Abstract
Background
Oral toxicities, such as mucositis and stomatitis, are some of the most significant and unavoidable side effects associated with anticancer therapies. In past decades, research has focused on newer targeted agents with the aim of decreasing the rates of side effects on healthy cells. Unfortunately, even targeted anticancer therapies show significant rates of toxicity on healthy tissue. mTOR inhibitors display some adverse events, such as hyperglycemia, hyperlipidemia, hypophosphatemia, hematologic toxicities, and mucocutaneous eruption, but the most important are still stomatitis and skin rash, which are often dose-limiting side effects.
Aim
This review was performed to answer the question “What is the incidence of stomatitis in patients treated with everolimus?”
Methods
We conducted a systematic search on the PubMed and Medline online databases using a combination of MESH terms and free text: “everolimus” (MESH) AND “side effects” OR “toxicities” OR “adverse events”. Only studies fulfilling the following inclusion criteria were considered eligible for inclusion in this study: performed on human subjects, reporting on the use of everolimus (even if in combination with other drugs or ionizing radiation), written in the English language, and reporting the incidence of side effects.
Results
The analysis of literature revealed that the overall incidence of stomatitis after treatment with everolimus was 42.6% (3,493) and that of stomatitis grade G1/2 84.02% (2,935), while G3/4 was 15.97% (558).
Conclusion
Results of the analysis showed that the incidence of stomatitis of grade 1 or 2 is higher than grade 3 or 4. However, it must be taken into account that it is not possible to say if side effects are entirely due to everolimus therapy or combinations with other drugs.
Introduction
Conventional anticancer therapy does not distinguish between normal and cancer cells. The damage inflicted on normal tissue have thus hampered this therapy.Citation1 The introduction of targeted-therapy molecules targeting specific enzymes, growth-factor receptors, and signal transducers has lowered the incidence of side effects,Citation2 significantly influencing patient quality of life and survival rates.Citation2mTOR is a therapeutic target for both solid and hematologic malignancies. It is part of a pathway that regulates protein biosynthesis, cell growth, and cell-cycle progression. Moreover, mTOR is the downstream effector of the PI3K–Akt–mTOR pathway, which regulates cell growth and metabolism and is involved in multiple processes.Citation1,Citation3–Citation5
Rapamycin and its analogues form the first generation of mTOR inhibitors. The action of these molecules is targeting a 289 kDa serine/threonine-protein kinase that is a component of the big family of PI3Ks. Rapamycin and its analogues work by inhibiting the activity of mTORC1 through binding to FKBP12 and the establishment of a ternary complex with mTOR.Citation6Actually, there are three available mTOR inhibitors: everolimus, temsirolimus, and ridaforolimus. Everolimus is currently used for the treatment of advanced hormone receptor–positive HER2-negative breast cancer together with exemestane, renal-cell carcinoma after failure of therapies based on sunitinib or sorafenib, progressive neuroendocrine tumors of pancreatic origin, in combination with exemestane, and subependymal giant-cell astrocytoma.
These drugs have a different spectrum of side effects compared to conventional chemotherapy. Common side effects are anemia, fatigue, hyperglycemia, hyperlipidemia, stomatitis, rash, and thrombocytopenia.Citation5,Citation7 The terms “oral mucositis” and “stomatitis” are both used to describe inflammation and ulceration of the oral mucosal lining due to chemotherapy or ionizing radiation. However, stomatitis associated with mTOR inhibitors should be considered a separate entity this often being designated as mTOR inhibitor–associated stomatitis (mIAS).Citation8,Citation9 Mouth lesions present as superficial, ovoid, well-demarcated singular or multiple ulcers with a grayish white pseudomembrane. Their size often does not exceed 0.5 cm in diameter. Lesions typically involve the nonkeratinized mucosa, like the inner aspect of the lips, the ventral and lateral surfaces of the tongue, and the soft palate. Ulcers generally develop in 5 days and usually heal spontaneously in 1 week, most frequently in the first cycle of mTOR-inhibitor therapy.Citation10
Methods
This review was performed to answer to the question “Which is the rate of incidence of stomatitis in patients treated with everolimus?” A systematic search on the online databases PubMed and Medline was conducted using a combination of MESH terms and free-text words: “everolimus” (MESH) AND “side effects” OR “toxicities” OR “adverse events”. The authors included in this study only reports that fulfilled certain criteria: performed on human subjects, everolimus alone or in combination with other drugs/ionizing radiation, written in the English language, and providing incidence of side effects. No restrictions were applied on year of publication. Reviews, case reports, and studies in vitro or performed on animal models were excluded. Information collected comprised name of first author, year, title, number of patients enrolled, and number and grade of events recorded. In addition, data were independently extracted by two authors (CA and LLM) and checked in a joint session.
Results
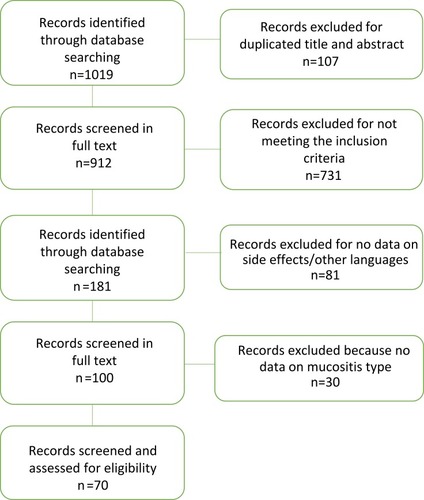
Our literature search yielded 1,019 potentially relevant studies. After elimination of duplicates, titles and abstracts of 912 potentially relevant studies were screened. Of these, 731 were not considered because they did not meet the inclusion criteria. A total of 181 studies were read in full text. Of these, only 100 reported on stomatitis or oral mucositis, and 30 were excluded due to lack of data (). Results howed that the overall incidence of stomatitis after treatment with everolimus was 42.6% (3,493), stomatitis grade G12 84.02% (2,935), and G34 15.97% (558, ).
Table 1 Papers About Everolimus And Stomatitis
Discussion
Targeted therapy includes those drugs that selectively inhibit a target that is mutated in malignant tissue, aiming to achieve preferential localization in the region of disease and thus an increase in local concentration. In particular, mTOR inhibitors work as signal-transduction inhibitors. Rapamycin, also named sirolimus, was the first mTOR inhibitor approved. It is an antiungal agent produced by Streptomyces hygroscopicus with immunosuppressive properties.Citation11 However, sirolimus has been show to have poor pharmacokinetic characteristics, and research has focused on the synthesis of analogues of rapamycin more suitable to therapy, such as everolimus, temsirolimus, and ridaforolimus. These molecules differ from sirolimus in their C-40-O positions, resulting in disparate pharmacokinetic and pharmacodynamic profiles.Citation12 This class of drugs is typically used for the treatment of solid tumors, such as renal-cell carcinoma, breast cancer, pancreatic neuroendocrine tumors, and tuberous sclerosis complex.Citation13–Citation17
Unlike results obtained from reviews about mucositis caused by conventional chemotherapy, in which mucositis is often severe and the most debilitating effect for patients,Citation18 our analysis showed that the incidence of stomatitis of grade 1 or 2 is higher than that of grade 3 or 4. These results are consistent with our previous work.Citation19 However, it must be taken into account that it is not possible to say if side effects are entirely due to everolimus therapy or combination with other drugs. Moreover, not all the papers included in this review specified the exact therapeutic regimen. mIAS generally sets in within a few weeks of initiating everolimus therapy. Grade 3 or 4 lesions may lead to dose interruption or reduction. Interfering with patientfood intake and diminishing quality of life, this kind of toxicity may cause treatment discontinuation or interruption.Citation20
mIAS is often evaluated using common scales employed in the evaluation of conventional oral mucositis (OMAS, 1999; NCI-CTCAE 2006, 2010). However, its clinical appearance tends to differ from conventional mucositis. A more specific mIAS scale was set by Boers-Doets and Lalla. According to this scale, lesions are evaluated depending on their duration, eg, a grade 3 lesion is an ulceration lasting >7 days.Citation21 Management of mIAS is still widely based on education of patients on oral hygiene measures, diet modifications, and pain management.Citation9,Citation22 Treatments used are often based on “magic” mouthwash, composed of lidocaine gel 2% × 30 g, doxycycline suspension 50 mg/5mL × 60 mL, and sucralfate oral suspension 1,000 mg/5 mL dissolved in sodium chloride 0.9% × 2,000 mL used for 3–15 days,Citation23 a sodium bicarbonate–based mouthwash combined with oral fluconazole,Citation24 or a combination of dexamethasone solution 0.5 mg/mL and miconazole 2% gel.Citation25 Another treatment is based on a combination of topical anesthetics, a magic mouthwash (composed of lidocaine, aluminum hydroxide, magnesium hydroxide, dimethicone suspension, diphenhydramine, equal parts) clobetasol gel 0.05%, dexamethasone 0.1 mg/mL, triamcinolone paste, intralesional triamcinolone, and systemic prednisone (1 mg/kg for 7 days). Management of mIAS nowadays is based on education of patients on oral hygiene measures, diet modifications, and pain management.Citation9 Rugo et al showed evidence of the efficacy of a prophylactic use of dexamethasone mouthwash in patients treated with everolimus plus exemestane for advanced or metastatic breast cancer.Citation26 The mouthwash was administered in combination with a prophylactic topical antifungal agent to prevent potential fungal infection.Citation26 It still cannot be determined which lesions will self-limit and which will reduce quality of life, leading to malnutrition and dose reduction in medically necessary treatment.
Disclosure
The authors report no conflicts of interest in this work
References
- Gomez-Pinillos A, Ferrari AC. mTOR signaling pathway and mTOR inhibitors in cancer therapy. Hematol Oncol Clin North Am. 2012;26(3):483–505, vii. doi:10.1016/j.hoc.2012.02.01422520976
- Keefe DM, Bateman EH. Tumor control versus adverse events with targeted anticancer therapies. Nat Rev Clin Oncol. 2011;9(2):98–109. doi:10.1038/nrclinonc.2011.19222182972
- Khokhar NZ, Altman JK, Platanias LC. Emerging roles for mammalian target of rapamycin inhibitors in the treatment of solid tumors and hematological malignancies. Curr Opin Oncol. 2011;23(6):578–586. doi:10.1097/CCO.0b013e32834b892d21892085
- Dabney R, Devine R, Sein N, George B. New agents in renal cell carcinoma. Target Oncol. 2014;9(3):183–193. doi:10.1007/s11523-013-0303-824243495
- Yuan R, Kay A, Berg WJ, Lebwohl D. Targeting tumorigenesis: development and use of mTOR inhibitors in cancer therapy. J Hematol Oncol. 2009;2:45. doi:10.1186/1756-8722-2-4519860903
- Meng LH, Zheng XF. Toward rapamycin analog (rapalog)-based precision cancer therapy. Acta Pharmacol Sin. 2015;36(10):1163–1169. doi:10.1038/aps.2015.6826299952
- Martins F, de Oliveira MA, Wang Q, et al. A review of oral toxicity associated with mTOR inhibitor therapy in cancer patients. Oral Oncol. 2013;49(4):293–298. doi:10.1016/j.oraloncology.2012.11.00823312237
- Sonis S, Treister N, Chawla S, Demetri G, Haluska F. Preliminary characterization of oral lesions associated with inhibitors of mammalian target of rapamycin in cancer patients. Cancer. 2010;116(1):210–215. doi:10.1002/cncr.2469619862817
- Boers-Doets CB, Raber-Durlacher JE, Treister NS, et al. Mammalian target of rapamycin inhibitor-associated stomatitis. Future Oncol. 2013;9(12):1883–1892. doi:10.2217/fon.13.14124295418
- Peterson DE, O’Shaughnessy JA, Rugo HS, et al. Oral mucosal injury caused by mammalian target of rapamycin inhibitors: emerging perspectives on pathobiology and impact on clinical practice. Cancer Med. 2016;5(8):1897–1907. doi:10.1002/cam4.2016.5.issue-827334013
- Sehgal SN, Baker H, Rapamycin VC. (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo). 1975;28(10):727–732. doi:10.7164/antibiotics.28.7271102509
- Liu Q, Thoreen C, Wang J, Sabatini D, Gray NS. mTOR mediated anti-cancer drug discovery. Drug Discov Today Ther Strateg. 2009;6(2):47–55. doi:10.1016/j.ddstr.2009.12.00120622997
- Awada A, Cardoso F, Fontaine C, et al. The oral mTOR inhibitor RAD001 (everolimus) in combination with letrozole in patients with advanced breast cancer: results of a phase I study with pharmacokinetics. Eur J Cancer. 2008;44(1):84–91. doi:10.1016/j.ejca.2007.10.00318039566
- Boers-Sonderen MJ, de Geus-Oei LF, Desar IM, et al. Temsirolimus and pegylated liposomal doxorubicin (PLD) combination therapy in breast, endometrial, and ovarian cancer: phase Ib results and prediction of clinical outcome with FDG-PET/CT. Target Oncol. 2014;9(4):339–347. doi:10.1007/s11523-014-0309-x24577626
- Hudes GR, Berkenblit A, Feingold J, Atkins MB, Rini BI, Dutcher J. Clinical trial experience with temsirolimus in patients with advanced renal cell carcinoma. Semin Oncol. 2009;36(Suppl 3):S26–S36. doi:10.1053/j.seminoncol.2009.10.01319963097
- Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363(19):1801–1811. doi:10.1056/NEJMoa100167121047224
- Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–456. doi:10.1016/S0140-6736(08)61039-918653228
- Borbasi S, Cameron K, Quested B, Olver I, To B, Evans D. More than a sore mouth: patients’ experience of oral mucositis. Oncol Nurs Forum. 2002;29(7):1051–1057. doi:10.1188/02.ONF.1051-105712183754
- Lo Muzio L, Arena C, Troiano G, Villa A. Oral stomatitis and mTOR inhibitors: a review of current evidence in 20,915 patients. Oral Dis. 2018;24(1–2):144–171. doi:10.1111/odi.1279529480626
- Rugo HS, Pritchard KI, Gnant M, et al. Incidence and time course of everolimus-related adverse events in postmenopausal women with hormone receptor-positive advanced breast cancer: insights from BOLERO-2. Ann Oncol. 2014;25(4):808–815. doi:10.1093/annonc/mdu00924615500
- Boers-Doets C, Lalla RV. The mIAS scale: a scale to measure mTOR inhibitor-associated stomatitis. Supp Care Cancer. 2013;21(Suppl):1.
- Ji YD, Aboalela A, Villa A. Everolimus-associated stomatitis in a patient who had renal transplant. BMJ Case Rep. 2016;2016.
- Kalogirou EM, Tosios KI, Piperi EP, Sklavounou A. mTOR inhibitor-associated stomatitis (mIAS) in three patients with cancer treated with everolimus. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119(1):e13–e19. doi:10.1016/j.oooo.2014.08.02325442249
- Ferte C, Soria JC, Penel N. Dose-levels and first signs of efficacy in contemporary oncology phase 1 clinical trials. PLoS One. 2011;6(3):e16633. doi:10.1371/journal.pone.001663321415927
- Nicolatou-Galitis O, Nikolaidi A, Athanassiadis I, Papadopoulou E, Sonis S. Oral ulcers in patients with advanced breast cancer receiving everolimus: a case series report on clinical presentation and management. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(2):e110–e116. doi:10.1016/j.oooo.2013.02.02223643584
- Rugo HS, Seneviratne L, Beck JT, et al.Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): a single-arm, phase 2 trial. Lancet Oncol. 2017;(18):654–662. doi:10.1016/S1470-2045(17)30109-228314691
- Amato RJ, Jac J, Giessinger S, Saxena S, Willis JP. A phase 2 study with a daily regimen of the oral mTOR inhibitor RAD001 (everolimus) in patients with metastatic clear cell renal cell cancer. Cancer. 2009;115(11):2438–2446. doi:10.1002/cncr.v115:1119306412
- Andre F, O’Regan R, Ozguroglu M, et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15(6):580–591. doi:10.1016/S1470-2045(14)70138-X24742739
- Angelousi A, Kamp K, Kaltsatou M, O’Toole D, Kaltsas G, de Herder W. Sequential everolimus and sunitinib treatment in pancreatic metastatic well-differentiated neuroendocrine tumours resistant to prior treatments. Neuroendocrinology. 2017;105(4):394–402. doi:10.1159/00045603528122378
- Armstrong AJ, Halabi S, Eisen T, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. Lancet Oncol. 2016;17(3):378–388. doi:10.1016/S1470-2045(15)00515-X26794930
- Bajetta E, Catena L, Fazio N, et al. Everolimus in combination with octreotide long-acting repeatable in a first-line setting for patients with neuroendocrine tumors: an ITMO group study. Cancer. 2014;120(16):2457–2463. doi:10.1002/cncr.2872624752410
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–529. doi:10.1056/NEJMoa110965322149876
- Baselga J, Semiglazov V, van Dam P, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27(16):2630–2637. doi:10.1200/JCO.2008.18.839119380449
- Bergmann L, Kube U, Doehn C, et al. Everolimus in metastatic renal cell carcinoma after failure of initial anti-VEGF therapy: final results of a noninterventional study. BMC Cancer. 2015;15:303. doi:10.1186/s12885-015-1309-725925846
- Besse B, Leighl N, Bennouna J, et al. Phase II study of everolimus-erlotinib in previously treated patients with advanced non-small-cell lung cancer. Ann Oncol. 2014;25(2):409–415. doi:10.1093/annonc/mdt53624368400
- Campone M, Levy V, Bourbouloux E, et al. Safety and pharmacokinetics of paclitaxel and the oral mTOR inhibitor everolimus in advanced solid tumours. Br J Cancer. 2009;100(2):315–321. doi:10.1038/sj.bjc.660485119127256
- Castellano D, Bajetta E, Panneerselvam A, et al. Everolimus plus octreotide long-acting repeatable in patients with colorectal neuroendocrine tumors: a subgroup analysis of the phase III RADIANT-2 study. Oncologist. 2013;18(1):46–53. doi:10.1634/theoncologist.2012-026323263288
- Chan JA, Blaszkowsky L, Stuart K, et al. A prospective, phase 1/2 study of everolimus and temozolomide in patients with advanced pancreatic neuroendocrine tumor. Cancer. 2013;119(17):3212–3218. doi:10.1002/cncr.2814223733618
- Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1814–1823. doi:10.1056/NEJMoa151001626406150
- Chow H, Ghosh PM, deVere White R, et al. A phase 2 clinical trial of everolimus plus bicalutamide for castration-resistant prostate cancer. Cancer. 2016;122(12):1897–1904. doi:10.1002/cncr.2992727019001
- Chung V, Frankel P, Lim D, et al. Phase Ib trial of mFOLFOX6 and everolimus (NSC-733504) in patients with metastatic gastroesophageal adenocarcinoma. Oncology. 2016;90(6):307–312. doi:10.1159/00044529727093189
- Ciruelos E, Vidal M, Martinez de Duenas E, et al. Safety of everolimus plus exemestane in patients with hormone-receptor-positive, HER2-negative locally advanced or metastatic breast cancer: results of phase IIIb BALLET trial in Spain. Clin Transl Oncol. 2017.
- Ciunci CA, Perini RF, Avadhani AN, et al. Phase 1 and pharmacodynamic trial of everolimus in combination with cetuximab in patients with advanced cancer. Cancer. 2014;120(1):77–85. doi:10.1002/cncr.2829424108668
- Colon-Otero G, Weroha SJ, Foster NR, et al. Phase 2 trial of everolimus and letrozole in relapsed estrogen receptor-positive high-grade ovarian cancers. Gynecol Oncol. 2017;146(1):64–68. doi:10.1016/j.ygyno.2017.04.02028461031
- Courtney KD, Manola JB, Elfiky AA, et al. A phase I study of everolimus and docetaxel in patients with castration-resistant prostate cancer. Clin Genitourin Cancer. 2015;13(2):113–123. doi:10.1016/j.clgc.2014.08.00725450031
- Deenen MJ, Klumpen HJ, Richel DJ, et al. Phase I and pharmacokinetic study of capecitabine and the oral mTOR inhibitor everolimus in patients with advanced solid malignancies. Invest New Drugs. 2012;30(4):1557–1565. doi:10.1007/s10637-011-9723-421809026
- Doi T, Muro K, Boku N, et al. Multicenter phase II study of everolimus in patients with previously treated metastatic gastric cancer. J Clin Oncol. 2010;28(11):1904–1910. doi:10.1200/JCO.2009.26.292320231677
- El-Madani M, Colomban O, Tod M, et al. EVESOR, a model-based, multiparameter, Phase I trial to optimize the benefit/toxicity ratio of everolimus and sorafenib. Future Oncol. 2017;13(8):679–693. doi:10.2217/fon-2016-035728076966
- Escudier B, Molinie V, Bracarda S, et al. Open-label phase 2 trial of first-line everolimus monotherapy in patients with papillary metastatic renal cell carcinoma: RAPTOR final analysis. Eur J Cancer. 2016;69:226–235. doi:10.1016/j.ejca.2016.08.00427680407
- Fazio N, Granberg D, Grossman A, et al. Everolimus plus octreotide long-acting repeatable in patients with advanced lung neuroendocrine tumors: analysis of the phase 3, randomized, placebo-controlled RADIANT-2 study. Chest. 2013;143(4):955–962. doi:10.1378/chest.12-110823187897
- Ferolla P, Brizzi MP, Meyer T, et al. Efficacy and safety of long-acting pasireotide or everolimus alone or in combination in patients with advanced carcinoids of the lung and thymus (LUNA): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2017;18(12):1652–1664. doi:10.1016/S1470-2045(17)30681-229074099
- Finn RS, Poon RT, Yau T, et al. Phase I study investigating everolimus combined with sorafenib in patients with advanced hepatocellular carcinoma. J Hepatol. 2013;59(6):1271–1277. doi:10.1016/j.jhep.2013.07.02923928403
- Fury MG, Sherman E, Haque S, et al. A phase I study of daily everolimus plus low-dose weekly cisplatin for patients with advanced solid tumors. Cancer Chemother Pharmacol. 2012;69(3):591–598. doi:10.1007/s00280-011-1734-521913034
- Ghobrial IM, Gertz M, Laplant B, et al. Phase II trial of the oral mammalian target of rapamycin inhibitor everolimus in relapsed or refractory Waldenstrom macroglobulinemia. J Clin Oncol. 2010;28(8):1408–1414. doi:10.1200/JCO.2009.24.099420142598
- Goldberg HJ, Harari S, Cottin V, et al. Everolimus for the treatment of lymphangioleiomyomatosis: a phase II study. Eur Respir J. 2015;46(3):783–794. doi:10.1183/09031936.0021071426113676
- Gong C, Zhao Y, Wang B, et al. Efficacy and safety of everolimus in Chinese metastatic HR positive, HER2 negative breast cancer patients: a real-world retrospective study. Oncotarget. 2017;8(35):59810–59822. doi:10.18632/oncotarget.v8i3528938684
- Grignani G, Palmerini E, Ferraresi V, et al. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: a non-randomised phase 2 clinical trial. Lancet Oncol. 2015;16(1):98–107. doi:10.1016/S1470-2045(14)71136-225498219
- Hainsworth JD, Spigel DR, Burris HA 3rd, Waterhouse D, Clark BL, Whorf R. Phase II trial of bevacizumab and everolimus in patients with advanced renal cell carcinoma. J Clin Oncol. 2010;28(13):2131–2136. doi:10.1200/JCO.2009.26.315220368560
- Hatano T, Chikaraishi K, Inaba H, Endo K, Egawa S. Outcomes of everolimus treatment for renal angiomyolipoma associated with tuberous sclerosis complex: A single institution experience in Japan. Int J Urol. 2016;23(10):833–838. doi:10.1111/iju.2016.23.issue-1027480662
- Hatano T, Atsuta M, Inaba H, Endo K, Egawa S. Effect of everolimus treatment for renal angiomyolipoma associated with tuberous sclerosis complex: an evaluation based on tumor density. Int J Clin Oncol. 2017.
- Hurvitz SA, Dalenc F, Campone M, et al. A phase 2 study of everolimus combined with trastuzumab and paclitaxel in patients with HER2-overexpressing advanced breast cancer that progressed during prior trastuzumab and taxane therapy. Breast Cancer Res Treat. 2013;141(3):437–446. doi:10.1007/s10549-013-2689-524101324
- Jerusalem G, Mariani G, Ciruelos EM, et al. Safety of everolimus plus exemestane in patients with hormone-receptor-positive, HER2-negative locally advanced or metastatic breast cancer progressing on prior non-steroidal aromatase inhibitors: primary results of a phase IIIb, open-label, single-arm, expanded-access multicenter trial (BALLET). Ann Oncol. 2016;27(9):1719–1725. doi:10.1093/annonc/mdw24927358383
- Jovanovic B, Mayer IA, Mayer EL, et al. A randomized Phase II neoadjuvant study of cisplatin, paclitaxel with or without everolimus in patients with stage II/III Triple-Negative Breast Cancer (TNBC): responses and long-term outcome correlated with increased frequency of DNA damage response gene mutations, TNBC subtype, AR status, and Ki67. Clin Cancer Res. 2017;23(15):4035–4045. doi:10.1158/1078-0432.CCR-16-305528270498
- Jozwiak S, Kotulska K, Berkowitz N, Brechenmacher T, Franz DN. Safety of everolimus in patients younger than 3 years of age: results from EXIST-1, a randomized, controlled clinical trial. J Pediatr. 2016;172:151–155 e151. doi:10.1016/j.jpeds.2016.01.02726858193
- Kato R, Obara W, Matsuura T, Kato Y, Iwasaki K, Fujioka T. Efficacy of everolimus in patients with advanced renal cell carcinoma refractory or intolerant to VEGFR-TKIs and safety compared with prior VEGFR-TKI treatment. Jpn J Clin Oncol. 2014;44(5):479–485. doi:10.1093/jjco/hyu01824688083
- Kim DW, Oh DY, Shin SH, et al. A multicenter phase II study of everolimus in patients with progressive unresectable adenoid cystic carcinoma. BMC Cancer. 2014;14:795. doi:10.1186/1471-2407-14-79525362970
- Kim JH, Lee W, Kim TN, Nam JK, Kim TH, Lee KS. Clinical outcomes of the sequential use of pazopanib followed by everolimus for the treatment of metastatic renal cell carcinoma: A multicentre study in Korea. Can Urol Assoc J. 2018;12(1):E15–E20. doi:10.5489/cuaj.464429173270
- Koutsoukos K, Bamias A, Tzannis K, et al. Real-world experience of everolimus as second-line treatment in metastatic renal cell cancer after failure of pazopanib. Onco Targets Ther. 2017;10:4885–4893. doi:10.2147/OTT29062235
- Kulke MH, Ruszniewski P, Van Cutsem E, et al. A randomized, open-label, phase 2 study of everolimus in combination with pasireotide LAR or everolimus alone in advanced, well-differentiated, progressive pancreatic neuroendocrine tumors: COOPERATE-2 trial. Ann Oncol. 2017;28(6):1309–1315. doi:10.1093/annonc/mdx07828327907
- Kumano M, Miyake H, Harada K, Fujisawa M. Sequential use of mammalian target of rapamycin inhibitors in patients with metastatic renal cell carcinoma following failure of tyrosine kinase inhibitors. Med Oncol. 2013;30(4):745. doi:10.1007/s12032-013-0745-y24122255
- Moscetti L, Vici P, Gamucci T, et al. Safety analysis, association with response and previous treatments of everolimus and exemestane in 181 metastatic breast cancer patients: a multicenter Italian experience. Breast. 2016;29:96–101. doi:10.1016/j.breast.2016.07.00527476084
- Motzer RJ, Alyasova A, Ye D, et al. Phase II trial of second-line everolimus in patients with metastatic renal cell carcinoma (RECORD-4). Ann Oncol. 2016;27(3):441–448. doi:10.1093/annonc/mdv61226681676
- Motzer RJ, Barrios CH, Kim TM, et al. Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol. 2014;32(25):2765–2772. doi:10.1200/JCO.2013.54.691125049330
- Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16(15):1473–1482. doi:10.1016/S1470-2045(15)00290-926482279
- Oh DY, Kim TW, Park YS, et al. Phase 2 study of everolimus monotherapy in patients with nonfunctioning neuroendocrine tumors or pheochromocytomas/paragangliomas. Cancer. 2012;118(24):6162–6170. doi:10.1002/cncr.2767522736481
- Ohtsu A, Ajani JA, Bai YX, et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol. 2013;31(31):3935–3943. doi:10.1200/JCO.2012.48.355224043745
- Ohyama K, Matsumoto Y, Amamizu H, et al. Association of coronary perivascular adipose tissue inflammation and drug-eluting stent-induced coronary hyperconstricting responses in pigs: (18)F-fluorodeoxyglucose positron emission tomography imaging study. Arterioscler Thromb Vasc Biol. 2017;37(9):1757–1764. doi:10.1161/ATVBAHA.117.30984328751570
- Panzuto F, Rinzivillo M, Fazio N, et al. Real-world study of everolimus in advanced progressive neuroendocrine tumors. Oncologist. 2014;19(9):966–974. doi:10.1634/theoncologist.2014-003725117065
- Park K, Lee JL, Ahn JH, et al. Efficacy and safety of everolimus in korean patients with metastatic renal cell carcinoma following treatment failure with a vascular endothelial growth factor receptor-tyrosine kinase inhibitor. Cancer Res Treat. 2014;46(4):339–347. doi:10.4143/crt.2013.15425036572
- Pavel M, Unger N, Borbath I, et al. Safety and QOL in patients with advanced NET in a Phase 3b expanded access study of everolimus. Target Oncol. 2016;11(5):667–675. doi:10.1007/s11523-016-0440-y27193465
- Quek R, Wang Q, Morgan JA, et al. Combination mTOR and IGF-1R inhibition: phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clin Cancer Res. 2011;17(4):871–879. doi:10.1158/1078-0432.CCR-10-262121177764
- Safra T, Kaufman B, Kadouri L, et al. Everolimus plus letrozole for treatment of patients with HR(+), HER2(-) advanced breast cancer progressing on endocrine therapy: an open-label, Phase II trial. Clin Breast Cancer. 2017.
- Salazar R, Garcia-Carbonero R, Libutti SK, et al. Phase II study of BEZ235 versus Everolimus in patients with mammalian target of rapamycin inhibitor-naive advanced pancreatic neuroendocrine tumors. Oncologist. 2017.
- Sarkaria JN, Galanis E, Wu W, et al. North Central Cancer Treatment Group Phase I trial N057K of everolimus (RAD001) and temozolomide in combination with radiation therapy in patients with newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2011;81(2):468–475. doi:10.1016/j.ijrobp.2010.05.06420864273
- Strickler JH, Starodub AN, Jia J, et al. Phase I study of bevacizumab, everolimus, and panobinostat (LBH-589) in advanced solid tumors. Cancer Chemother Pharmacol. 2012;70(2):251–258. doi:10.1007/s00280-012-1911-122744359
- Sun JM, Kim JR, Do IG, et al. A phase-1b study of everolimus plus paclitaxel in patients with small-cell lung cancer. Br J Cancer. 2013;109(6):1482–1487. doi:10.1038/bjc.2013.46723963141
- Takahashi K, Uchida K, Yoshimura N, et al. Efficacy and safety of concentration-controlled everolimus with reduced-dose cyclosporine in Japanese de novo renal transplant patients: 12-month results. Transplant Res. 2013;2(1):14. doi:10.1186/2047-1440-2-1423866828
- Tobinai K, Ogura M, Maruyama D, et al. Phase I study of the oral mammalian target of rapamycin inhibitor everolimus (RAD001) in Japanese patients with relapsed or refractory non-Hodgkin lymphoma. Int J Hematol. 2010;92(4):563–570. doi:10.1007/s12185-010-0707-520972652
- Vlahovic G, Meadows KL, Uronis HE, et al. A phase I study of bevacizumab, everolimus and panitumumab in advanced solid tumors. Cancer Chemother Pharmacol. 2012;70(1):95–102. doi:10.1007/s00280-012-1889-822638798
- Wang M, Popplewell LL, Collins RH Jr., et al. Everolimus for patients with mantle cell lymphoma refractory to or intolerant of bortezomib: multicentre, single-arm, phase 2 study. Br J Haematol. 2014;165(4):510–518. doi:10.1111/bjh.2014.165.issue-424579926
- Werner D, Atmaca A, Pauligk C, Pustowka A, Jager E, Al-Batran SE. Phase I study of everolimus and mitomycin C for patients with metastatic esophagogastric adenocarcinoma. Cancer Med. 2013;2(3):325–333. doi:10.1002/cam4.7723930209
- Wolpin BM, Hezel AF, Abrams T, et al. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol. 2009;27(2):193–198. doi:10.1200/JCO.2008.18.951419047305
- Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26(26):4311–4318. doi:10.1200/JCO.2008.16.785818779618
- Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–523. doi:10.1056/NEJMoa100929021306238
- Yee KW, Zeng Z, Konopleva M, et al. Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2006;12(17):5165–5173. doi:10.1158/1078-0432.CCR-06-076416951235