOncotarget2015621184601846825962960
- VoloshynaIBesanaACastilloMTREK-1 is a novel molecular target in prostate cancerCancer Res20086841197120318281496
- BrandTSimrickSLPoonKLSchindlerRFThe cAMP-binding Popdc proteins have a redundant function in the heartBiochem Soc Trans201442229530124646234
- StanRVStructure of caveolaeBiochim Biophys Acta Mol Cell Res200517463334348
- BusijaARPatelHHInselPACaveolins and cavins in the trafficking, maturation, and degradation of caveolae: implications for cell physiologyAm J Physiol Cell Physiol20173124C459C47728122734
- YinHLiuTZhangYYangBCaveolin proteins: a molecular insight into diseaseFront Med201610439740427896622
- SongKSSchererPETangZExpression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteinsJ Biol Chem19962712515160151658663016
- KasaharaTHaraNBilimVTomitaYTsutsuiTTakahashiKImmunohistochemical studies of caveolin-3 in germ cell tumors of the testisUrol Int2002691636812119442
- KimDKimHKooJSExpression of caveolin-1, Caveolin-2 and caveolin-3 in thyroid cancer and stromaPathobiology201279111022236542
- SotgiaFCasimiroMCBonuccelliGLoss of caveolin-3 induces a lactogenic microenvironment that is protective against mammary tumor formationAm J Pathol2009174261362919164602
- HuCHardeeDMinnearFMembrane fusion by VAMP3 and plasma membrane t-SNAREsExp Cell Res2007313153198320917651732
- RiggsKAHasanNHumphreyDRegulation of integrin endocytic recycling and chemotactic cell migration by syntaxin 6 and VAMP3 interactionJ Cell Sci2012125Pt 163827383922573826
- KeanMJWilliamsKCSkalskiMVAMP3, syntaxin-13 and SNAP23 are involved in secretion of matrix metalloproteinases, degradation of the extracellular matrix and cell invasionJ Cell Sci2009122Pt 224089409819910495
- LuftmanKHasanNDayPHardeeDHuCSilencing of VAMP3 inhibits cell migration and integrin-mediated adhesionBiochem Biophys Res Commun20093801657019159614
- HagerHARobertsRJCrossEEProux-GillardeauxVBaderDMIdentification of a novel Bves function: regulation of vesicular transportEMBO J201029353254520057356
- MelotteVLentjesMHvan den BoschSMN-Myc downstream- regulated gene 4 (NDRG4): a candidate tumor suppressor gene and potential biomarker for colorectal cancerJ Natl Cancer Inst20091011391692719535783
- KotipatruniRPFerraroDJRenXNDRG4, the N-Myc downstream regulated gene, is important for cell survival, tumor invasion and angiogenesis in meningiomasIntegr Biol (Camb)20124101185119722869042
- BeneshECMillerPMPfaltzgraffERBves and NDRG4 regulate directional epicardial cell migration through autocrine extracellular matrix depositionMol Biol Cell201324223496351024048452
- VasagiriNKutalaVKStructure, function, and epigenetic regulation of BNIP3: a pathophysiological relevanceMol Biol Rep201441117705771425096512
- ZhangJNeyPARole of BNIP3 and NIX in cell death, autophagy, and mitophagyCell Death Differ200916793994619229244
- ChourasiaAHMacleodKFTumor suppressor functions of BNIP3 and mitophagyAutophagy201511101937193826315353
- BrandTSchindlerRNew kids on the block: the Popeye domain containing (POPDC) protein family acting as a novel class of cAMP effector proteins in striated muscleCell Signal20174015616528939104
- BoukensBJChristoffelsVMPopeye proteins: muscle for the aging sinus nodeJ Clin Invest2012122381081322354173
- WuMLiYHeXMutational and functional analysis of the BVES gene coding region in Chinese patients with non-syndromic tetralogy of FallotInt J Mol Med201331489990323403794
- HanPFuYYanWTianDBVES decreases liver cancer cell extrusion in a 3D cell culture modelGastroenterology20151484S1017S1017
- FadulJRosenblattJThe forces and fates of extruding cellsCurr Opin Cell Biol201854667129727745
- GuYSheaJSlattumGDefective apical extrusion signaling contributes to aggressive tumor hallmarksElife20154e0406925621765
- GuYRosenblattJNew emerging roles for epithelial cell extrusionCurr Opin Cell Biol201224686587023044222
