Abstract
Background
Cells within breast cancer stem cell populations have been confirmed to have a CD44+CD24− phenotype. Strong expression of CD44 plays a critical role in numerous types of human cancers. CD44 is involved in cell differentiation, adhesion, and metastasis of cancer cells.
Methods
In this study, we reduced CD44 expression in CD44+CD24− breast cancer stem cells and investigated their sensitivity to an antitumor drug. The CD44+CD24− breast cancer stem cells were isolated from breast tumors; CD44 expression was downregulated with siRNAs followed by treatment with different concentrations of the antitumor drug.
Results
The proliferation of CD44 downregulated CD44+CD24− breast cancer stem cells was decreased after drug treatment. We noticed treated cells were more sensitive to doxorubicin, even at low doses, compared with the control groups.
Conclusions
It would appear that expression of CD44 is integral among the CD44+CD24− cell population. Reducing the expression level of CD44, combined with doxorubicin treatment, yields promising results for eradicating breast cancer stem cells in vitro. This study opens a new direction in treating breast cancer through gene therapy in conjunction with chemotherapy.
Background
Cancer stem cells have been considered to be persistent in malignant tissues. The existence of cancer stem cells has been recently confirmed in solid tumors of the brain, prostate, pancreas, liver, colon, head and neck, lung, and skin.Citation1–Citation6 Breast cancer stem cells were identified as a cell population with a CD44+CD24− phenotype. This finding proved that as few as 100 cells with this phenotype could efficiently generate new tumors, while 20,000 cells without such marker expression did not form tumors.Citation7 The presence of a breast cancer stem cell population explained the minimal efficiency and high recurrence of conventional breast cancer treatments; breast cancer stem cells are able to resist chemotherapy and radiotherapy treatment. To date, various strategies have been developed to target these stem cells, utilizing differentiation and antitumor drug resistance therapy. We postulate that using antitumor drug resistance therapy to support chemotherapy would be a potential approach for more efficient cancer treatment. Numerous reports have shown that drug resistance involved inhibiting the expression of the ABCG2 protein.Citation8–Citation13 ABCG2 is a drug transporter on the membrane surface of cells. Inhibition of the expression of this channel results in an increase in the sensitivity of cells to antitumor drugs.
In this study, we wanted to evaluate the role of other genes and proteins in limiting propagation of CD44+CD24− breast cancer stem cells. The adhesion molecule, CD44, is a cell surface transmembrane glycoprotein involved in lymphocyte activation, recirculation and homing, adhesion of extracellular matrix, angiogenesis, and cell proliferation, differentiation, and migration.Citation14 These properties are associated with the pathologic activities of cancer cells. As reported by Al-Hajj et al,Citation7 cells that were strongly positive for CD44 and negative for CD24 (CD44+CD24−/low) had tumorigenic and metastatic abilities in breast tumor tissue. We postulated that CD44 was a critical protein for breast cancer stem cells to retain their survival, multipotency, and other important properties, especially drug resistance.
Methods
Cell culture and isolation of CD44+CD24− cells
Isolation and in vitro expansion of stem cells were carried out with breast tumor specimens obtained from consenting patients. Tumor biopsies were obtained at hospitals, then transferred to our laboratory. The biopsy samples were washed 3–4 times with phosphate-buffered saline (PBS), supplemented with 1X antibiotics and an antimycotic (Sigma-Aldrich, St Louis, MO), and homogenized into small (approximately 1–2 mm3) fragments. Homogenized samples were resuspended in M171 medium (Invitrogen, Carlsbad, CA) containing mammary epithelial growth supplement (MEGS; Invitrogen) and seeded into 35-mm culture dishes (Nunc, Roskilde, Denmark). Dishes were incubated at 37°C/5% CO2 and medium was refreshed every third day.
When confluency reached 70%, candidates for breast cancer stem cells were plated at a concentration of 1000 cells/mL in serum-free DMEM-F12, supplemented with 10 ng/mL basic fibroblast growth factor (bFGF), 20 ng/mL epidermal growth factor (EGF), 5 ng/mL insulin, and 0.4% bovine serum albumin (BSA). Cells grown under these conditions were nonadherent and formed spherical clusters of cells designated ‘spheres’ or ‘mammospheres,’ and were enzymatically dissociated every 3 days by incubation in a 0.25% trypsin-EDTA solution (Sigma-Aldrich) for 2 minutes at 37°C to achieve a single cell suspension.
To purify the CD44+CD24− cell population, 1 mL cell suspensions in PBS (107 cells) were double stained with 20 μL anti CD44-FITC and 20 μL anti CD24–PE. Samples were incubated in the dark and at room temperature for 45 minutes. CellQuest Pro software (BD Bioscience, Franklin Lakes, NJ) application was used to identify the CD44+CD24−/low cell population (). This population was sorted into a 50 mL tube coated with 1 mg/mL BSA. CD44+CD24− cells were harvested by centrifugation (3000 rpm, 5 minutes) and the population recovered by adherent culture in DMEM/F12 supplemented with 10% fetal bovine serum (FBS) and 1X antibiotic-mycotic (Sigma-Aldrich). The purity was confirmed by flow cytometry and samples with higher than 90% CD44+CD24− cells were used for further experiments.
Transient transfection of siRNA
The CD44 small interfering RNA (siRNA) sequences were 5′-AGC TCT GAG CAT CGG ATT T-3′, 5′-TGG CTG ATC ATC TTG GCA T-3′, and 5′-CAC CTC CCA GTA TGA CAC A-3′. The siRNAs were transiently transfected with an siRNA Transfection kit (Santa Cruz Biotechnology, Inc, Santa Cruz, CA) following the manufacturer’s instructions. Briefly, 1 μg of the siRNA was mixed with 1 mL transfection medium and transfection reagent supplied with the kit, and incubated with 2 × 105 adherent cells for 5–7 hours at 37°C/5% CO2. The medium was replaced with DMEM/F12 supplemented with 20% FBS and 1% antibiotic-antimycotic, and incubated at 37°C/5% CO2 for 18–24 hours. Finally, cells were cultured in fresh DMEM/F12 containing 10% FBS and 1% antibiotic-antimycotic. After 48 hours of incubation, cells were ready for expression analysis of CD44.
RT-PCR
Approximately 107 cells in the control and experimental groups were collected for total RNA isolation using a GeneJet RNA purification kit (Fermentas International Inc, Thermo Fisher Scientific, Ottawa, ON) according to the manufacturer’s instructions. The concentration of RNA was measured using a Biophotometer (Eppendorf, Hamburg, Germany). One-step RT-PCR was carried out using an Access RT-PCR system kit (Promega, Madison, WI). The RT-PCR was performed in a 50 μL volume with a 45°C incubation for 30 minutes initially, followed by a 5-minute incubation at 95°C, then 30 cycles of 94°C for 45 seconds, 59°C for 30 seconds, 72°C for 45 seconds, and a final extension step at 72°C for 5 minutes after cycle 30. The primer sequences used for amplification of a 250 bp CD44 fragment were 5′-ACA GCA CAG ACA GAA TCC CTG-3′ (forward) and 5′-TCT TCT GCC CAC ACC TTC TCC-3′ (reverse). Amplification of a 94 bp fragment of the GAPDH gene was carried out with primers 5′-ACA GTC AGC CGC ATC TTC TT-3′ (forward) and 5′-ACG ACC AAA TCC GTT GAC TC-3′ reverse. The PCR products were separated on a 2% (w/v) agarose gel stained with ethidium bromide and visualized under UV light (GelDot it, UVP, Upland, CA).
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde and washed three times with PBS. Fixed cells were incubated with a mouse antihuman CD44 antibody followed by a fluorescein isothiocyanate (FITC)-conjugated goat antimouse antibody (Santa Cruz Biotechnology Inc). For all the immunocytochemistry assays, negative staining controls involved omitting the primary antibody. Nuclei were stained with Hoescht 33342 (Sigma-Aldrich). Images were captured using a Carl Zeiss microscope and a monochromatic cooled camera (Carl Zeiss, Oberkochen, Germany).
Antitumor drug treatment assay
Breast cancer stem cells (CD44+CD24−) with and without CD44 downregulation were seeded at a density of 0.4 × 104 cells per well in 24-well plates (Nunc) in DMEMF12/10% FBS. After a 24-hour culture period, cells were treated with 0, 1, 3, and 6 μg/mL doxorubicin (Sigma-Aldrich) for 48 hours. These cells were used to analyze the cell cycle, proliferation, and apoptosis.
Apoptosis and cell cycle analysis
Apoptosis was investigated by flow cytometry using annexin V and propidium iodide (PI; BD Biosciences), while cell cycle analysis was carried according to following protocols. Cells in groups were washed twice in PBS and fixed in cold 70% ethanol for at least 3 hours at 4°C. Subsequently, cells were washed in PBS twice and stained with 1 mL of PI (20 μg/mL). A 50 μL volume of RNase A (10 μg/mL) was added to samples and incubated for 3 hours at 4°C. Stained cells were analyzed by flow cytometry using CellQuest Pro software.
Proliferation assay
Cells (5 × 105 cells/well) were seeded into 96-well microplates in DMEM/F12 supplemented with 10% FBS and 1% antibiotic-mycotic and incubated for 1, 3, and 5 days at 37°C/5% CO2. A 10 μL volume of MTT (5 mg/mL) was added to each well for and left for 4 hours, then 150 μL DMSO was added to each well. The absorbance was measured with a multimode reader (Beckman Coulter, Brea, CA) at 595 nm and 620 nm. Samples were analyzed in triplicate.
Statistical analysis
All experiments were performed in triplicate. The significance of differences between mean values was assessed by the t-test and ANOVA. A P-value <0.05 was considered to be significant. Data were analyzed by Statgraphics software (v 7.0; Statgraphics Graphics System, Warrenton, VA).
Results
Isolation of CD44+CD24− cells from breast cancer biopsies
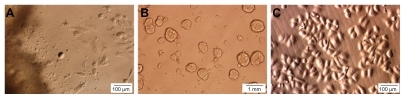
We carried out primary culture of 21 tumors from 21 different patients, 15 samples exhibiting tumors that had grown with many single cells surrounding the tumor tissue. Cells from 15 samples were left to freely propagate until the culture reached 80% confluence. In most of the samples, single cells appeared around day 5, with the earliest at day 3. In the next stage, cells proliferated rapidly and combined clones were achieved at day 15. Two types of cells were observed in primary culture: epithelial cells with a bean shape and large nucleus, and stromal cells containing a smaller nucleus that were long, like fibroblasts (). When 15 primary cell samples were analyzed for the markers CD44 and CD24, all had a small population of cells positive for CD44 and negative or weakly positive for CD24. On average, this population comprised 3.59% ± 1.65% of the total of cells derived from primary culture, the lowest proportion being 1.25% and the highest 7.12% (n = 15). The results also showed that approximately 50% of cells were positive for CD24. However, >90% of cells were negative for CD44. Flow cytometry analysis indicated that most cells that were positive for CD44 were negative or weakly positive for CD24. When culturing primary cells in the mammosphere medium, many mammospheres were appearing in culture after 14 days (). Mammospheres were disaggregated by trypsin and seeded under adherent conditions with uniform morphology ().
Expression of CD44 after downregulation CD44+CD24− cells
Following siRNA transfection, total RNA was extracted to evaluate the expression of CD44 in transfected cells. Electrophoresis indicated a significant change in RT-PCR CD44 products before and after downregulation, which did not occur for the internal control gene (). The band intensity of PCR products analyzed by GelAnalyzer software showed that the signal of CD44 band decreased by nearly half compared with before knockdown (). This showed that CD44 siRNA transfection efficiently silenced RNA copies of CD44, thus reducing CD44 messenger RNA (mRNA) level in treated cells. To investigate the decrease in expression of CD44, we performed protein quantification by flow cytometry, which showed that the number of CD44 positive cells was reduced from 93.65% ± 2.34% to 6.18% ± 2.39% (n = 3) (). The immunocytochemistry results revealed a similar trend after siRNA transfection ().
Figure 2 Expression of CD44 was markedly decreased after transfection with CD44 siRNA. The CD44 knockdown CD44+CD24− cells exhibited decreased expression of CD44 at a transcriptional level as confirmed by RT-PCR (A) and band intensity on agarose gel analyzed with GelAnalyzer software (E). At the translational level, this was confirmed by flow cytometry (C) and compared with cells where knockdown did not occur (B). The CD44 knockdown cells proliferated slowly with a decrease in the total number of cells compared with normal cells (D).
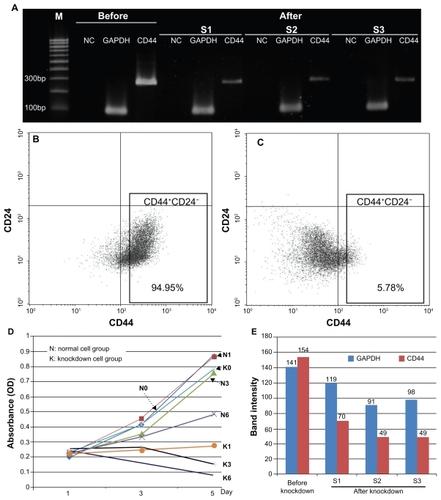
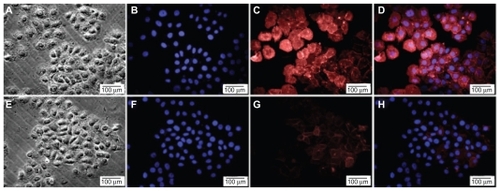
Figure 3 Expression of CD44 was decreased after transfection with CD44 siRNA. The CD44+CD24− cells strongly expressed CD44 protein (A, B, C, D) and CD44 was weakly expressed after knockdown (E, F, G, H). A, E) cells before and after siRNA transfection. B, F) nuclei were stained with Hoescht 33342. C, G) cells were stained with anti-CD44 antibody. D) merged picture of B and C. H) merged picture of F and G.
Characteristics of CD44+CD24− cells following CD44 downregulation and treatment with doxorubicin
CD44 knockdown CD44+CD24− cells slowly proliferated after treatment with doxorubicin
Treatment with doxorubicin had a suppressive effect on proliferation of CD44+CD24− cells where CD44 expression had been downregulated, whereas no change was seen in the control group. and show that when doxorubicin was absent, proliferation rate was similar in the two groups with or without downregulation of CD44 after 3–5 days in culture. The effects of doxorubicin on normal cells were not observable at concentrations of 0, 1, and 3 μg/mL. At 6 μg/mL, proliferation inhibition became apparent; however, at this concentration, cells still proliferated at a moderate speed, with the OD value increasing from 0.24 ± 0.06 at day 1 to 0.49 ± 0.03 at day 5. In contrast, for the CD44 knockdown cells, doxorubicin effects were noticeable at concentrations of 1, 3, and 6 μg/mL. At a concentration of 1 μg/mL, cells grew at the same rate as cells without doxorubicin treatment and as well as those with 1 μg/mL growth inhibitor. At concentrations of 3 and 6 μg/mL, cells showed no sign of proliferation, with necrosis observed. This led to a significant reduction in cell numbers in comparison with day 1. These data show that CD44+CD24− cells had strong resistance to doxorubicin. The resistance to doxorubicin was decreased by downregulation of CD44. Consequently, after treatment at a concentration of 1 μg/mL, proliferation of CD44 knockdown cells was inhibited and higher concentrations of doxorubicin caused cell death ().
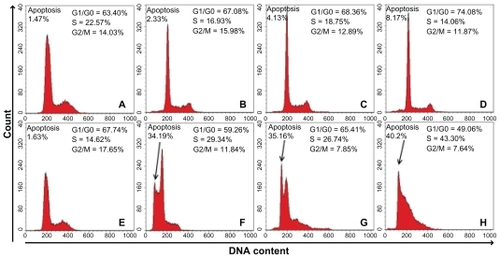
CD44 knockdown CD44+CD24− cells showed increased levels of apoptosis and alterations in the cell cycle
As shown in , downregulation of CD44 expression caused apoptosis when doxorubicin was added. When the concentration of doxorubicin was 1, 3, and 6 μg/mL, the proportion of dead cells increased gradually to 2.33%, 4.13%, and 8.17%, respectively (). The apoptotic effect was obvious in CD44 knockdown cells, peaking at 34.19% dead cells when treated with 1 μg/mL doxorubicin. The level of apoptosis rose steadily and peaked at 40.2% when doxorubicin was used at a concentration of 6 μg/mL (). Additionally, cells underwent cell cycle alteration, with the majority of normal cells in the G1/G0 phase and 10%–20% of cells in the S and G2/M phase. Unlike normal cells, CD44 knockdown cells remained in the S phase, leading to a reduction in the proportion of cells in G0/G1 and G2/M. When a high concentration of agents was used, more cells terminated in the S phase ().
Discussion
Breast cancer stem cells have been identified to have the CD44+CD24− phenotype.Citation7 This is a rare population in malignant breast tumors, with numerous reports indicating that they are the origin of breast tumors. Breast cancer stem cells with this profile were shown to be capable of tumorigenicity, metastasis, and drug resistance.Citation15–Citation17 Based on the knowledge that CD44 plays a critical role in tumorigenesis from other cancer studies, this work was performed to evaluate and compare the effects of a tumor-killing agent on CD44+CD24− breast cancer stem cells with knocked down CD44.
We isolated breast cancer cells with a CD44+CD24− phenotype from malignant tumors and enriched mammospheres prior to harvesting and purifying cells of interest. We were able to obtain a CD44+CD24− cell population with a purity >90.05%. The highest and lowest levels of purity were 94.54% and 85.12%, respectively (n = 15). Of the 21 samples, we selected the purest sample (94.54%) for further experiments.
To decrease expression of CD44, we used siRNA; following CD44 siRNA transfection, cells were subjected to RT-PCR quantification and drug effects were investigated. Transfection of CD44 siRNA into CD44+CD24− cells reduced expression of the CD44. The semi-quantitative RT-PCR analysis and electrophoresis showed that CD44 mRNAs remaining in the cells was much lower compared with the original cells, while expression of the GAPDH internal control was unaltered. At a translational level, we used flow cytometry and immunocytochemistry to assess CD44 expression. Results from these two techniques confirmed a noticeable reduction in CD44 expression. These results are consistent with a previous report that also used CD44 siRNA to monitor CD44 expression.Citation18
Cells verified as CD44 knockdown cells were treated with doxorubicin and compared with normal cells to confirm the role of CD44 in the breast cancer stem cell population. Doxorubicin is commonly used to treat some leukemias and Hodgkin’s lymphoma, as well as cancers of the bladder, breast, stomach, lung, ovaries, thyroid, soft tissue sarcoma, multiple myeloma, and others. Moreover because doxorubicin is extruded out via ABCG2 pumps, it is easy to evaluate the role of CD44 downregulation in further experiments. Doxorubicin is therefore used in much research, especially on antitumor drug resistance.
The knockdown cells were more sensitive to doxorubicin compared with the original cells. Even at high doxorubicin concentrations, the drug failed to kill normal CD44+CD24− cells, whereas it caused apoptosis in the CD44 siRNA-transfected cells. This implies that the reduction of CD44 expression levels was associated with a decrease in drug resistance. Reduction of CD44 expression made breast cancer stem cells more sensitive to anticancer agents.
CD44 is a transmembrane proteinCitation19 synthesized in multiple isoforms by mRNA splicing. Although CD44 lacks domains critical for signal transmission, it has been demonstrated that it is necessary for homing, and contains some characteristics of leukemia stem cells.Citation20,Citation21 There is some evidence that CD44 supports independent adhesion in vitro and tumor development as well as metastasis in solid tumors.Citation22–Citation24 The tumor-stimulating function of CD44 occurs through costimulation with signals by growth factor receptors such as EGF receptor-2 (Her2), EGF receptor 1 (Her1), and hepatocyte growth factor (Met).Citation25 CD44 was considered as a useful marker to detect and enrich cancer stem cells.Citation7,Citation26–Citation29 CD44-positive cells are tumorigenic and highly metastatic.Citation7 Additionally, the CD44 marker has also been used to isolate prostate,Citation30,Citation31 pancreas,Citation4 and colorectalCitation32 cancer stem cells.
One feature of cancer stem cells (CSCs) in tumors is high expression of ABC transporters, especially ABCG2. The ABC transporters protect cells from the effects of drugs through drug excretion. Thus, CSCs, with their special biological properties, are able to resist drugs, even those used in chemotherapy.Citation33 In clinical treatment, chemotherapy can abolish most of the cells in a solid tumor. However, a small population of CSCs are drug resistant, possibly due to the presence of many ABC transporters that help to pump antitumor drugs out of the cells. Increased expression of ABC transporters in CSCs led to export of Hoechst 33342 and Rhodamine 123, which were detected by flow cytometry. Those cells that expelled Hoechst 33342 as detected by flow cytometry were called a side population. In comparison with cells sensitive to drugs, the CD44+CD24− cell population has a high level of ABCG2. Therefore, several studies using siRNA demonstrated that ABCG2 gene knockdown can ablate the capacity for drug resistance.
In this current study, reduction of CD44 expression alleviated the drug-resistant ability in breast cancer stem cells. This showed that there was a correlation between drug resistance and CD44 expression or signal pathways related to this protein. Reducing CD44 expression inhibited proliferation and caused apoptosis, which was also confirmed in CD44-positive liver cancer cellsCitation34,Citation35 and prostate cancer cells.Citation36
Based on the results of this study, we propose that CD44 can be a target for treating cancers, especially breast cancer. Using CD44 siRNAs against breast cancer stem cells with the CD44+CD24− phenotype may be an efficient gene therapy strategy for breast cancer. However the application of gene therapy to knock out CD44 still faces various challenges as there are many cells in the human body positive for CD44. Therefore intratumoral gene therapy with a polyethylenimine/siRNA CD44 plasmid DNA complex would be the best choice in this case. In this strategy, DNA plasmids containing the CD44 sequence that can transcript to CD44 siRNAs are mixed with polyethylenimine. Then this complex can be directly injected into breast tumors. Polyethylenimine is used as a reagent to facilitate plasmids into the tumor cells.
Conclusions
Strong expression of CD44 in a breast cancer stem cell population with a CD44+CD24− phenotype plays a pivotal role in proliferation and drug resistance of malignant cells. In this study, the molecular mechanisms and signal pathways responsible for proliferation and drug resistance were downregulated by knocking down CD44. Our data suggest that decreasing CD44 expression made cancer cells more sensitive to anticancer drugs. A combination of gene therapy to reduce CD44 expression and chemotherapy mitigated drug resistance and caused apoptosis in treated cells. This could be a new targeted strategy to eradicate breast cancer stem cells.
Acknowledgments
This work was funded by grants from the Vietnam National Project about Breast Cancer, Ministry of Science and Technology, Vietnam. We thank the Oncology Hospital at Ho Chi Minh for supplying the malignant breast cancer tumors.
Disclosure
The authors report no conflicts of interest in this work.
References
- Antón AparicioLMCassinello EspinosaJGarcía CampeloRGómez VeigaFDíaz PradoSAparicio GallegoGProstate carcinoma and stem cellsClin Transl Oncol200792667617329217
- EramoALottiFSetteGIdentification and expansion of the tumorigenic lung cancer stem cell populationCell Death Differ200815350451418049477
- GlinskyGVStem cell origin of death-from-cancer phenotypes of human prostate and breast cancersStem Cell Rev200731799317873385
- LiCHeidtDGDalerbaPIdentification of pancreatic cancer stem cellsCancer Res20076731030103717283135
- PrinceMESivanandanRKaczorowskiAIdentification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinomaProc Natl Acad Sci U S A2007104397397817210912
- SeoDCSungJMChoHJGene expression profiling of cancer stem cell in human lung adenocarcinoma A549 cellsMol Cancer200767518034892
- Al-HajjMWichaMSBenito-HernandezAMorrisonSJClarkeMFProspective identification of tumorigenic breast cancer cellsProc Natl Acad Sci U S A200310073983398812629218
- Ahmed-BelkacemAPozzaAMacalouSPérez-VictoriaJMBoumendjelADi PietroAInhibitors of cancer cell multidrug resistance mediated by breast cancer resistance protein (BCRP/ABCG2)Anticancer Drugs200617323924316520651
- CalcagnoAMFostelJMToKKSingle-step doxorubicin-selected cancer cells overexpress the ABCG2 drug transporter through epigenetic changesBr J Cancer20089891515152418382425
- LagasJSvan der KruijssenCMvan de WeteringKBeijnenJHSchinkelAHTransport of diclofenac by breast cancer resistance protein (ABCG2) and stimulation of multidrug resistance protein 2 (ABCC2)-mediated drug transport by diclofenac and benzbromaroneDrug Metab Dispos200937112913618845662
- LouHDeanMTargeted therapy for cancer stem cells: the patched pathway and ABC transportersOncogene20072691357136017322922
- LvHHeZLiuXYuanJYuYChenZReversal of BCRP-mediated multidrug resistance by stable expression of small interfering RNAsJ Cell Biochem20071021758117372930
- PriebschARompeFTönniesHComplete reversal of ABCG2- depending atypical multidrug resistance by RNA interference in human carcinoma cellsOligonucleotides200616326327416978089
- AdamiaSMaxwellCAPilarskiLMHyaluronan and hyaluronan synthases: potential therapeutic targets in cancerCurr Drug Targets Cardiovasc Haematol Disord20055131415720220
- CalcagnoAMSalcidoCDGilletJPProlonged drug selection of breast cancer cells and enrichment of cancer stem cell characteristicsJ Natl Cancer Inst2010102211637165220935265
- FillmoreCMKuperwasserCHuman breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapyBreast Cancer Res2008102R2518366788
- LacerdaLPusztaiLWoodwardWAThe role of tumor initiating cells in drug resistance of breast cancer: Implications for future therapeutic approachesDrug Resist Updat2010134–59910820739212
- VerkaikNSTrapmanJRomijnJCVan der KwastTHVan SteenbruggeGJDown-regulation of CD44 expression in human prostatic carcinoma cell lines is correlated with DNA hypermethylationInt J Cancer19908034394439935187
- AruffoAStamenkovicIMelnickMUnderhillCBSeedBCD44 is the principal cell surface receptor for hyaluronateCell1990617130313131694723
- JinLHopeKJZhaiQSmadja-JoffeFDickJETargeting of CD44 eradicates human acute myeloid leukemic stem cellsNat Med200612101167117416998484
- KrauseDSLazaridesKvon AndrianUHVan EttenRARequirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cellsNat Med200612101175118016998483
- BarbourAPReederJAWalshMDFawcettJAntalisTMGotleyDCExpression of the CD44v2-10 isoform confers a metastatic phenotype: importance of the heparan sulfate attachment site CD44v3Cancer Res200363488789212591743
- WeberGFBronsonRTIlaganJCantorHSchmitsRMakTWAbsence of the CD44 gene prevents sarcoma metastasisCancer Res20026282281228611956084
- YuQTooleBPStamenkovicIInduction of apoptosis of metastatic mammary carcinoma cells in vivo by disruption of tumor cell surface CD44 functionJ Exp Med199718612198519969396767
- PontaHShermanLHerrlichPACD44: from adhesion molecules to signalling regulatorsNat Rev Mol Cell Biol200341334512511867
- DouJPanMWenPIsolation and identification of cancer stem-like cells from murine melanoma cell linesCell Mol Immunol20074646747218163959
- HurtEMKawasakiBTKlarmannGJThomasSBFarrarWLCD44+CD24(−) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosisBr J Cancer200898475676518268494
- WrightMHCalcagnoAMSalcidoCDCarlsonMDAmbudkarSVVarticovskiLBrca1 breast tumors contain distinct CD44+/CD24− and CD133+ cells with cancer stem cell characteristicsBreast Cancer Res2008101R1018241344
- YangYMChangJWBladder cancer initiating cells (BCICs) are among EMA-CD44v6+ subset: novel methods for isolating undetermined cancer stem (initiating) cellsCancer Invest200826772573318608209
- CollinsATBerryPAHydeCStowerMJMaitlandNJProspective identification of tumorigenic prostate cancer stem cellsCancer Res20056523109461095116322242
- PatrawalaLCalhounTSchneider-BroussardRHighly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cellsOncogene200625121696170816449977
- DalerbaPDyllaSJParkIKPhenotypic characterization of human colorectal cancer stem cellsProc Natl Acad Sci U S A200710424101581016317548814
- SpillaneJBHendersonMACancer stem cells: a reviewANZ J Surg200777646446817501888
- HenryJCParkJKJiangJmiR-199a-3p targets CD44 and reduces proliferation of CD44 positive hepatocellular carcinoma cell linesBiochem Biophys Res Commun2010403112012521055388
- XieZChoongPFPoonLFInhibition of CD44 expression in hepatocellular carcinoma cells enhances apoptosis, chemosensitivity, and reduces tumorigenesis and invasionCancer Chemother Pharmacol200862694995718259754
- YangKTangYHabermehlGKIczkowskiKAStable alterations of CD44 isoform expression in prostate cancer cells decrease invasion and growth and alter ligand binding and chemosensitivityBMC Cancer2010101620074368