Abstract
Gastric cancer (GC) is one of the leading causes of tumor-related mortality. In addition to surgery and endoscopic resection, systemic therapy remains the main treatment option for GC, especially for advanced-stage disease and for cases not suitable for surgical therapy. Hence, improving the efficacy of systemic therapy is still an urgent problem to overcome. In the past decade, the essential roles of microRNAs (miRNAs) in tumor treatment have been increasingly recognized. In particular, miRNAs were recently shown to reverse the resistance to chemotherapy drugs such as 5-fluorouracil, cisplatin, and doxorubicin. Synthesized nanoparticles loaded with mimics or inhibitors of miRNAs can directly target tumor cells to suppress their growth. Moreover, exosomes may serve as promising safe carriers for mimics or inhibitors of miRNAs to treat GC. Some miRNAs have also been shown to play roles in the mechanism of action of other anti-tumor drugs. Therefore, in this review, we highlight the research progress on microRNA-based therapy in GC and discuss the challenges and prospects associated with this strategy. We believe that microRNA-based therapy has the potential to offer a clinical benefit to GC patients, and this review would contribute to and motivate further research to promote this field toward this ultimate goal.
Introduction
Gastric cancer (GC) has an extremely high rate of mortality and is ranked as one of the leading causes of cancer-related death worldwide.Citation1,Citation2 Besides adequate surgical resection and endoscopic resection, adjuvant and neoadjuvant therapies are generally used to improve the disease-free survival and overall survival of patients with GC.Citation3,Citation4 However, it remains an important challenge to develop more effective systematic treatments for GC, especially for patients with inoperable disease at diagnosis or recurrent disease after resection. The development of resistance to standard chemotherapy drugs such as 5-fluorouracil (5-FU), cisplatin, and doxorubicin has been the main cause of chemotherapy failure. Moreover, first-line targeted therapies such as trastuzumab and ramucirumab are not suitable for the majority of patients with GC.Citation5,Citation6 Hence, extensive research effort has focused on solving this current treatment challenge by gaining a better understanding of the underlying pathogenic mechanisms and conducting preclinical studies for ultimate clinical application.
In the last decade, exploration of the roles of miRNAs in carcinogenesis, treatment response, and as potential therapeutic targets has attracted widespread attention, with substantial progress made in better understanding mechanisms.Citation7 On the basis of mechanism-related studies, there has been increasing effort made to further explore the practical applications of these fundamental findings, which have revealed the essential roles of microRNAs in tumor therapy.Citation8 For example, with respect to drug resistance, over-expression of miR-939 was shown to enhance 5-FU-induced chemosensitivity by suppressing cellular growth and inducing apoptosis both in vitro and in vivo.Citation9 Moreover, caudally injected exosomes loaded with the inhibitor of miR-214 (exo-anti-214) could reverse the chemoresistance to cisplatin in a nude mouse model of GC.Citation10 In addition, synthetic carriers have been used to deliver the confirmed anti-tumor miRNAs to the precise tumor site so as to directly inhibit GC, such as “PPP”, which was constructed by modifying phenylboronic acid onto the surface of a polyamidoamine dendrimer and poly(ethylene glycol)-poly(ε-caprolactone) copolymers (PEG-PCL) nanoparticles coated with trastuzumab.Citation11,Citation12 Indeed, these studies have led to a major advance in the clinical application of miRNAs. Moreover, these findings suggested that in addition to their function as biomarkers, miRNAs can also play roles in the treatment of tumors. Nevertheless, despite these many important findings, there has been no study that summarizes these recent advances in microRNA-based therapy for GC.
Therefore, in this review, we mainly focus on the advances in microRNA-based therapies for GC, including chemotherapy, targeted therapy, tumor immunotherapy, and others. In particular, we highlight the key miRNAs reported to affect the actions of commonly used chemotherapy drugs, which will facilitate further research on drug resistance. Moreover, the listed carriers that loaded mimics or inhibitors of miRNAs can emphasize the promising applications of anti-tumor miRNAs in targeted therapy and immunotherapy, which should promote their valuable consideration for clinical practice.
microRNA-Based Chemotherapy
Fluorouracil Resistance
According to the National Comprehensive Cancer Network clinical practice guidelines in GC, 5-FU is recommended as the first-line anti-tumor drug. Several miRNAs have been identified to participate in the anti-tumor mechanism of 5-FU by decreasing the expression levels of various target genes. For example, overexpression of miR-939, miR-623, miR-429, miR-204, miR-124 or miR-31 was shown to enhance 5-FU-induced chemosensitivity by compromising tumor cell growth or inducing apoptosis.Citation9,Citation13–Citation19 Among the above-mentioned miRNAs, miR-939 and miR-204 were the only two miRNAs with confirmed ability of reversing drug resistance both in vitro and in vivo, providing more convening evidence than others.Citation9,Citation15 A role of miR-31 in chemosensitivity was reported by two separate research groups and may have more obvious effects.Citation17–Citation19 Moreover, reduced miR-6785-5p, miR-193-3p, or miR-147 expression could also increase the chemosensitivity of GC to 5-FU.Citation20–Citation22 Interestingly, miR-193-3p and miR-147 both target phosphatase and tensin homolog (PTEN) to exert their functions in GC.Citation21,Citation22 In addition to miR-193-3p and miR-147, PTEN was identified as the target gene of numerous other miRNAs and to play a role in other drug resistance and treatment mechanisms of GC, which will be discussed in further detail below. The full list of miRNAs reported to be involved in the 5-FU resistance in GC to date is provided in .
Table 1 miRNAs Involved in the 5-Fluorouracil (5-FU) Resistance in Gastric Cancer
Cisplatin Resistance
Cisplatin (DDP) is another first-line chemotherapy drug, and the mechanism of cisplatin resistance has been the most frequently studied according to our review of the literature. As shown in , the majority of reported miRNAs (34 at the time of writing this review) can reverse DDP resistance, whereas some miRNAs (13 included in this review) could induce DDP resistance. Overexpression of miR-421, miR-320a, miR-192-5p, miR-181a, miR-148a-3p, miR-145 and miR-let-7b, and reduced expression of miR-135b-5p and miR-21-5p could enhance the chemosensitivity to DDP, which was observed in both GC cell lines and in animal tumor models.Citation23–Citation30 These miRNAs could become more promising targets for further clinical research after resolving some key technical problems, such as selecting suitable carrier for mimics and inhibitors of miRNAs. Moreover, although miR-218, miR-200c, miR-198, miR34a, and miR-30a were only investigated in cell lines, each of these microRNAs was reported to be involved in DDP resistance in more than one separate study,Citation32–Citation40 suggesting that they warrant further attention.
Table 2 miRNAs Involved in the Cisplatin (DDP) Resistance in Gastric Cancer
The miRNAs reported to be involved in the mechanism of DDP resistance mainly influence the phosphatidylinositol 3-kinase (PI3K)/AKT, mitogen-activated protein kinase (MAPK), and nuclear factor kappa B (NF-κB) signaling pathway.Citation29,Citation30,Citation38,Citation39,Citation41–Citation49 However, different miRNAs may have opposite effects on the same pathway. For example, miR-206 targets MAPK to enhance the chemosensitivity to DDP, and miR-135b-5p targets mammalian ste20-like kinase 1 (MST1) through the MAPK signaling pathway to induce drug resistance.Citation29,Citation43 Thus, it may be necessary to consider the antagonistic effects of different miRNAs in reversing drug resistance in future studies. In addition, some genes were identified to be targets of more than one miRNA, such as survivin (miR-218, miR-34a), PTEN (miR-106a, miR-21-5p), P-glycoprotein (P-gp) (miR-129, miR-30a), and insulin-like growth factor 1 receptor (IGF1R) (miR-1271, miR-143).Citation30,Citation32,Citation38,Citation46,Citation48,Citation50–Citation53 Thus, it will be worth considering whether there is a synergistic effect between different miRNAs in future research on DDP resistance. Furthermore, some long non-coding RNAs (HOTAIR, MALAtl, CASC2) and circular RNAs (hsa_circ0081143, circAKT3) were found to function as competing endogenous RNAs in the regulation of cisplatin resistance.Citation36,Citation39,Citation45,Citation54–Citation56 These findings also provide more possibilities for microRNA-based therapies in GC treatment.
Other Single-Drug Resistance
Some studies have also indicated roles of miRNAs in the resistance of other clinically used chemotherapy drugs, including doxorubicin (DOX; also named adriamycin, ADR), paclitaxel (PTX), oxaliplatin (OXA), vincristine (VCR), taxol, and docetaxel (). DOX, as an anthracycline-based anti-tumor agent, is widely used for the treatment of various solid tumors such as GC. Increased expression levels of miR-494, miR-422a, miR-140, miR-103/107, and miR-16-1, and reduced levels of miR-501-5p and miR-21-5p promoted the chemosensitivity to DOX (for more details, refer to Table 3).Citation57–Citation64 Among these, miR-103 and miR-107 were the only miRNAs demonstrated to have effects on resistance both in vitro and in vivo.Citation60 Another important miRNA that is taking the spotlight in chemotherapy resistance research is miR-21. miR-21-5p targets PTEN to induce both DOX and DDP resistance.Citation30,Citation48,Citation64 Other studies showed that miR-21 targets P-gp to induce PTX resistance.Citation65 Notably, PTEN is not only a target gene of miR-21-5p but is also a target of miR-193-3p, miR-147, and miR-106a.Citation21,Citation22,Citation46 Through targeting PTEN, miRNAs regulate 5-FU, DDP, and DOX resistance in GC.Citation21,Citation22,Citation30,Citation46,Citation48,Citation64 Hence, we speculated that miR-21-5p and its target gene PTEN may play essential and broad roles in regulating drug resistance in GC.
Table 3 miRNAs Involved in Other Drug Resistance in Gastric Cancer
As shown in , limited studies have been conducted to investigate the roles of miRNAs in the resistance mechanisms to other chemotherapy drugs. MiR-155-5p, miR-34c-5p, and miR-21 were found to be related to PTX resistance;Citation65–Citation67 miR-361-3p, miR-135a, and miR-27a affect OXA resistance;Citation68–Citation70 miR-1284 and miR-647 regulated VCR resistance;Citation71,Citation72 and miR-200a inhibited taxol resistance, while miR-361-5p suppressed docetaxel resistance.Citation73,Citation74 Among the above-mentioned miRNAs, only miR-135a, miR-1284, and miR-647 were studied both in vitro and in vivo.Citation69,Citation71,Citation72 Interestingly, miR-361-3p and miR-361-5p were reported to regulate the resistance of different drugs.Citation68,Citation74 This indicates that to best exploit the potential of microRNA-based tumor therapies, we need to pay attention to both the 3p- and 5p-arms of the same miRNA in addition to considering the functions of different miRNAs.
Multidrug Resistance
Although chemotherapy resistance is a challenge for all cancer treatments, it is considered to be a particularly more complex problem in the clinical treatment of GC. In addition to single-drug resistance, multidrug resistance (MDR) may be even more common in clinical practice. Some studies have focused specifically on the roles of miRNAs in MDR (). Both in vitro and in vivo studies confirmed that miR-508-5p, miR-23b-3p, and miR-590-5p strongly regulate MDR.Citation75–Citation79 Shang’s group revealed that the restoration of miR-508-5p reversed the 5-FU, DDP, DOX, and VCR resistance of a xenograft in an animal model by targeting ATP binding cassette subfamily B member 1 (ABCB1) and zinc ribbon domain-containing 1 (ZNRD1).Citation75 The same group further identified that the miR-27b-CCNG1-P53-miR-508-5p axis regulated the MDR of GC.Citation76 They transplanted SGC7901/VCR cells into the right flanks of mice, which were then treated with an agomir for miR-27b or control oligonucleotides before 5-FU or DDP therapy, demonstrating that miR-27b overexpression reversed drug resistance. An et alCitation77 found that miR-23b-3p sensitized GC cells to 5-FU, DDP, and VCR by targeting autophagy-related gene 12 (ATG12) and high-mobility group box 2 (HMGB2). Subsequently, another study showed that the long non-coding RNA MALAT1 regulates autophagy-associated chemoresistance via the miR-23b-3p/ATG12 pathway.Citation78 Shen et alCitation79 found that miR-590-5p reduces the sensitivity of GC cells to DDP and PTX. To further verify the effect of miR-590-5p, stable miR-590-5p-expressing SGC7901 cells were inoculated into nude mice and the tumor-bearing mice were treated with DDP, demonstrating that miR-590-5p reduced chemosensitivity. Compared with other reported miRNAs related to MDR (see for the full list), there is more sufficient and reliable preclinical evidence for these three above-mentioned miRNAs, demonstrating their suitability for further research toward their potential clinical application.
Table 4 miRNAs Involved in Multidrug Resistance (MDR) in Gastric Cancer
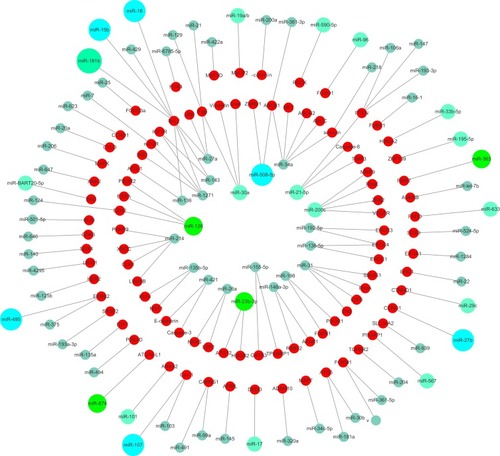
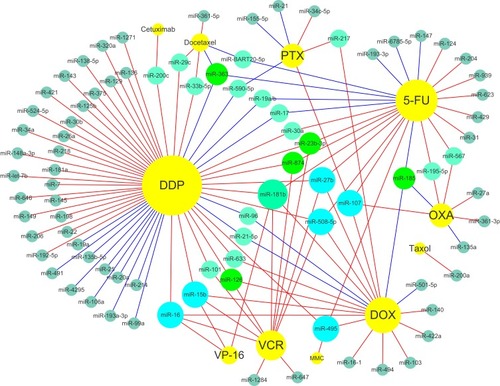
schematically illustrates the proposed relationship between chemotherapy drugs and relevant miRNAs based on the results of studies of single-drug resistance and MDR. shows the relationship between miRNAs that have an effect on drug resistance and their target genes. It is now clear that some miRNAs can regulate the resistance of different drugs simultaneously. Therefore, this review should be beneficial for the design of future drug resistance studies to select the most suitable miRNAs according to different clinical application purposes. Given the pace of this field along with continuous advances in sequencing technologies, we also believe that more miRNAs that participate in the drug resistance mechanisms will be discovered with further research.
Figure 1 Schematic diagram showing the proposed relationship between chemotherapy drugs and relevant miRNAs based on the results of studies of single-drug resistance and MDR. Increased miRNAs (red lines connected) and reduced miRNAs (blue lines connected) could enhance chemosensitivity.
Abbreviations: DDP, cisplatin; 5-FU, 5-fluorouracil; VCR, vincristine; DOX (ADR), doxorubicin (adriamycin); PTX, paclitaxel; OXA, oxaliplatin; L-OHP, oxaliplatin; VP-16, etoposide; MMC, mitomycin.
microRNA-Based Targeted Therapy
Thus far, the majority of research focused on microRNA-based targeted therapy has focused on miRNAs that affect the resistance to targeted drugs (trastuzumab and lapatinib) and development of new types of transport carriers (synthetic nanoparticles/compounds and exosomes).
Trastuzumab and Lapatinib
Trastuzumab as a HER2-targeting antibody has been successfully used in combination with chemotherapy for the treatment of HER2-neu overexpressing GC. Trastuzumab resistance is considered to be a difficult problem in clinical application. Increased miR-223, miR-200c, and reduced miR-21 were reported to reverse trastuzumab resistance via different mechanisms.Citation80,Citation81 MiR-223 could modulate apoptosis to enhance the sensitivity of a HER2-positive GC cell line to trastuzumab through targeting F-box and WD repeat domain-containing 7 (FBXW7),Citation80 whereas miR-200c inhibits TGF-β-induced epithelial–mesenchymal transition to restore trastuzumab sensitivity through inhibiting zinc finger E-box binding homeobox 1 (ZEB1) and ZEB2,Citation81 and miR-21 significantly suppressed trastuzumab-induced apoptosis and decreased the sensitivity of GC cells to trastuzumab through regulating PTEN expression.Citation82 In addition to the above three miRNAs, Sun et alCitation83 reported a correlation between miR-125b and trastuzumab resistance according to the clinicopathologic characteristics of patients with GC. Nishida et alCitation84 reported that the inhibitory effect of miR-125a-5p on GC proliferation was enhanced in combination with trastuzumab. As an alternative strategy, nanoparticles coated with trastuzumab were used as carriers to transfer miRNAs or chemotherapy drugs to the target, which will be discussed further in the following section.
Lapatinib, a type of tyrosine kinase inhibitor, is also commonly used in GC targeted treatment. The resistance to lapatinib can be reversed by miR-494.Citation85 Like trastuzumab, lapatinib can induce miR-16 upregulation in GC sensitive cells via inhibition of c-Myc and the PI3K/AKT and Erk1/2 pathways.Citation86
Synthetic Nanoparticles and Compounds
As mentioned above, some studies have explored the applications of nanoparticles loaded with mimics/inhibitors of miRNAs or some other tumor-targeting compounds. Song’s team constructed a tumor-targeted gene carrier, PPP, through modification of phenylboronic acid onto the surface of a polyamidoamine dendrimer.Citation11 The carrier PPP showed favorable miRNAs binding and condensation ability, protected miRNAs against nuclease degradation, and mediated the cellular uptake of miRNAs.Citation11 Jang’s team used nanovesicles containing poly (l-lysine-graft-imidazole) (PLI)/miRNA complexes (NVs/miR) to systemically deliver miRNA to the target site.Citation87 Incorporation of hydrophilic PEG molecules on the nanoparticle surface could further prolong the blood circulation. Li’s team loaded sorafenib (SRF) and all-trans retinoic acid (ATRA) in PEGylated solid lipid nanoparticles (SLNs) composed of Gelucire, and loaded miRNA onto the surface of the SLNs based on the electrostatic interaction.Citation88 Liu’s team employed intelligent gelatinases-stimuli nanoparticles to co-deliver miRNA and docetaxel to inhibit cancer stem cells.Citation89 Hu’s team and Wu’s team each introduced PCL-PEG nanoparticles coated with trastuzumab (HER-PEG-PCL NPs) to control the delivery of the inhibitor of miRNA.Citation12,Citation90 These studies, respectively, verified the inhibitory function of miR-34a, miR-21, miR-542-3p, and miR-200c on GC in vitro or in vivo. Importantly, these strategies involve the use of synthetic nanoparticles and compounds to attempt to solve the problem of improving microRNAs-targeted transport to tumors so as to promote the clinical application of microRNA-based targeted therapies.
Exosomes
Exosomes are nanosized extracellular membrane-derived vesicles that are secreted by various cells. In 2007, miRNAs were first identified to be transferred in exosomes.Citation91 In a study on the DDP resistance mechanism, miR-21 was identified within the exosomes secreted by tumor-associated macrophages and could be directly transferred from macrophages to GC cells to promote DDP resistance.Citation30 Similarly, the exosomal transfer of miR-501-5p from DOX-resistant GC SGC7901/ADR cells to SGC7901 cells enhanced recipient cell growth, metastasis, and chemoresistance.Citation62 Moreover, the exosomal delivery of miR-155-5p was suggested to induce chemoresistant phenotypes from paclitaxel-resistant GC cells to sensitive cells.Citation66 Given the potential of genetic exchange between cells via exosomes, they have been considered as an alternative promising tool for therapeutic purposes, including in microRNA-based treatment. To support this possibility, one study transfected inhibitors of miR-214 and a negative control into HEK293T cells, and exosomes extracted from the cell medium after 48 h were verified to contain the inhibitor of miR-214, which reversed the resistance to DDP both in GC cells and in vivo.Citation10 In applications for reversing drug resistance or directly suppressing tumors, exosomes containing mimics or inhibitors of miRNAs are likely safer than synthetic nanoparticles and compounds. However, more research is needed to solve exosome-related technical problems, such as the lack of methods to interfere with the packaging of cargo or with vesicle release, before microRNA-based targeted therapies can become a reality.Citation92
microRNA-Based Tumor Immunotherapy
In the past decade, tumor immunotherapy has made great progress in both scientific research and clinical application. Novel drugs target immune checkpoint molecules, such as programmed cell death protein 1 (PD-1), PD-1 ligand cytotoxic, and T lymphocyte antigen-4 (CTLA-4), and augment T-cell activity. The use of immune checkpoint inhibitors has been approved in several tumors, and they are showing remarkable clinical effects. However, only a limited number of GC patients respond to tumor immunotherapy. Few studies have focused on the functions of miRNAs in immunotherapy for GC. Fan et alCitation93 reported that Cbl proto-oncogene B (Cbl-b) as a target gene of miR-940 interacted and ubiquitinated signal transducer and activator of transcription 5a (STAT5a) and down-regulated the expression of STAT5a and anti-programmed death ligand-1 (PD-L1). The miR-940/Cbl-b/STAT5a/PD-L1 axis promoted the proliferation and migration of gastric cancer cells. Accordingly, similar researches can perhaps provide some new ideas and conjectures for microRNA-based immunotherapies of GC.
microRNA-Based Other Therapies
The upstream regulatory mechanism of miRNAs, such as hypermethylation in the promoter region and transcription factor dysregulation, affects the expression of miRNAs. Some miRNAs play key roles in GC tumorigenesis, proliferation, metastasis, and recurrence. In theory, many genes and miRNAs in these processes could become potential therapeutic targets. However, there are too many possible miRNAs and related mechanisms involved in GC to give a detailed description of them in just one paper. Some miRNAs, regarded as potential therapeutic targets, have also had been reviewed elsewhere.Citation94–Citation96 Our review focused more on discussing miRNAs that are more promising for clinical use. We believe that miRNAs that have an effect on GC treatment (including chemotherapy, targeted therapy, immunotherapy, and others) could also become core targets in microRNA-based therapies. It is reported that some drugs with anti-tumor effects may play roles by altering the expression of miRNAs and their related pathways. For example, cinobufacini, which is widely used in the treatment of advanced cancers, was reported to suppress cell proliferation and induce apoptosis in GC by regulating the miR-494-BAG-1 (BCL2 associated athanogene 1) axis.Citation97,Citation98 summarizes the 24 microRNAs reported to be involved in the mechanism of other anti-tumor drugs in GC treatment. Some miRNAs, such as miR-4670-5p, miR-940, miR-493, miR-30e, and miR-7, have been shown to have anti-tumor effects both in vitro and in vivo,Citation99–Citation102 and other miRNAs, such as miR-494, miR-124, miR-34a, miR-21, and miR-9, have been investigated in more than one study.Citation97–Citation114
Table 5 miRNAs Involved in Treatment Mechanisms of Anti-Tumor Drugs
Future Prospects and Challenges
Since Ma et alCitation115 first reported that miRNAs affected the biological behavior of tumors in 2007, more and more functions of miRNAs have been described and more and more miRNAs have been identified in different tumors, including GC. With the continual development of research on miRNAs, scientists are no longer satisfied with exploration of mechanisms but are now actively trying to realize the goal of clinical application and translation of achievements from the laboratory to clinical practice. Hence, microRNAs-based therapy is expected to become a promising research direction and ultimately benefit patients with GC.
The goal of the present review was not only to summarize the relevant literature and advances on the roles of miRNAs in GC treatment, but to further attempt at answering several existing questions. First, we wondered which aspects of GC treatment will microRNA-based therapies be best suited for. As reviewed herein, miRNAs can function in reversing drug resistance, directly inhibiting tumors, or enhancing the anti-tumor effects of drugs. Here, we have mainly focused on the potential for curbing drug resistance and the enhancing the anti-tumor effects of drugs. In terms of directly inhibiting tumors, we have highlighted only some key representative studies, as this field is extremely rich and therefore a comprehensive review is outside of the overall scope. Second, we have tried to assess which miRNAs are most suitable for therapeutic applications. We believe that the miRNAs verified in animal experiments and in more than one study are more reliable for further investigation. Of course, we also acknowledge that this perspective is limited by the extent and scope of current research. Therefore, further verification and screening are needed in future studies. Third, we want to determine the best method to realize the utilization of these miRNAs. Synthesized oligonucleotides of miRNA mimics or inhibitors have been used in an animal tumor model for several years with good results. However, before clinical application, more safety, targeting, and stability issues of this approach need to be considered. Recently, the application of synthetic nanoparticles/compounds and exosomes provides more promising possibilities; however, these techniques also require the combined efforts of doctors, pharmacists, and scientists of other disciplines.
Overall, we believe that this field is currently at an explosive stage of discovery, and hope that this review will promote further applications of this research to realize the ultimate goal of improving the quality of life and outcome of patients with GC.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgment
We are grateful to Dr Pu-Jun Gao who is the doctoral supervisor of Xu Zhao for his guidance in revising this manuscript.
Disclosure
All authors declare they have no conflicts of interest.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.v68.630207593
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.2133826808342
- Van CE, Sagaert X, Topal B, et al. Gastric cancer. Lancet. 2016;388(60):2654–2664. doi:10.1016/S0140-6736(16)30354-327156933
- Orditura M, Galizia G, Sforza V, et al. Treatment of gastric cancer. World J Gastroenterol. 2014;20(7):1635–1649. doi:10.3748/wjg.v20.i7.163524587643
- Murahashi S, Takahari D, Wakatsuki T, et al. A retrospective analysis of ramucirumab monotherapy in previously treated Japanese patients with advanced or metastatic gastric adenocarcinoma. Int J Clin Oncol. 2018;23(1):92–97. doi:10.1007/s10147-017-1192-028913565
- Bang YJ, Van CE, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a Phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi:10.1016/S0140-6736(10)61121-X20728210
- Chen Z, Li Z, Soutto M, et al. Integrated analysis of mouse and human gastric neoplasms identifies conserved microRNA networks in gastric carcinogenesis. Gastroenterology. 2019;156(4):1127–1139. doi:10.1053/j.gastro.2018.11.05230502323
- Miroshnichenko S, Patutina O. Enhanced inhibition of tumorigenesis using combinations of miRNA-targeted therapeutics. Front Pharmacol. 2019;10:488. doi:10.3389/fphar.2019.0048831156429
- Zhang JX, Xu Y, Gao Y, et al. Decreased expression of miR-939 contributes to chemoresistance and metastasis of gastric cancer via dysregulation of SLC34A2 and Raf/MEK/ERK pathway. Mol Cancer. 2017;16(1):18.28114937
- Wang X, Zhang H, Bai M, et al. Exosomes serve as nanoparticles to deliver anti-miR-214 to reverse chemoresistance to cisplatin in gastric cancer. Mol Ther. 2018;26(3):774–783. doi:10.1016/j.ymthe.2018.01.00129456019
- Song Z, Liang X, Wang Y, et al. Phenylboronic acid-functionalized polyamidoamine-mediated miR-34a delivery for the treatment of gastric cancer. Biomater Sci. 2019;7(4):1632–1642. doi:10.1039/C8BM01385C30720809
- Hu N, Yin JF, Ji Z, et al. Strengthening gastric cancer therapy by trastuzumab-conjugated nanoparticles with simultaneous encapsulation of anti-miR-21 and 5-fluorouridine. Cell Physiol Biochem. 2017;44(6):2158–2173. doi:10.1159/00048595529241186
- Jiang L, Yang W, Bian W, et al. MicroRNA-623 targets cyclin D1 to inhibit cell proliferation and enhance the chemosensitivity of cells to 5-fluorouracil in gastric cancer. Oncol Res. 2018;27(1):19–27. doi:10.3727/096504018X1519346924050829495973
- Zhu P, Zhang J, Zhu J, et al. MiR-429 induces gastric carcinoma cell apoptosis through Bcl-2. Cell Physiol Biochem. 2015;37(4):1572–1580. doi:10.1159/00043852426513239
- Li LQ, Pan D, Chen Q, et al. Sensitization of gastric cancer cells to 5-FU by microRNA-204 through targeting the TGFBR2-mediated epithelial to mesenchymal transition. Cell Physiol Biochem. 2018;47(4):1533–1545. doi:10.1159/00049087129940566
- Xie L, Zhang Z, Tan Z, et al. MicroRNA-124 inhibits proliferation and induces apoptosis by directly repressing EZH2 in gastric cancer. Mol Cell Biochem. 2014;392(1–2):153–159. doi:10.1007/s11010-014-2028-024658854
- Sun KK, Shen XJ, Yang D, et al. MicroRNA-31 triggers G2/M cell cycle arrest, enhances the chemosensitivity and inhibits migration and invasion of human gastric cancer cells by downregulating the expression of zeste homolog 2 (ZH2). Archi Biochem Biophys. 2019;663:269–275. doi:10.1016/j.abb.2019.01.023
- Korourian A, Roudi R, Shariftabrizi A, et al. MicroRNA-31 inhibits RhoA-mediated tumor invasion and chemotherapy resistance in MKN-45 gastric adenocarcinoma cells. Exp Biol Med (Maywood). 2017;242(18):1842–1847. doi:10.1177/153537021772846028836853
- Korourian A, Madjd Z, Roudi R, et al. Induction of miR-31 causes increased sensitivity to 5-FU and decreased migration and cell invasion in gastric adenocarcinoma. Bratisl Lek Listy. 2019;120(1):35–39. doi:10.4149/BLL_2019_00530685990
- Yu C, Chen DQ, Liu HX, et al. Rosmarinic acid reduces the resistance of gastric carcinoma cells to 5-fluorouracil by downregulating FOXO4-targeting miR-6785-5p. Biomed Pharmacother. 2019;109:2327–2334. doi:10.1016/j.biopha.2018.10.06130551491
- Jian B, Li Z, Xiao D, et al. Downregulation of microRNA-193-3p inhibits tumor proliferation migration and chemoresistance in human gastric cancer by regulating PTEN gene. Tumour Biol. 2016;37(7):8941–8949. doi:10.1007/s13277-015-4727-x26753960
- Shen J, Niu W, Zhang H, et al. Downregulation of microRNA-147 inhibits cell proliferation and increases the chemosensitivity of gastric cancer cells to 5-fluorouracil by directly targeting PTEN. Oncol Res. 2018;26(6):901–911. doi:10.3727/096504017X1506190253371528950928
- Ge X, Liu X, Lin F, et al. MicroRNA-421 regulated by HIF-1alpha promotes metastasis, inhibits apoptosis, and induces cisplatin resistance by targeting E-cadherin and caspase-3 in gastric cancer. Oncotarget. 2016;7(17):24466–24482. doi:10.18632/oncotarget.822827016414
- Ge X, Cui H, Zhou Y, et al. MiR-320a modulates cell growth and chemosensitivity via regulating ADAM10 in gastric cancer. Mol Med Rep. 2017;16(6):9664–9670. doi:10.3892/mmr.2017.781929152656
- Xie X, Huang N, Zhang Y, et al. MiR-192-5p reverses cisplatin resistance by targeting ERCC3 and ERCC4 in SGC7901/DDP cells. J Cancer. 2019;10(4):1039–1051. doi:10.7150/jca.2581430854110
- Zhao J, Nie Y, Wang H, et al. MiR-181a suppresses autophagy and sensitizes gastric cancer cells to cisplatin. Gene. 2016;576(2 Pt 2):828–833. doi:10.1016/j.gene.2015.11.01326589846
- Li B, Wang W, Li Z, et al. MicroRNA-148a-3p enhances cisplatin cytotoxicity in gastric cancer through mitochondrial fission induction and cyto-protective autophagy suppression. Cancer Lett. 2017;410:212–227. doi:10.1016/j.canlet.2017.09.03528965855
- Han X, Zhang JJ, Han ZQ, et al. Let-7b attenuates cisplatin resistance and tumor growth in gastric cancer by targeting AURKB. Cancer Gene Ther. 2018;25(11–12):300–308. doi:10.1038/s41417-018-0048-830237418
- Zhou J, Chen Q. Poor expression of microRNA-135b results in the inhibition of cisplatin resistance and proliferation and induces the apoptosis of gastric cancer cells through MST1-mediated MAPK signaling pathway. FASEB J. 2019;33(3):3420–3436. doi:10.1096/fj.201800618RRR30576232
- Zheng P, Chen L, Yuan X, et al. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36(1):53. doi:10.1186/s13046-017-0528-y28407783
- Zhi X, Tao J, Xiang G, et al. APRIL induces cisplatin resistance in gastric cancer cells via activation of the NF-κB pathway. Cell Physiol Biochem. 2015;35(2):571–585. doi:10.1159/00036972025612651
- Zhang Z, Kong Y, Yang W, et al. MicroRNA-218 enhances gastric cancer cell cisplatin sensitivity by targeting survivin. Exp Ther Med. 2018;16(6):4796–4802. doi:10.3892/etm.2018.680230542435
- Zhang XL, Shi HJ, Wang JP, et al. MicroRNA-218 is upregulated in gastric cancer after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy and increases chemosensitivity to cisplatin. World J Gastroenterol. 2014;20(32):11347–11355. doi:10.3748/wjg.v20.i32.1134725170221
- Jiang T, Dong P, Li L, et al. MicroRNA-200c regulates cisplatin resistance by targeting ZEB2 in human gastric cancer cells. Oncol Rep. 2017;38(1):151–158. doi:10.3892/or.2017.565928534959
- Ghasabi M, Majidi J, Mansoori B, et al. The effect of combined miR-200c replacement and cisplatin on apoptosis induction and inhibition of gastric cancer cell line migration. J Cell Physiol. 2019. doi:10.1002/jcp.28823
- Huang X, Li Z, Zhang Q, et al. Circular RNA AKT3 upregulates PIK3R1 to enhance cisplatin resistance in gastric cancer via miR-198 suppression. Mol Cancer. 2019;18(1):71. doi:10.1186/s12943-019-0969-330927924
- Gu J, Li X, Li H, et al. MicroRNA-198 inhibits proliferation and induces apoptosis by directly suppressing FGFR1 in gastric cancer. Biosci Rep. 2019;39:6. doi:10.1042/BSR20181258
- Cao W, Yang W, Fan R, et al. MiR-34a regulates cisplatin-induce gastric cancer cell death by modulating PI3K/AKT/survivin pathway. Tumour Biol. 2014;35(2):1287–1295. doi:10.1007/s13277-013-1171-724068565
- Cheng C, Qin Y, Zhi Q, et al. Knockdown of long non-coding RNA HOTAIR inhibits cisplatin resistance of gastric cancer cells through inhibiting the PI3K/Akt and Wnt/β-catenin signaling pathways by up-regulating miR-34a. Int J Biol Macromol. 2018;107(Pt B):2620–2629. doi:10.1016/j.ijbiomac.2017.10.15429080815
- Zhang Z, Kong Y, Yang W, et al. Upregulation of microRNA-34a enhances the DDP sensitivity of gastric cancer cells by modulating proliferation and apoptosis via targeting MET. Oncol Rep. 2016;36(4):2391–2397. doi:10.3892/or.2016.501627513895
- Yan R, Li K, Yuan DW, et al. Downregulation of microRNA-4295 enhances cisplatin-induced gastric cancer cell apoptosis through the EGFR/PI3K/Akt signaling pathway by targeting LRIG1. Int J Oncol. 2018;53(6):2566–2578. doi:10.3892/ijo.2018.459530320337
- Zhou N, Qu Y, Xu C, et al. Upregulation of microRNA-375 increases the cisplatin-sensitivity of human gastric cancer cells by regulating ERBB2. Exp Ther Med. 2016;11(2):625–630. doi:10.3892/etm.2015.292026893657
- Chen Z, Gao YJ, Hou RZ, et al. MicroRNA-206 facilitates gastric cancer cell apoptosis and suppresses cisplatin resistance by targeting MAPK2 signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(1):171–180.
- Shao L, Chen Z, Soutto M, et al. Helicobacter pylori-induced miR-135b-5p promotes cisplatin resistance in gastric cancer. FASEB J. 2019;33(1):264–274. doi:10.1096/fj.201701456RR29985646
- Yan J, Dang Y, Liu S, et al. LncRNA HOTAIR promotes cisplatin resistance in gastric cancer by targeting miR-126 to activate the PI3K/AKT/MRP1 genes. Tumour Biol. 2016;37(12):16345–16355. doi:10.1007/s13277-016-5448-5
- Fang Y, Shen H, Li H, et al. MiR-106a confers cisplatin resistance by regulating PTEN/Akt pathway in gastric cancer cells. Acta Biochim Biophys Sin (Shanghai). 2013;45(11):963–972. doi:10.1093/abbs/gmt10624108762
- Xin L, Yang WF, Zhang HT, et al. The mechanism study of lentiviral vector carrying methioninase enhances the sensitivity of drug-resistant gastric cancer cells to Cisplatin. Br J Cancer. 2018;118(9):1189–1199. doi:10.1038/s41416-018-0043-829576621
- Yang SM, Huang C, Li XF, et al. MiR-21 confers cisplatin resistance in gastric cancer cells by regulating PTEN. Toxicology. 2013;306:162–168. doi:10.1016/j.tox.2013.02.01423466500
- Zhu M, Zhou X, Du Y, et al. MiR-20a induces cisplatin resistance of a human gastric cancer cell line via targeting CYLD. Mol Med Rep. 2016;14(2):1742–1750. doi:10.3892/mmr.2016.541327357419
- Lu C, Shan Z, Li C, et al. MiR-129 regulates cisplatin-resistance in human gastric cancer cells by targeting P-gp. Biomed Pharmacother. 2017;86:450–456. doi:10.1016/j.biopha.2016.11.13928012924
- Du X, Liu B, Luan X, et al. MiR-30 decreases multidrug resistance in human gastric cancer cells by modulating cell autophagy. Exp Ther Med. 2018;15(1):599–605. doi:10.3892/etm.2017.535429375703
- Yang M, Shan X, Zhou X, et al. MiR-1271 regulates cisplatin resistance of human gastric cancer cell lines by targeting IGF1R, IRS1, mTOR, and BCL2. Anticancer Agents Med Chem. 2014;14(6):884–891. doi:10.2174/187152061466614052816131824875127
- Zhuang M, Shi Q, Zhang X, et al. Involvement of miR-143 in cisplatin resistance of gastric cancer cells via targeting IGF1R and BCL2. Tumour Biol. 2015;36(4):2737–2745. doi:10.1007/s13277-014-2898-525492481
- Xue M, Li G, Fang X, et al. hsa_circ_0081143 promotes cisplatin resistance in gastric cancer by targeting miR-646/CDK6 pathway. Cancer Cell Int. 2019;19:25. doi:10.1186/s12935-019-0737-x30733646
- Xi Z, Si J, Nan J. LncRNA MALAT1 potentiates autophagy-associated cisplatin resistance by regulating the microRNA-30b/autophagy-related gene 5 axis in gastric cancer. Int J Oncol. 2019;54(1):239–248. doi:10.3892/ijo.2018.460930365113
- Li Y, Lv S, Ning H, et al. Down-regulation of CASC2 contributes to cisplatin resistance in gastric cancer by sponging miR-19a. Biomed Pharmacother. 2018;108:1775–1782.30372881
- Peng QP, Du DB, Ming Q, et al. MicroRNA 494 increases chemosensitivity to doxorubicin in gastric cancer cells by targeting phosphodiesterases 4D. Cell Mol Biol (Noisy-Le-Grand). 2018;64(15):62–66. doi:10.14715/cmb/2017.64.15.10
- Zhou Z, Lin Z, He Y, et al. The long noncoding RNA D63785 regulates chemotherapy sensitivity in human gastric cancer by targeting miR-422a. Mol Ther Nucleic Acids. 2018;12:405–419. doi:10.1016/j.omtn.2018.05.02430195778
- Zou J, Xu Y. MicroRNA-140 inhibits cell proliferation in gastric cancer cell line HGC-27 by suppressing SOX4. Med Sci Monit. 2016;22:2243–2252. doi:10.12659/MSM.89663327353653
- Zhang Y, Qu X, Li C, et al. MiR-103/107 modulates multidrug resistance in human gastric carcinoma by downregulating Cav-1. Tumour Biol. 2015;36(4):2277–2285. doi:10.1007/s13277-014-2835-725407491
- Zhao D, Zhang Y and Song L. MiR-16-1 targeted silences far upstream element binding protein 1 to advance the chemosensitivity to adriamycin in gastric cancer. Pathol Oncol Res. 2018;24(3):483–488. doi:10.1007/s12253-017-0263-x28667493
- Liu X, Lu Y, Xu Y, et al. Exosomal transfer of miR-501 confers doxorubicin resistance and tumorigenesis via targeting of BLID in gastric cancer. Cancer Lett. 2019;459:122–134. doi:10.1016/j.canlet.2019.05.03531173853
- Xu YC, Liu X, Li M, et al. A novel mechanism of doxorubicin resistance and tumorigenesis mediated by MicroRNA-501-5p-Suppressed BLID. Mol Ther Nucleic Acids. 2018;12:578–590. doi:10.1016/j.omtn.2018.06.01130195794
- Chen J, Zhou C, Li J, et al. MiR-21-5p confers doxorubicin resistance in gastric cancer cells by targeting PTEN and TIMP3. Int J Mol Med. 2018;41(4):1855–1866. doi:10.3892/ijmm.2018.340529393355
- Jin B, Liu Y, Wang H. Antagonism of miRNA-21 sensitizes human gastric cancer cells to paclitaxel. Cell Biochem Biophys. 2015;72(1):275–282. doi:10.1007/s12013-014-0450-227040946
- Wang M, Qiu R, Yu S, et al. Paclitaxel-resistant gastric cancer MGC-803 cells promote epithelial-to-mesenchymal transition and chemoresistance in paclitaxel-sensitive cells via exosomal delivery of miR-155-5p. Int J Oncol. 2019;54(1):326–338. doi:10.3892/ijo.2018.460130365045
- Wu H, Huang M, Lu M, et al. Regulation of microtubule-associated protein tau (MAPT) by miR-34c-5p determines the chemosensitivity of gastric cancer to paclitaxel. Cancer Chemother Pharmacol. 2013;71(5):1159–1171. doi:10.1007/s00280-013-2108-y23423488
- Wu X, Zheng Y, Han B, et al. Long noncoding RNA BLACAT1 modulates ABCB1 to promote oxaliplatin resistance of gastric cancer via sponging miR-361. Biomed Pharmacother. 2018;99:832–838. doi:10.1016/j.biopha.2018.01.13029710482
- Yan LH, Chen ZN, Li L, et al. MiR-135a promotes gastric cancer progression and resistance to oxaliplatin. Oncotarget. 2016;7(43):70699–70714. doi:10.18632/oncotarget.1220827683111
- Zhao Q, Li Y, Tan BB, et al. HIF-1α induces multidrug resistance in gastric cancer cells by inducing miR-27a. PLoS One. 2015;10(8):e0132746. doi:10.1371/journal.pone.013274626292288
- Cao W, Wei W, Zhan Z, et al. MiR-1284 modulates multidrug resistance of gastric cancer cells by targeting EIF4A1. Oncol Rep. 2016;35(5):2583–2591. doi:10.3892/or.2016.464326936591
- Cao W, Wei W, Zhan Z, et al. Regulation of drug resistance and metastasis of gastric cancer cells via the microRNA647-ANK2 axis. Int J Mol Med. 2018;41(4):1958–1966. doi:10.3892/ijmm.2018.338129328428
- Liu X, Du P, Han L, et al. Effects of miR-200a and FH535 combined with taxol on proliferation and invasion of gastric cancer. Pathol Res Pract. 2018;214(3):442–449. doi:10.1016/j.prp.2017.12.00429482988
- Tian L, Zhao Z, Xie L, et al. MiR-361-5p suppresses chemoresistance of gastric cancer cells by targeting FOXM1 via the PI3K/Akt/mTOR pathway. Oncotarget. 2018;9(4):4886–4896. doi:10.18632/oncotarget.2351329435149
- Shang Y, Zhang Z, Liu Z, et al. MiR-508-5p regulates multidrug resistance of gastric cancer by targeting ABCB1 and ZNRD1. Oncogene. 2014;33(25):3267–3276. doi:10.1038/onc.2013.29723893241
- Shang Y, Feng B, Zhou L, et al. The miR27b-CCNG1-P53-miR-508-5p axis regulates multidrug resistance of gastric cancer. Oncotarget. 2016;7(1):538–549. doi:10.18632/oncotarget.v7i126623719
- An Y, Zhang Z, Shang Y, et al. MiR-23b-3p regulates the chemoresistance of gastric cancer cells by targeting ATG12 and HMGB2. Cell Death Dis. 2015;6:e1766. doi:10.1038/cddis.2015.12325996293
- YiRen H, YingCong Y, Sunwu Y, et al. Long noncoding RNA MALAT1 regulates autophagy associated chemoresistance via miR-23b-3p sequestration in gastric cancer. Mol Cancer. 2017;16(1):174. doi:10.1186/s12943-017-0743-329162158
- Shen B, Yu S, Zhang Y, et al. MiR-590-5p regulates gastric cancer cell growth and chemosensitivity through RECK and the AKT/ERK pathway. Onco Targets Ther. 2016;9:6009–6019. doi:10.2147/OTT27757042
- Eto K, Iwatsuki M, Watanabe M, et al. The sensitivity of gastric cancer to trastuzumab is regulated by the miR-223/FBXW7 pathway. Int J Cancer. 2015;136(7):1537–1545. doi:10.1002/ijc.v136.725159729
- Zhou X, Men X, Zhao R, et al. MiR-200c inhibits TGF-β-induced-EMT to restore trastuzumab sensitivity by targeting ZEB1 and ZEB2 in gastric cancer. Cancer Gene Ther. 2018;25(3–4):68–76. doi:10.1038/s41417-017-0005-y29302045
- Eto K, Iwatsuki M, Watanabe M, et al. The microRNA-21/PTEN pathway regulates the sensitivity of HER2-positive gastric cancer cells to trastuzumab. Ann Surg Oncol. 2014;21(1):343–350. doi:10.1245/s10434-013-3325-724154840
- Sui M, Jiao A, Zhai H, et al. Upregulation of miR-125b is associated with poor prognosis and trastuzumab resistance in HER2-positive gastric cancer. Exp Ther Med. 2017;14(1):657–663. doi:10.3892/etm.2017.454828672982
- Nishida N, Mimori K, Fabbri M, et al. MicroRNA-125a-5p is an independent prognostic factor in gastric cancer and inhibits the proliferation of human gastric cancer cells in combination with trastuzumab. Clin Cancer Res. 2011;17(9):2725–2733. doi:10.1158/1078-0432.CCR-10-213221220473
- Yu Y, Yu X, Liu H, et al. MiR-494 inhibits cancer-initiating cell phenotypes and reverses resistance to lapatinib by downregulating FGFR2 in HER2-positive gastric cancer. Int J Mol Med. 2018;42(2):998–1007. doi:10.3892/ijmm.2018.368029786108
- Venturutti L, Cordo RR, Rivas MA, et al. MiR-16 mediates trastuzumab and lapatinib response in ErbB-2-positive breast and gastric cancer via its novel targets CCNJ and FUBP1. Oncogene. 2016;35(48):6189–6202. doi:10.1038/onc.2016.15127157613
- Jang E, Kim E, Son HY, et al. Nanovesicle-mediated systemic delivery of microRNA-34a for CD44 overexpressing gastric cancer stem cell therapy. Biomaterials. 2016;105:12–24. doi:10.1016/j.biomaterials.2016.07.03627497057
- Li T, Zhang Y, Meng YP, et al. MiR-542-3p appended sorafenib/all-trans retinoic acid (ATRA)-loaded lipid nanoparticles to enhance the anticancer efficacy in gastric cancers. Pharm Res. 2017;34(12):2710–2719. doi:10.1007/s11095-017-2202-729181687
- Liu Q, Li RT, Qian HQ, et al. Targeted delivery of miR-200c/DOC to inhibit cancer stem cells and cancer cells by the gelatinases-stimuli nanoparticles. Biomaterials. 2013;34(29):7191–7203. doi:10.1016/j.biomaterials.2013.06.00423806972
- Wu FL, Zhang J, Li W, et al. Enhanced antiproliferative activity of antibody-functionalized polymeric nanoparticles for targeted delivery of anti-miR-21 to HER2 positive gastric cancer. Oncotarget. 2017;8(40):67189–67202. doi:10.18632/oncotarget.1806628978026
- Valadi H, Ekstrom K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi:10.1038/ncb159617486113
- Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. doi:10.1016/j.jconrel.2015.07.03026241750
- Fan Y, Che X, Hou K, et al. MiR-940 promotes the proliferation and migration of gastric cancer cells through up-regulation of programmed death ligand-1 expression. Exp Cell Res. 2018;373(1–2):180–187. doi:10.1016/j.yexcr.2018.10.01130367831
- Polakovicova I, Jerez S, Wichmann IA, et al. Role of microRNAs and exosomes in helicobacter pylori and epstein-barr virus associated gastric cancers. Front Microbiol. 2018;9:636. doi:10.3389/fmicb.2018.0063629675003
- Matsuzaki J, Suzuki H. Role of MicroRNAs-221/222 in digestive systems. J Clin Med. 2015;4(8):1566–1577. doi:10.3390/jcm408156626258795
- Xie M, Dart DA, Owen S, et al. Insights into roles of the miR-1, −133 and −206 family in gastric cancer (Review). Oncol Rep. 2016;36(3):1191–1198. doi:10.3892/or.2016.490827349337
- Shen Z, Li Y, Zhao C, et al. MiR-494-BAG-1 axis is involved in cinobufacini-induced cell proliferation and apoptosis in gastric cancer. Mol Med Rep. 2018;17(5):7435–7441. doi:10.3892/mmr.2018.878829568970
- Zhou RP, Chen G, Shen ZL, et al. Cinobufacin suppresses cell proliferation via miR-494 in BGC-823 gastric cancer cells. Asian Pac J Cancer Prev. 2014;15(3):1241–1245. doi:10.7314/APJCP.2014.15.3.124124606447
- Mikami J, Kurokawa Y, Takahashi T, et al. Antitumor effect of antiplatelet agents in gastric cancer cells: an in vivo and in vitro study. Gastric Cancer. 2016;19(3):817–826. doi:10.1007/s10120-015-0556-226486505
- Jia X, Li N, Peng C, et al. MiR-493 mediated DKK1 down-regulation confers proliferation, invasion and chemo-resistance in gastric cancer cells. Oncotarget. 2016;7(6):7044–7054. doi:10.18632/oncotarget.v7i626799283
- Ye Y, Fang Y, Xu W, et al. 3,3ʹ-Diindolylmethane induces anti-human gastric cancer cells by the miR-30e-ATG5 modulating autophagy. Biochem Pharmacol. 2016;115:77–84. doi:10.1016/j.bcp.2016.06.01827372603
- Cao D, Jiang J, Tsukamoto T, et al. Canolol inhibits gastric tumors initiation and progression through COX-2/PGE2 pathway in K19-C2mE transgenic mice. PLoS One. 2015;10(3):e0120938. doi:10.1371/journal.pone.012093825781635
- Xu S, Li D, Li T, et al. MiR-494 sensitizes gastric cancer cells to TRAIL treatment through downregulation of survivin. Cell Physiol Biochem. 2018;51(5):2212–2223. doi:10.1159/00049586730537730
- Wang X, Li Y, Dai Y, et al. Sulforaphane improves chemotherapy efficacy by targeting cancer stem cell-like properties via the miR-124/IL-6R/STAT3 axis. Sci Rep. 2016;6:36796. doi:10.1038/srep3679627824145
- Zheng YB, Xiao GC, Tong SL, et al. Paeoniflorin inhibits human gastric carcinoma cell proliferation through up-regulation of microRNA-124 and suppression of PI3K/Akt and STAT3 signaling. World J Gastroenterol. 2015;21(23):7197–7207. doi:10.3748/wjg.v21.i23.719726109806
- Zhou Y, Ding BZ, Lin YP, et al. MiR-34a, as a suppressor, enhance the susceptibility of gastric cancer cell to luteolin by directly targeting HK1. Gene. 2018;644:56–65. doi:10.1016/j.gene.2017.10.04629054762
- Wu H, Huang M, Liu Y, et al. Luteolin Induces apoptosis by up-regulating miR-34a in human gastric cancer cells. Technol Cancer Res Treat. 2015;14(6):747–755. doi:10.7785/tcrt.2012.50043424988056
- Wang G, Liu G, Ye Y, et al. Upregulation of miR-34a by diallyl disulfide suppresses invasion and induces apoptosis in SGC-7901 cells through inhibition of the PI3K/Akt signaling pathway. Oncol Lett. 2016;11(4):2661–2667. doi:10.3892/ol.2016.426627073535
- Mohammadian F, Abhari A, Dariushnejad H, et al. Upregulation of mir-34a in AGS gastric cancer cells by a PLGA-PEG-PLGA chrysin nano formulation. Asian Pac J Cancer Prev. 2015;16(18):8259–8263. doi:10.7314/APJCP.2015.16.18.825926745070
- Li H, Cheng J, Mao Y, et al. MiR-21 inhibits the effects of cyclooxygenase-2 inhibitor NS398 on apoptosis and invasion in gastric cancer cells. Onco Targets Ther. 2015;8:3245–3253. doi:10.2147/OTT.S9001226604791
- Yao SS, Han L, Tian ZB, et al. Celastrol inhibits growth and metastasis of human gastric cancer cell MKN45 by down-regulating microRNA-21. Phytother Res. 2019;33(6):1706–1716. doi:10.1002/ptr.635930989726
- Zhang W, Tan Y, Ma H. Combined aspirin and apatinib treatment suppresses gastric cancer cell proliferation. Oncol Lett. 2017;14(5):5409–5417. doi:10.3892/ol.2017.685829142602
- Kiani S, Akhavan-Niaki H, Fattahi S, et al. Purified sulforaphane from broccoli (Brassica oleracea var. italica) leads to alterations of CDX1 and CDX2 expression and changes in miR-9 and miR-326 levels in human gastric cancer cells. Gene. 2018;678:115–123. doi:10.1016/j.gene.2018.08.02630096452
- Mohammadian F, Pilehvar SY, Zarghami F, et al. Upregulation of miR-9 and Let-7a by nanoencapsulated chrysin in gastric cancer cells. Artif Cells Nanomed Biotechnol. 2017;45(6):1–6. doi:10.1080/21691401.2017.1391824
- Ma L. Teruya FJ and Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449(7163):682–688. doi:10.1038/nature0617417898713
- Wang J, Xue X, Hong H, et al. Upregulation of microRNA-524-5p enhances the cisplatin sensitivity of gastric cancer cells by modulating proliferation and metastasis via targeting SOX9. Oncotarget. 2017;8(1):574–582. doi:10.18632/oncotarget.1347927880941
- Lee SD, Yu D, Lee DY, et al. Upregulated microRNA-193a-3p is responsible for cisplatin resistance in CD44(+) gastric cancer cells. Cancer Sci. 2019;110(2):662–673. doi:10.1111/cas.1389430485589
- Li X, Liang J, Liu YX, et al. MiR-149 reverses cisplatin resistance of gastric cancer SGC7901/DDP cells by targeting FoxM1. Pharmazie. 2016;71(11):640–643. doi:10.1691/ph.2016.669629441968
- Ning J, Jiao Y, Xie X, et al. MiR-138-5p modulates the expression of excision repair cross-complementing proteins ERCC1 and ERCC4, and regulates the sensitivity of gastric cancer cells to cisplatin. Oncol Rep. 2019;41(2):1131–1139. doi:10.3892/or.2018.690730535472
- Yu L, Zhou GQ, Li DC. MiR-136 triggers apoptosis in human gastric cancer cells by targeting AEG-1 and BCL2. Eur Rev Med Pharmacol Sci. 2018;22(21):7251–7256. doi:10.26355/eurrev_201811_1625930468468
- Zhang X, Yao J, Guo K, et al. The functional mechanism of miR-125b in gastric cancer and its effect on the chemosensitivity of cisplatin. Oncotarget. 2018;9(2):2105–2119. doi:10.18632/oncotarget.2324929416757
- Zhang Y, Xu W, Ni P, et al. MiR-99a and miR-491 regulate cisplatin resistance in human gastric cancer cells by targeting CAPNS1. Int J Biol Sci. 2016;12(12):1437–1447. doi:10.7150/ijbs.1652927994509
- Wang LL, Zhang XH, Zhang X, et al. MiR-30a increases cisplatin sensitivity of gastric cancer cells through suppressing epithelial-to-mesenchymal transition (EMT). Eur Rev Med Pharmacol Sci. 2016;20(9):1733–1739.27212164
- Wen L, Cheng F, Zhou Y, et al. MiR-26a enhances the sensitivity of gastric cancer cells to cisplatin by targeting NRAS and E2F2. Saudi J Gastroenterol. 2015;21(5):313–319. doi:10.4103/1319-3767.16620626458859
- He J, Qi H, Chen F, et al. MicroRNA-25 contributes to cisplatin resistance in gastric cancer cells by inhibiting forkhead box O3a. Oncol Lett. 2017;14(5):6097–6102. doi:10.3892/ol.2017.698229113252
- Qian X, Xu W, Xu J, et al. Enolase 1 stimulates glycolysis to promote chemoresistance in gastric cancer. Oncotarget. 2017;8(29):47691–47708. doi:10.18632/oncotarget.v8i2928548950
- Xu N, Lian YJ, Dai X, et al. MiR-7 increases cisplatin sensitivity of gastric cancer cells through suppressing mTOR. Technol Cancer Res Treat. 2017;16(6):1022–1030.
- Huang H, Tang J, Zhang L, et al. MiR-874 regulates multiple-drug resistance in gastric cancer by targeting ATG16L1. Int J Oncol. 2018;53(6):2769–2779. doi:10.3892/ijo.2018.459330320370
- Pang X, Zhou Z, Yu Z, et al. Foxo3a-dependent miR-633 regulates chemotherapeutic sensitivity in gastric cancer by targeting Fas-associated death domain. RNA Biol. 2019;16(2):233–248. doi:10.1080/15476286.2019.156566530628514
- Zhang F, Li K, Yao X, et al. A miR-567-PIK3AP1-PI3K/AKT-c-Myc feedback loop regulates tumour growth and chemoresistance in gastric cancer. EBioMedicine. 2019;44:311–321. doi:10.1016/j.ebiom.2019.05.00331078520
- Li N, Han M, Zhou N, et al. MicroRNA-495 confers increased sensitivity to chemotherapeutic agents in gastric cancer via the Mammalian Target of Rapamycin (mTOR) signaling pathway by interacting with human Epidermal Growth Factor Receptor 2 (ERBB2). Med Sci Monit. 2018;24:5960–5972. doi:10.12659/MSM.90945830147110
- Zhang PF, Sheng LL, Wang G, et al. MiR-363 promotes proliferation and chemo-resistance of human gastric cancer via targeting of FBW7 ubiquitin ligase expression. Oncotarget. 2016;7(23):35284–35292. doi:10.18632/oncotarget.916927167197
- Wang H, Qin R, Guan A, et al. HOTAIR enhanced paclitaxel and doxorubicin resistance in gastric cancer cells partly through inhibiting miR-217 expression. J Cell Biochem. 2018;119(9):7226–7234. doi:10.1002/jcb.v119.929856087
- Chang L, Guo F, Wang Y, et al. MicroRNA-200c regulates the sensitivity of chemotherapy of gastric cancer SGC7901/DDP cells by directly targeting RhoE. Pathol Oncol Res. 2014;20(1):93–98. doi:10.1007/s12253-013-9664-723821457
- Nie H, Mu J, Wang J, et al. MiR-195-5p regulates multidrug resistance of gastric cancer cells via targeting ZNF139. Oncol Rep. 2018;40(3):1370–1378. doi:10.3892/or.2018.652429956811
- Tan B, Li Y, Zhao Q, et al. ZNF139 increases multidrug resistance in gastric cancer cells by inhibiting miR-185. Biosci Rep. 2018;38:5. doi:10.1042/BSR20181023
- Zhu W, Shan X, Wang T, et al. MiR-181b modulates multidrug resistance by targeting BCL2 in human cancer cell lines. Int J Cancer. 2010;127(11):2520–2529. doi:10.1002/ijc.2526020162574
- Wang P, Li Z, Liu H, et al. MicroRNA-126 increases chemosensitivity in drug-resistant gastric cancer cells by targeting EZH2. Biochem Biophys Res Commun. 2016;479(1):91–96. doi:10.1016/j.bbrc.2016.09.04027622325
- Teng R, Hu Y, Zhou J, et al. Overexpression of Lin28 decreases the chemosensitivity of gastric cancer cells to oxaliplatin, paclitaxel, doxorubicin, and fluorouracil in part via microRNA-107. PLoS One. 2015;10(12):e0143716. doi:10.1371/journal.pone.014371626636340
- Bao J, Xu Y, Wang Q, et al. MiR-101 alleviates chemoresistance of gastric cancer cells by targeting ANXA2. Biomed Pharmacother. 2017;92:1030–1037. doi:10.1016/j.biopha.2017.06.01128609840
- Lang C, Xu M, Zhao Z, et al. MicroRNA-96 expression induced by low-dose cisplatin or doxorubicin regulates chemosensitivity, cell death and proliferation in gastric cancer SGC7901 cells by targeting FOXO1. Oncol Lett. 2018;16(3):4020–4026. doi:10.3892/ol.2018.912230128023
- Yang X, Zhao Q, Yin H, et al. MiR-33b-5p sensitizes gastric cancer cells to chemotherapy drugs via inhibiting HMGA2 expression. J Drug Target. 2017;25(7):653–660. doi:10.1080/1061186X.2017.132322028436711
- Li C, Zou J, Zheng G, et al. MiR-30a decreases multidrug resistance (MDR) of gastric cancer cells. Med Sci Monit. 2016;22:4509–4515. doi:10.12659/MSM.898415
- Wang Y, Liu C, Luo M, et al. Chemotherapy-induced miRNA-29c/catenin-δ signaling suppresses metastasis in gastric cancer. Cancer Res. 2015;75(7):1332–1344. doi:10.1158/0008-5472.CAN-14-078725634213
- Fang Q, Chen X, Zhi X. Long non-coding RNA (LncRNA) urothelial carcinoma associated 1 (UCA1) increases multi-drug resistance of gastric cancer via downregulating miR-27b. Med Sci Monit. 2016;22:3506–3513. doi:10.12659/MSM.90068827694794
- Kim H, Choi H, Lee SK. Epstein-Barr virus miR-BART20-5p regulates cell proliferation and apoptosis by targeting BAD. Cancer Lett. 2015;356(2 Pt B):733–742. doi:10.1016/j.canlet.2014.10.02325449437
- Zhu F, Wu Q, Ni Z, et al. MiR-19a/b and MeCP2 repress reciprocally to regulate multidrug resistance in gastric cancer cells. Int J Mol Med. 2018;42(1):228–236. doi:10.3892/ijmm.2018.358129568890
- Wu DM, Hong XW, Wang LL, et al. MicroRNA-17 inhibition overcomes chemoresistance and suppresses epithelial-mesenchymal transition through a DEDD-dependent mechanism in gastric cancer. Int J Biochem Cell Biol. 2018;102:59–70. doi:10.1016/j.biocel.2018.06.00729953965
- Xia L, Zhang D, Du R, et al. MiR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J Cancer. 2008;123(2):372–379. doi:10.1002/ijc.2350118449891
- Xing Z, Yu L, Li X, et al. Anticancer bioactive peptide-3 inhibits human gastric cancer growth by targeting miR-338-5p. Cell Biosci. 2016;6:53. doi:10.1186/s13578-016-0112-827688872
- Zhao H, Zhao D, Tan G, et al. Bufalin promotes apoptosis of gastric cancer by down-regulation of miR-298 targeting bax. Int J Clin Exp Med. 2015;8(3):3420–3428.26064232
- You HY, Xie XM, Zhang WJ, et al. Berberine modulates cisplatin sensitivity of human gastric cancer cells by upregulation of miR-203. In Vitro Cell Dev Biol Anim. 2016;52(8):857–863. doi:10.1007/s11626-016-0044-y27142767
- Zhang W, Wang Y, Zhu Z, et al. Propofol inhibits proliferation, migration and invasion of gastric cancer cells by up-regulating microRNA-195. Int J Biol Macromol. 2018;120(Pt A):975–984. doi:10.1016/j.ijbiomac.2018.08.17330171944
- Zhang F, Ma C. Kaempferol suppresses human gastric cancer SNU-216 cell proliferation, promotes cell autophagy, but has no influence on cell apoptosis. Braz J Med Biol Res. 2019;52(2):e7843. doi:10.1590/1414-431x2018784330785478
- Xiang F, Pan C, Kong Q, et al. Ursolic acid inhibits the proliferation of gastric cancer cells by targeting miR-133a. Oncol Res. 2014;22(5–6):267–273. doi:10.3727/096504015X1441023848668526629938
- Shekari N, Javadian M, Ghasemi M, et al. Synergistic beneficial effect of docosahexaenoic acid (DHA) and docetaxel on the expression level of matrix metalloproteinase-2 (MMP-2) and microRNA-106b in gastric cancer. J Gastrointest Cancer. 2019. doi:10.1007/s12029-019-00205-0
- Mohammadian F, Pilehvar SY, Mofarrah M, et al. Down regulation of miR-18a, miR-21 and miR-221 genes in gastric cancer cell line by chrysin-loaded PLGA-PEG nanoparticles. Artif Cells Nanomed Biotechnol. 2016;44(8):1972–1978. doi:10.3109/21691401.2015.112961526772615
- Mohammadian F, Pilehvar SY, Alipour S, et al. Chrysin Alters microRNAs expression levels in gastric cancer cells: possible molecular mechanism. Drug Res (Stuttg). 2017;67(9):509–514. doi:10.1055/s-0042-11964728628924
- Jia J, Zhan D, Li J, et al. The contrary functions of lncRNA HOTAIR/miR-17-5p/PTEN axis and Shenqifuzheng injection on chemosensitivity of gastric cancer cells. J Cell Mol Med. 2019;23(1):656–669. doi:10.1111/jcmm.1397030338929
- Zhu C, Huang Q, Zhu H. Melatonin inhibits the proliferation of gastric cancer cells through regulating the miR-16-5p-Smad3 pathway. DNA Cell Biol. 2018;37(3):244–252. doi:10.1089/dna.2017.404029359963